AbstractPurpose:This study aimed to evaluate the ultrasonographic findings for various types of vascular closure devices (VCDs) immediately after the angiographic procedure and at 6-month follow-up.
Methods:We included 18 VCDs including Angio-Seal (n=4), FemoSeal (n=8), ExoSeal (n=3), Perclose (n=2), and StarClose (n=1) in this study. Four patients were implanted with 2 VCDs at the each side of bilateral femoral arteries, while the remaining 8 patients were inserted 1 VCD at the right femoral artery. Ultrasonography was performed within 10 days and at approximately 6 months after the angiographic procedure. Ultrasonographic morphology of the attached VCD and its relationship with the arterial wall were analyzed.
Results:Initial ultrasonography revealed the attached VCD as the relevant unique structure with successful deployment and hemostasis. Follow-up ultrasonography demonstrated partial absorption of hemostatic materials in cases of Angio-Seal (n=3), FemoSeal (n=5), and ExoSeal (n=3), changes in the soft tissue surrounding the femoral artery in case of Angio-Seal (n=1), arterial intimal hyperplasia in cases of FemoSeal (n=3), and no gross changes as compared with the initial ultrasonographic findings in cases of Perclose (n=2) and StarClose (n=1).
With the evolution of medical technology, interventional radiology has become a key component of modern diagnostic and treatment methods [1]. In recent times, with increasing numbers of patients suffering from vascular diseases, the number of percutaneous endovascular procedures has correspondingly increased, with the majority of clinicians and patients preferring these less invasive and less risky procedures over major surgery [2].
Hemostasis at the puncture site has a major impact on patient recovery and turnaround time. Manual compression has been widely used as an efficient tool for hemostasis. Vascular closure devices (VCDs) were developed in the 1990s as a faster and more convenient means of achieving hemostasis [1]. VCDs allow for the immediate closure of the arteriotomy site using various methods, such as collagen plugs, sutures, and clips. Sometimes, puncture sites are associated with certain complications, including simple hematoma, pseudoaneurysm, and distal ischemia [1]. Although VCDs are easy to use in the clinical setting, these associated complications are detected based on the patients’ subjective complaints; in the absence of such complaints, dangerous complications may remain undiagnosed. Therefore, the assessment of the puncture site following VCD deployment is essential in order to avoid overlooking any serious morbidity.
Since the femoral artery is located superficially, it is easily accessible for ultrasonographic examination. Ultrasonography can identify vascular flow, which helps in confirming simple hematomas, pseudoaneurysms, and distal vascular poor runoff. Furthermore, ultrasonography provides a simple and convenient diagnostic approach that can be performed before patient ambulation. Until now, the ultrasonographic studies for various types of VCDs have been seldom reported. Without knowledge of the ultrasonographic findings of the VCDs, the normal VCDs can be misinterpreted as the complication during ultrasonographic exam. Vice versa , the associated complications with the applied VCDs can be misinterpreted as normal.
Therefore, this study aimed to demonstrate the in vivo ultrasonography findings for various types of VCDs and to compare the initial and follow-up ultrasonographic findings for each VCD.
This study was approved by Inje University Busan Paik Hospital Institutional Review Board for human research. Informed consent was waived. From May 10, 2012, to June 25, 2012, 12 patients (1 male, 11 females; mean age, 56.3 years; range, 42 to 79 years) who admitted for neurovascular catheterization procedures and underwent femoral artery punctures were included in this study. None of them had any coagulation disorders. Overall, 16 VCDs were deployed in the 12 patients as follows: 8 patients received only 1 VCD in the right femoral artery, while 2 VCDs were employed in the remaining 4 patients, with 1 VCD in the right and left femoral artery each. The applied VSDs were 5 to 6F in this study.
Ultrasonographic studies, including gray-scale and color Doppler, were performed by 2 experienced radiologists using the iU22 model (Philips Medical Systems, Bothell, WA, USA) and a 5-12 MHz linear probe. Ultrasonographic findings for the VCD sites were initially investigated within 10 days after the angiographic procedure, and follow-up ultrasonography was performed at approximately 6 months after the angiographic procedure. Ultrasonographic findings from the femoral puncture sites were evaluated for the identification of the structure changes of the deployed VCD, vascular patency, perivascular and overlying subcutaneous tissue change and the presence of complications.
In this study, 5 types of commercially available VCDs (Angio-Seal, St. Jude Medical, St. Paul, MN, USA; FemoSeal, St. Jude Medical; ExoSeal, Cordis Europe, Waterloo, Belgium; Perclose, Abbott Labs, Redwood City, CA, USA; and StarClose, Abbott Vascular, Santa Clara, CA, USA) were evaluated. The Angio-Seal group comprised 4 cases; the FemoSeal group, 8 cases; the ExoSeal group, 3 cases; the Perclose group, 2 cases; and the StarClose group, 1 case.
On initial ultrasonography, except for Perclose, each type of the attached VCDs was clearly visualized based on their unique structures; clustered hypoechoic lesions over the arterial wall, suggesting the collagen plug were observed in the Angio-Seal group; intra-arterial linear echogenicity, suggesting the intraarterial anchor plate in the FemoSeal group; extravascular tubular hypoechogenicity, suggesting the hematostatic plug in the ExoSeal group; very hyperechogenic line at the perivascular area, suggesting the metal clip in the StarClose group. No major complications, such as inappropriate application of the VCD, unsatisfactory hemostasis, puncture site infection, or disruption of distal arterial flow, were observed.
Follow-up ultrasonograms demonstrated various changes as compared to the initial ultrasonogram; these were listed in Table 1. No adverse findings, such as femoral artery stenosis, were observed.
The Angio-Seal group (n=4) showed partial absorption of hemostatic material (clustered hypoechoic lesions over the arterial wall) in 3 cases (Fig. 1) and perivascular soft-tissue change in 1 case. In the FemoSeal group (n=8), partial absorption of hemostatic material (intra-arterial linear echogenicity) was observed in 5 cases and hypoechoic thickening of the arterial intima with partial absorption of hemostatic material was demonstrated in 3 cases (Fig. 2). The ExoSeal group (n=3) showed partial absorption of hemostatic material (extravascular tubular hypoechogenicity) in all the cases (Fig. 3). The Perclose group (n=2) and StarClose group (n=1) showed no changes as compared to the initial ultrasonographic findings (Figs. 4, 5).
VCDs were introduced in the early 1990s as a method for hemostasis in order to reduce the procedure time, labor, bed rest period, and patient discomfort associated with manual compression. At present, two types of VCDs are available, i.e., active and passive. A passive VCD indirectly acts at the puncture site, utilizing an external patch or balloon coated with prothrombotic material. Thus, it does not achieve prompt hemostasis (<5 minutes) or shorten the time to ambulation. In contrast, an active VCD directly acts at the puncture site, utilizing suture devices, collagen plug devices, or clips [3]. In the present study, only active VCDs were used in all the enrolled patients, as described below.
The Angio-Seal is a bioabsorbable collagen closure device, composed of an intra-arterial anchor, small bovine collagen plug, and absorbable traction suture. The intra-arterial anchor is a T-shaped material composed of copolymers of polylactic and polyglycolic acids. The anchor and collagen plug are inserted into the femoral site; after deployment, the anchor and collagen plug work in tandem to compress the puncture site effectively. In the previous study using a porcine aorta, the collagen plug over the artery was identified on the ultrasonography [4]. Like that, clustered hypoechoic lesions above the arterial wall on initial ultrasonography of the present study may be the collagen plus, although the histologic confirmation about the lesion was not preformed. The hypoechoic lesions above the artery were not completely absorbed at 6 months of follow-up in all the cases of the present study. Further, the perivascular soft tissue change, manifested oval hypoechoic lesion on ultrasonography was combined in one case. It can be postulated that tissue reaction was induced by the collagen plug, which may act as an obstacle in repuncturing the same femoral artery later.
The FemoSeal comprises a bioabsorbable polymer anchor plate and an outer disc. The anchor seal is deployed within the artery, while the outer locking disc is placed on the outer wall of the artery [5]. Initial ultrasonography of the applied FemoSeal successfully showed a linear echogenic intra-arterial lesion, which looked like the appearance of the bioabsorbable anchor plate. After 6 months, the lesion regarded as the bioabsorbable materials had partially disappeared (8/8), while some of them (3/8) coexisted with hypoechoic thickening of the arterial intima. In the previous article [6], intimal hyperplasia occurred after vessel injury, which was manifested as the hypoechoic thickening of the arterial intima. Intimal hyperplasia is a physiologic response to injury to the blood vessel wall and results from vascular surgery and endovascular intervention. In the present study, the hypoechoic thickening of the intima may be an intimal hyperplasia and be caused by vessel wall reaction to the larger intra-arterial anchor plate. However, no serious luminal narrowing with intimal hyperplasia was observed in the present study.
The ExoSeal is advanced through a preexisting standard procedural sheath with an indicator wire loop deployed inside the artery. A tubular bioabsorbable plug is released on the surface of the vessel without an intra-arterial anchor [5]. On the initial ultrasonographic exam of this study, the tubular hypoechoic lesions at the extruluminal area of the femoral artery, which looked like the appearance of the bioabsorbable plug were demonstrated, and on the follow-up exam they were resorbed partially.
The Perclose device is a suture-mediated closure system. After positioning the device appropriately, a braided 3-0 polyester suture is deployed, and the device is removed after the knot has been tightened around the puncture site. These suture-attached needles puncture through the artery and are engaged by the barrel of the delivery system. Once engaged in the arterial wall, the sutures are manually tied in a conventional surgical slipknot, which is pushed down toward the surface of the arteriotomy to achieve suture-mediated closure [2]. In this study, the initial and follow-up ultrasonography showed no definite findings related to the VCD.
The StarClose device features a flexible nitinol clip measuring 4 mm in diameter, which is designed to close femoral arteriotomies created by sheaths up to 6F in size. An implantable nitinol clip is engaged in the adventitial layer of the vessel, where its tines grasp the edges of arteriotomy and draw them together to close the puncture hole. In contrast to collagen-based devices, the nitinol clip used in the StarClose device is reported to have a low profile in bioreactivity and in inducing inflammatory soft tissue reaction [2]. A study involving the StarClose VCD has demonstrated the linear hyperechogenicity outside of the vascular wall on the ultrasonographic exam with no episodes of arterial or venous thrombosis or stenosis [7]. The present study also demonstrated the very hyperechoic line outside the vascular wall on the initial ultrasonography for the StarClose VCD, which was not changed on follow-up ultrasonography.
This study had limitations. First, the small number of patients was enrolled. Second, the number of each VCD was uneven. Third, the histologic confirmation about the ultrasonographic findings of VCDs was not performed. Moreover, there are few previous ultrasonographic or histologic studies about the VCDs except Angio-Seal and StarClose. Thus, there is little definite evidence to interpret the ultrasonographic findings. However, because the initial ultrasonographic appearance of each VCD was very similar to the unique structure of the each VCD, the findings of intra-arterial linear echogenicity in the FemoSeal group and extravascular tubular hypoechogenicity in the ExoSeal group were interpreted as the hemostatic materials of the VCDs. It requires a further histologic study.
In conclusion, the initial ultrasonographic evaluation of each VCD reflected its unique structure, with most of them being easily distinguishable. In follow-up ultrasonography, various changes in the affected vessels were observed. These results are expected to be helpful to differentiate the normal VCD and complicated VCDs. Further studies may be required for evaluating the long-term outcomes for each VCD type.
AcknowledgementsThis study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.
References1. Das R, Ahmed K, Athanasiou T, Morgan RA, Belli AM. Arterial closure devices versus manual compression for femoral haemostasis in interventional radiological procedures: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2011;34:723–738.
2. Bechara CF, Annambhotla S, Lin PH. Access site management with vascular closure devices for percutaneous transarterial procedures. J Vasc Surg 2010;52:1682–1696.
3. Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle DM, Mayeda GS. Review of vascular closure devices. J Invasive Cardiol 2010;22:599–607.
4. Corkill RA, Hughes PM, Elford JC, Roobottom CA. The in vitro and in vivo ultrasonographic appearances of the angio-seal percutaneous closure device. Clin Radiol 2002;57:930–936.
5. Byrne RA, Cassese S, Linhardt M, Kastrati A. Vascular access and closure in coronary angiography and percutaneous intervention. Nat Rev Cardiol 2013;10:27–40.
6. Schaberle W. Ultrasonography in vascular diagnosis: a therapy oriented textbook and atlas. 2nd ed. Berlin: Springer-Verlag, 2011.
Angio-Seal closure device for a 66-year-old woman.Photographies show Angio-Seal closure device (A) and its deployment into the silicone tube (B). Angio-Seal is composed of an intra-arterial anchor (arrowheads) and a small bovine collagen plug (arrow). After deployment, the anchor and collagen plug worked in tandem to compress the puncture site effectively. Initial longitudinal gray-scale ultrasonogram of the Angio-Seal deployed at the left femoral artery (C) shows a hypoechoic collagen plug (arrows) outside of the vessel wall. Follow-up ultrasonogram (D) reveals partial resorption of the collagen plug (arrow).
 Fig. 1.FemoSeal closure device for a 55-year-old man.Photographies show FemoSeal closure device (A) and its deployment into the silicone tube (B). FemoSeal comprises a relatively large bioabsorbable polymer anchor plate (arrowheads) and a relatively small outer disc (arrow). The anchor plate was deployed within the artery, while the outer locking disc was placed on the outer wall of the artery. Initial longitudinal gray-scale ultrasonogram of the FemoSeal deployed at the right femoral artery (C) reveals a linear-shaped echogenic anchor (arrows) at the intraluminal area of the femoral artery. Follow-up ultrasonogram (D) shows intimal hyperplasia with partial resorption of the anchor (arrows).
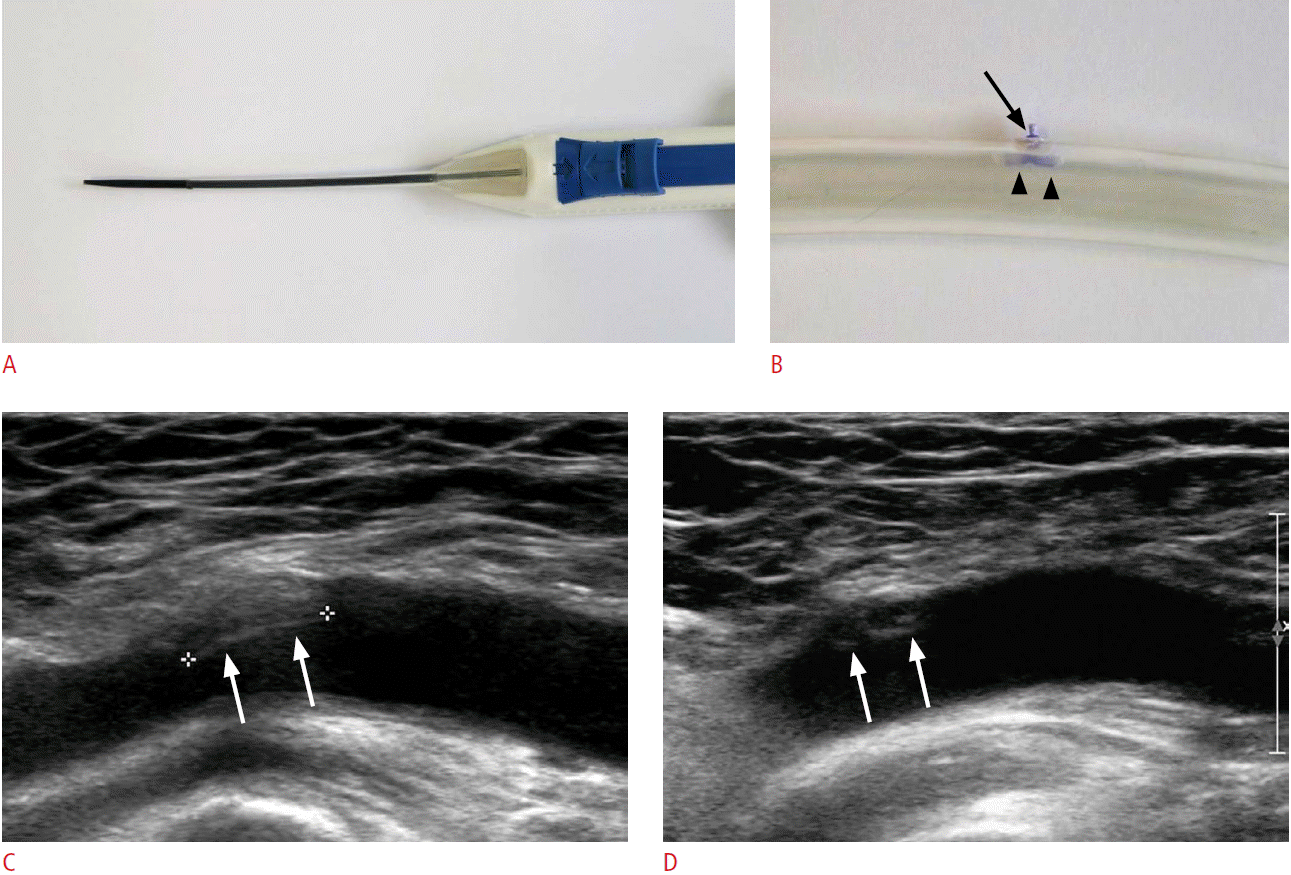 Fig. 2.ExoSeal closure device for a 43-year-old man.Photographies show ExoSeal closure device (A) and its deployment into the silicone tube (B). ExoSeal is composed of a tubular bioabsorbable plug (arrow). The ExoSeal was advanced through a pre-existing standard procedural sheath with an indicator wire loop deployed inside the artery. The hemostatic bioabsorbable plug was released on the surface of the vessel without an intra-arterial anchor. Initial longitudinal grayscale ultrasonogram of the ExoSeal deployed at the right femoral artery (C) shows a hypoechoic extravascular hemostatic plug (arrows) outside of the femoral artery. Follow-up ultrasonogram (D) reveals partial resorption of the hemostatic plug (arrows).
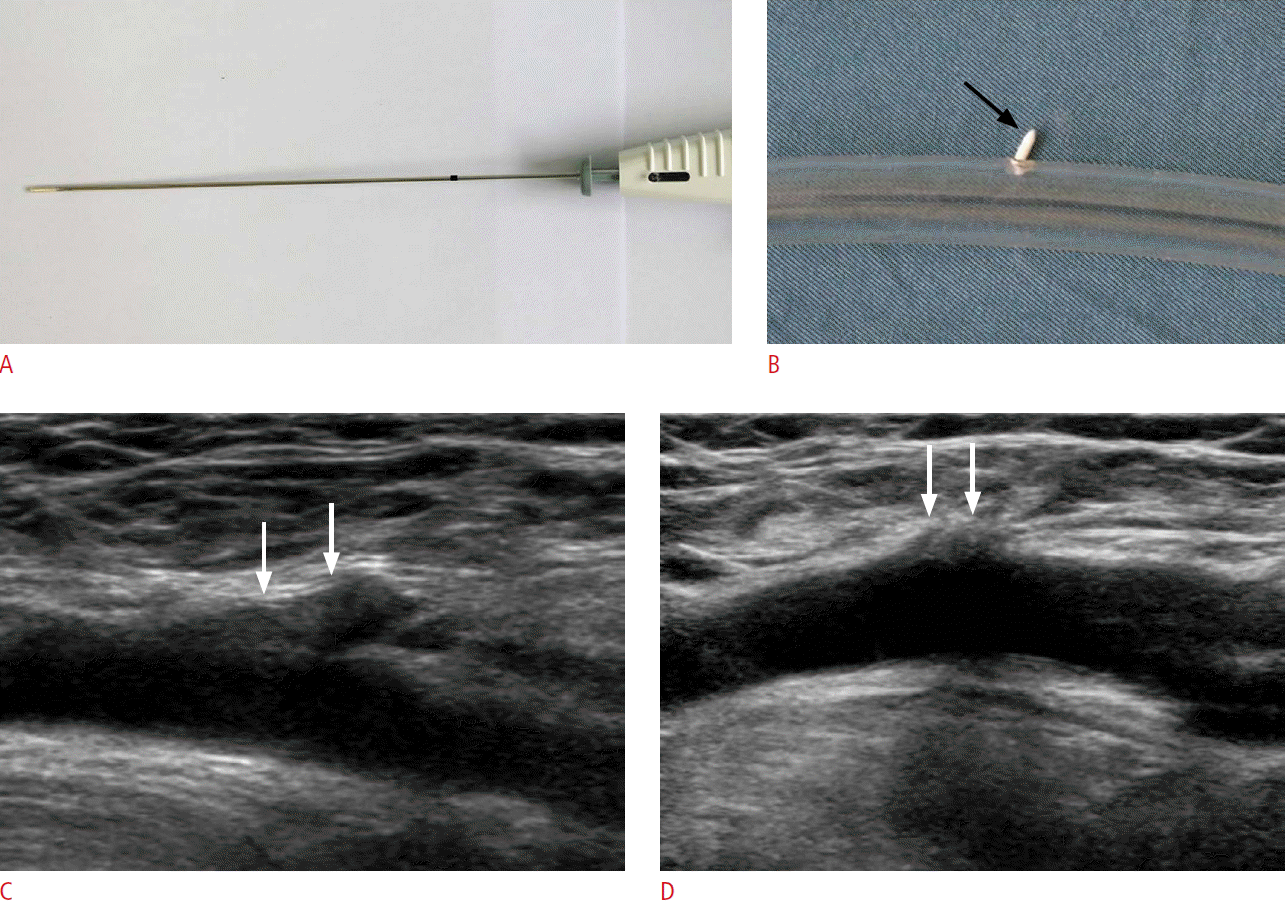 Fig. 3.Perclose closure device for a 61-year-old woman.Photographies show Perclose closure device (A) and its deployment into the fabric (B). The Perclose is a suture-mediated closure system. After positioning the device appropriately, a braided 3-0 polyester suture was deployed, and the device was removed after the knot (arrow) has been tightened around the puncture site. Initial longitudinal gray-scale ultrasonogram of the Perclose deployed at the right femoral artery (C) shows no definite abnormality except the very subtle perivascular hyperechogenicity (arrows). Follow-up ultrasonogram (D) demonstrates no findings inside and outside of the vessels.
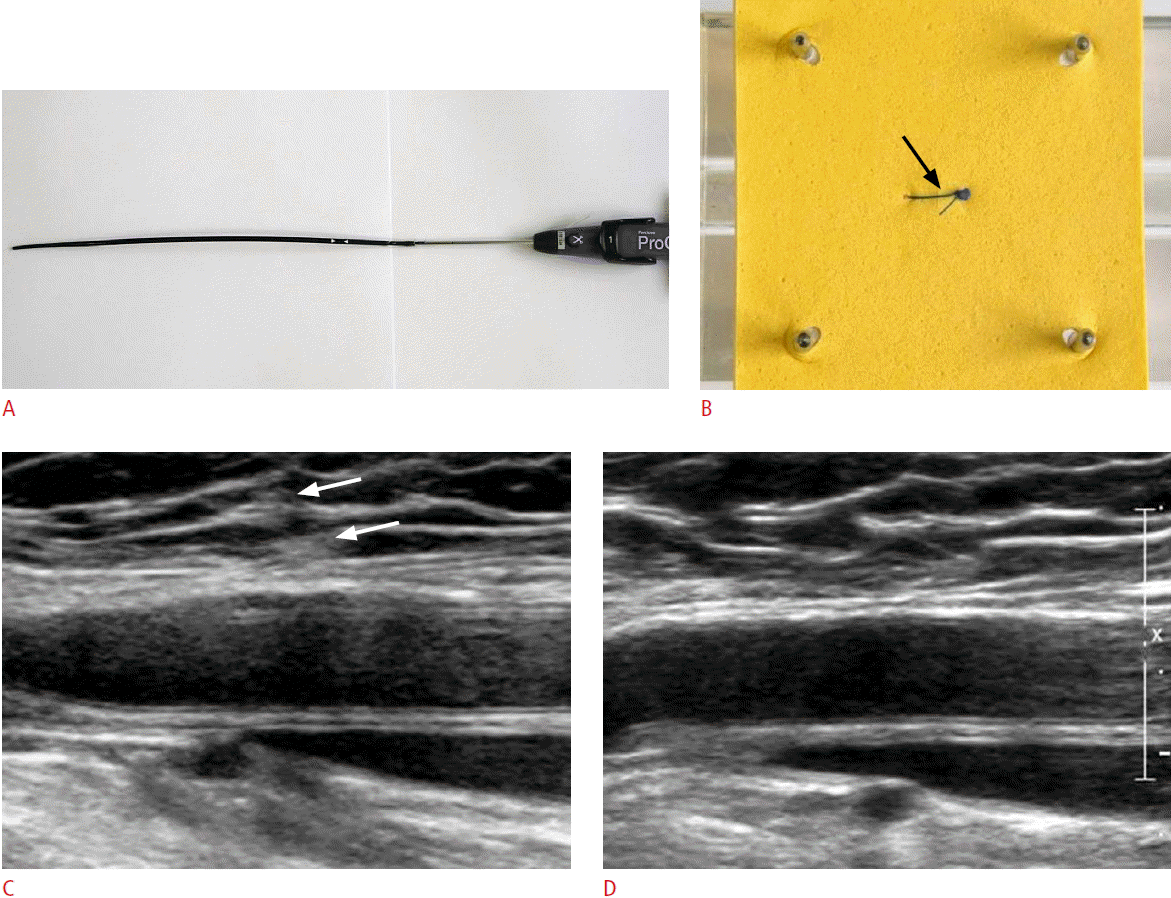 Fig. 4.StarClose closure device for a 48-year-old woman.Photographies show StarClose closure device (A) and its deployment into the fabric (B). The StarClose consists of a flexible nitinol clip measuring 4 mm in diameter (arrow), which was engaged in the adventitial layer of the vessel. Initial longitudinal gray-scale ultrasonogram of the StarClose deployed at the right femoral artery (C) shows a hyperechoic linear metal clip (arrow) at the extraluminal area of the right femoral artery. Follow-up ultrasonogram (D) reveals no specific change in the echogenic metal clip (arrow) at the femoral artery or the perivascular soft tissue.
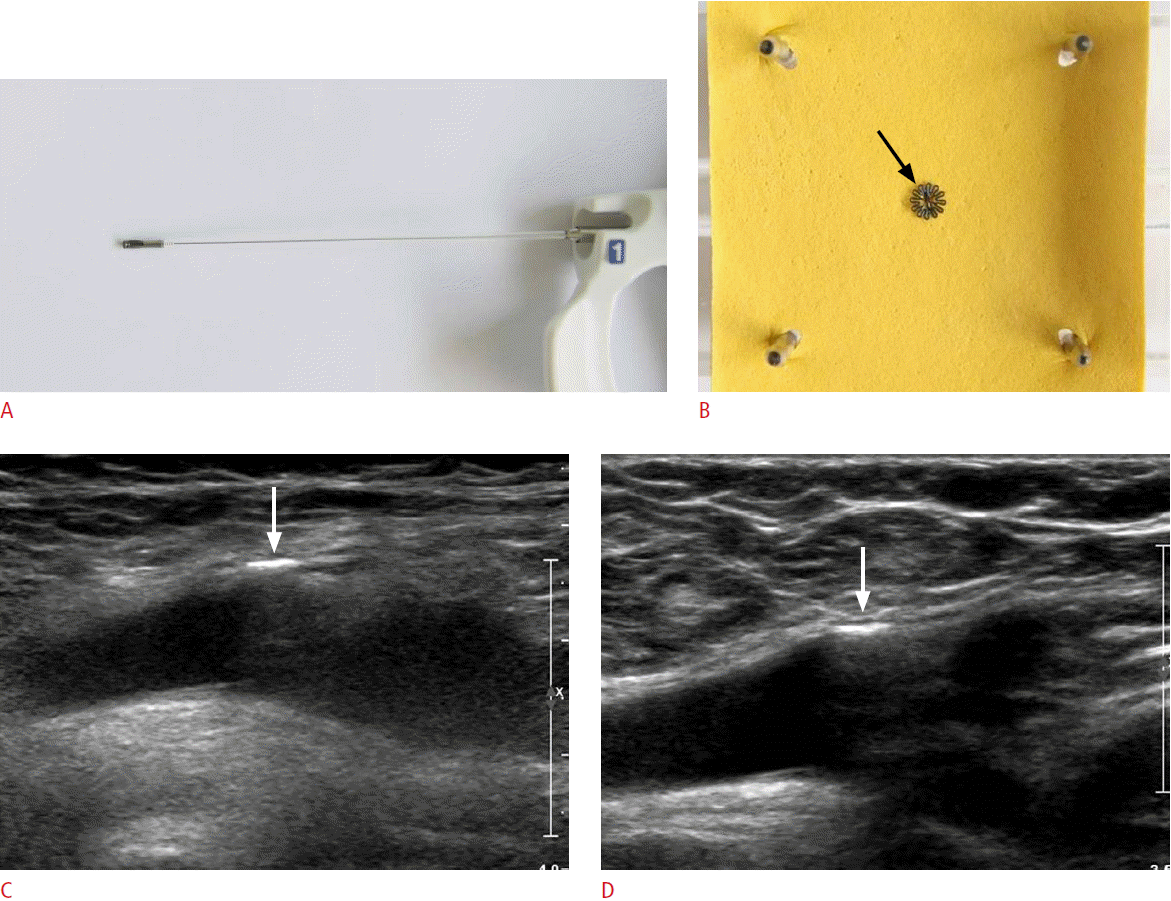 Fig. 5.Table 1.Follow-up periods and ultrasonographic findings of vascular closure devices
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




