Cheon: Intraoperative neurosonography revisited: effective neuronavigation in pediatric neurosurgery
Abstract
Intraoperative ultrasonography (IOUS) is a widely used noninvasive method to evaluate the morphology, vasculature, and pathologies of the brain. The advantages of IOUS include realtime depiction of neuroanatomy, accurate localization and characterization of a lesion, reduced surgical exploration and surgical time, and presumably decreased patient morbidity. IOUS is useful in the intraoperative monitoring of lesion resection as well as intraoperative localization and characterization of focal parenchymal lesions. This review aims to provide an overview of the clinical application of IOUS in pediatric intracranial neurosurgery.
Keywords: Monitoring, intraoperative; Neuronavigation; Neurosurgery; Pediatrics; Ultrasonography
Introduction
Image-guided neurosurgery based on neuronavigation is widely used and has become an essential tool for intracranial neurosurgery in recent decades [ 1- 3]. However, the accuracy of neuronavigation systems based on preoperative imaging data is limited due to intraoperative changes such as “brain shift.” Brain shift may be caused by various factors, including the effect of gravity on the brain, loss of cerebrospinal fluid (CSF), pneumocephalus, brain swelling, and surgical procedures [ 4, 5]. Both intraoperative magnetic resonance imaging (MRI) and intraoperative computed tomography (CT) are able to address this limitation [ 6, 7]. Unfortunately, these techniques require long image acquisition times, enormous economic investment, special surgical equipment, and specialized workspaces. In addition, intraoperative CT is rarely acceptable for pediatric patients because of the ionizing radiation [ 2]. Further improvements in imaging modalities, such as advanced intraoperative ultrasonography (IOUS), present a promising alternative. IOUS is truly produced in real time. The ability to depict realtime anatomic data during a surgical procedure is very useful for surgical decision making. IOUS is an effective way to localize and characterize juxtacortical lesions and to assist with the monitoring of the surgical field for the improvement of resectability and reduction of the risk of parenchymal injury [ 2, 8, 9]. The aim of this study was to review the clinical application of IOUS and to show representative cases of IOUS as an effective tool for neuronavigation.
Basic Considerations
IOUS requires a high-end ultrasound machine, combined with a 5-7-MHz sector transducer and a 7-12-MHz linear transducer, for optimal lesion detection and depiction. The transducer should be wrapped in a sterile sheath and applied directly to the intact dura or the exposed brain through craniostomy. The use of sterile saline before the application of the transducer improved the acoustic coupling ( Fig. 1). An overview of the cerebral structures is easily obtained using a sector transducer with deep penetration to identify reliable anatomic landmarks. Both the anechoic CSF in the lateral ventricle and the echogenic falx cerebri are useful landmarks for lesion orientation. During the examination, the insonation depth can be sequentially reduced to provide more detailed observations [ 8- 10]. With a relatively high-frequency linear transducer, the low-echoic cortical ribbon can be differentiated from the brighter echogenicity of the subcortical white matter ( Fig. 2). Color Doppler ultrasonography is useful for the evaluation of tumor vascularity. It can provide important anatomical information about the location of feeding and draining vessels and the displacement of normal brain vessels ( Fig. 3). The power mode may be recommended for the depiction of low-flow vessels within tumors. The pulse repetition frequency should be set as low as possible to avoid aliasing in normal vessels [ 11, 12].
Localization of Brain Tumors
The goal of cerebral glioma surgery is not only maximal but also safe tumor resection; that is, the maximum neurologically permissible tumor volume should be resected, but eloquent areas must be spared and neurological damage minimized. These two antagonistic goals can be achieved through accurate localization of the extent of tumors, and intraoperative mapping of eloquent areas required for functions such as language, sensation, or movement. The main advantage of IOUS in brain tumor surgery lies in its realtime property. It can generate a complete overview of the tumor and the surrounding structures within seconds ( Fig. 4). In deepseated lesions, IOUS can be used to find a suitable cleavage plane for resection. It also allows sonographic guidance of a tumor biopsy or puncture of the cystic tumor components. IOUS can repeatedly evaluate the extent and location of residual tumorous tissue at any stage of the operation. This greatly facilitates both resection control and the goal of safe but maximum volume reduction. In diffuse infiltrating gliomas, the extent of resection is associated with survival and ultrasonography has the potential to increase the extent of resection and thereby increase survival [ 11- 14]. The evaluation of echogenicity depends on the tumor components. Most tumors are hyperechoic when compared with the surrounding brain tissue. In gliomas, the echogenic patterns change with increasing tumor grades. High-grade gliomas often show a heterogeneous pattern with a hypoechoic necrotic portion ( Fig. 5) [ 15]. Intratumoral calcification, as is often found in meningiomas, oligodendrogliomas, craniopharyngiomas, or cavernous angiomas, can be easily depicted as hyperechoic dots or nodules within the lesion ( Fig. 6). In contrast, peritumoral edema cannot be depicted as easily, because it is isoechogenic in comparison to the surrounding brain parenchyma ( Fig. 5B) [ 2, 16]. IOUS imaging can be used in brain tumor surgery to identify tumor remnants. The resection cavity should be flooded with sterile saline solution to obtain an appropriate sonic window ( Fig. 7). By integrating ultrasonography data into the neuronavigational system, we can mark and display sonographically detected residual tumors in the microscope image superimposed on the brain surface [ 2, 3, 14, 16, 17].
Localization of Focal Cortical Dysplasia
Medically intractable focal epilepsy is an important health issue affecting 0.15%-0.2% of the population. Focal cortical dysplasia (FCD) is the most common underlying cause in these patients [ 18]. The ability to define and completely excise the dysplastic cortex is the most important factor for determining surgical outcomes in patients with FCD [ 19, 20]. Because imaging plays such a vital role in preoperative planning in patients with intractable epilepsy, there is strong interest in the use of high-end technologies to improve the rate of lesion detection. Despite the use of modern imaging techniques, the intraoperative definition of cortical dysplasia can be challenging. During surgery, it is difficult for the surgeon to distinguish dysplastic lesions from the normal cortex for several reasons: first, visual and tactile differentiation can be difficult; second, the spatial accuracy of neuronavigation based on preoperative MRI images may be weakened by brain shift, and electrocorticographic recordings only provide two-dimensional information about the epileptogenic zone [ 21, 22]. There is thus a need for unique intraoperative imaging methods. IOUS can depict cortical dysplasia with high resolution. This is particularly true in type-IIB FCD. Type-IIB FCD is usually the subtype of FCD best visualized by MRI ( Fig. 8), whereas type-1 FCD lesions are more difficult to visualize. The extent to which IOUS can contribute to resection control and the postoperative outcomes will therefore depend on further comparative studies. MRI-negative focal symptomatic epilepsy constitutes only 16% of the cases with focal symptomatic epilepsy and has a surgical success rate of only 38% [ 23]. The surgical outcome depends on the extent of cortical dysplasia resection required [ 24]. Chassoux et al. [ 25] reported a high rate of freedom from seizures (88%) following surgery in this group; however, 20% of the patients underwent a further procedure.
Drainage of Entrapped Ventricle
The symptoms of hydrocephalus can be relieved using ventriculostomy. In this procedure, an external ventricular drain (EVD) placed into the cerebral ventricles removes excess CSF. In approximately 50% of the cases, free-handed EVD cannulation results in misplacement, increasing the risk of iatrogenic complications. Current technical approaches to improve ventriculostomy guidance are often considered too complex or inaccurate [ 26]. IOUS guidance can be used to detect and localize the targeted ventricle. A transfontanelle approach is good for the visualization of the catheter tip in the lateral ventricle ( Fig. 9), and both the transfontanelle and the transmastoid approaches are appropriate for the visualization of the catheter tip in the entrapped lateral ventricle or the fourth ventricle [ 26- 28].
Recent Developments
Contrast-enhanced ultrasonography has shown promising results for both displaying and monitoring tumor vascularization. Intraoperative contrast-enhanced ultrasonography is useful, particularly in tumors with ill-defined borders on B-mode ultrasonography, in highlighting the lesion and its boundaries, and in possibly differentiating between the tumor and the edematous brain tissue [ 29]. A novel technique of elasticity imaging, called shearwave elastography (SWE), could be employed to improve the detection of epileptogenic foci. This is a noninvasive tissue elasticity imaging technique that maps tissue stiffness in real time. Using intraoperative SWE, we can outline dysplastic lesions as abnormally stiff lesions compared to the normal brain [ 30]. Ultrasound-linked neuronavigation plays an important role in the improvement of surgical accuracy when applied for the detection of brain shift. Real-time intraoperative fusion imaging between preoperative imaging and IOUS for virtual navigation allows true real-time feedback during surgery and is less expensive and time-consuming than other intraoperative imaging techniques. Moreover, the adjustments are very helpful in correcting brain shift and tissue distortion [ 31].
Limitations
IOUS requires the knowledge of neuroradiologic abnormalities that are not routinely evaluated by ultrasonography. Radiologists who performed IOUS should be familiar with CT and magnetic resonance (MR) anatomy as well as ultrasound anatomy. The application of IOUS may be limited by difficulties in distinguishing a space-occupying lesion from normal tissue and lesions can be obscured by the surrounding edema. The ultrasonograms may sometimes be difficult to interpret in the later stage of operation in terms of whether there are remnant lesions because of surgically induced ultrasound artifacts. Its role may also be diminished by the widespread use of intraoperative CT and MRI. Additionally, IOUS guidance by a radiologist requires a large time commitment.
Conclusion
IOUS is a noninvasive imaging modality that improves lesion detection and localization and contributes to resection control in pediatric tumor and epilepsy surgery. It also improves the safety and accuracy of ventricular drainage, particularly in cases of complicated ventricle dilation. IOUS has an alternative role in combination with MR and CT techniques. Large-scale studies are recommended to validate the utility of intraoperative sonography compared with intraoperative as well as preoperative MRI or CT.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
1. Preuss M, Werner P, Barthel H, Nestler U, Christiansen H, Hirsch FW, et al. Integrated PET/MRI for planning navigated biopsies in pediatric brain tumors. Childs Nerv Syst 2014;30:1399–1403.   2. Ulrich NH, Burkhardt JK, Serra C, Bernays RL, Bozinov O. Resection of pediatric intracerebral tumors with the aid of intraoperative realtime 3-D ultrasound. Childs Nerv Syst 2012;28:101–109.   3. Roth J, Beni-Adani L, Biyani N, Constantini S. Classical and realtime neuronavigation in pediatric neurosurgery. Childs Nerv Syst 2006;22:1065–1071.   4. Nimsky C, Ganslandt O, Kober H, Buchfelder M, Fahlbusch R. Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery 2001;48:1082–1089.   5. Fahlbusch R, Ganslandt O, Nimsky C. Intraoperative imaging with open magnetic resonance imaging and neuronavigation. Childs Nerv Syst 2000;16:829–831.   6. Jolesz FA. Future perspectives in intraoperative imaging. Acta Neurochir Suppl 2003;85:7–13.   7. Broggi G, Ferroli P, Franzini A, Dones L, Marras C, Marchetti M, et al. CT-guided neurosurgery: preliminary experience. Acta Neurochir Suppl 2003;85:101–104.   8. van Leyen K, Klotzsch C, Harrer JU. Brain tumor imaging with transcranial sonography: state of the art and review of the literature. Ultraschall Med 2011;32:572–581.   9. Quencer RM, Montalvo BM. Intraoperative cranial sonography. Neuroradiology 1986;28:528–550.   11. Lam AM, Newell DW. Intraoperative use of transcranial Doppler ultrasonography. Neurosurg Clin N Am 1996;7:709–722.   12. Seibert JJ, Avva R, Hronas TN, Mocharla R, Vanderzalm T, Cox K, et al. Use of power Doppler in pediatric neurosonography: a pictorial essay. Radiographics 1998;18:879–890.   13. Cohen HL, Haller JO. Advances in perinatal neurosonography. AJR Am J Roentgenol 1994;163:801–810.   14. El Beltagy MA, Aggag M, Kamal M. Role of intraoperative ultrasound in resection of pediatric brain tumors. Childs Nerv Syst 2010;26:1189–1193.   15. Wang J, Liu X, Hou WH, Dong G, Wei Z, Zhou H, et al. The relationship between intra-operative ultrasonography and pathological grade in cerebral glioma. J Int Med Res 2008;36:1426–1434.   17. Bai HM, Wang WM, Li TD, He H, Shi C, Guo XF, et al. Three core techniques in surgery of neuroepithelial tumors in eloquent areas: awake anaesthesia, intraoperative direct electrical stimulation and ultrasonography. Chin Med J (Engl) 2011;124:3035–3041.  18. Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe: a systematic review. Eur J Neurol 2005;12:245–253.   20. Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, et al. Terminology and classification of the cortical dysplasias. Neurology 2004;62(6 Suppl 3):S2–S8.  21. Luders H, Awad I, Burgess R, Wyllie E, Van Ness P. Subdural electrodes in the presurgical evaluation for surgery of epilepsy. Epilepsy Res Suppl 1992;5:147–156.  22. Miller D, Knake S, Bauer S, Krakow K, Pagenstecher A, Sure U, et al. Intraoperative ultrasound to define focal cortical dysplasia in epilepsy surgery. Epilepsia 2008;49:156–158.   23. Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol 2009;66:1491–1499.   24. Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg 2004;101:55–65.   25. Chassoux F, Landre E, Mellerio C, Turak B, Mann MW, Daumas-Duport C, et al. Type II focal cortical dysplasia: electroclinical phenotype and surgical outcome related to imaging. Epilepsia 2012;53:349–358.   26. Strowitzki M, Moringlane JR, Steudel W. Ultrasound-based navigation during intracranial burr hole procedures: experience in a series of 100 cases. Surg Neurol 2000;54:134–144.   27. Metellus P, Hsu W, Kharkar S, Kapoor S, Scott W, Rigamonti D. Accuracy of percutaneous placement of a ventriculoatrial shunt under ultrasonography guidance: a retrospective study at a single institution. J Neurosurg 2009;110:867–870.   28. Phillips SB, Gates M, Krishnamurthy S. Strategic placement of bedside ventriculostomies using ultrasound image guidance: report of three cases. Neurocrit Care 2012;17:255–259.   30. Chan HW, Pressler R, Uff C, Gunny R, St Piers K, Cross H, et al. A novel technique of detecting MRI-negative lesion in focal symptomatic epilepsy: intraoperative ShearWave elastography. Epilepsia 2014;55:e30–e33.   31. Prada F, Del Bene M, Mattei L, Lodigiani L, DeBeni S, Kolev V, et al. Preoperative magnetic resonance and intraoperative ultrasound fusion imaging for real-time neuronavigation in brain tumor surgery. Ultraschall Med 2014 Nov 27 [Epub]. http://dx.doi.org/10.1055/s-0034-1385347.  
Intraoperative ultrasonography procedure.
A 10-year-old girl presented with cerebellar pilocytic astrocytoma. The transducer is applied directly to the exposed cerebellum through craniostomy. Sterile saline solution filled the craniostomy defect to improve the acoustic coupling.
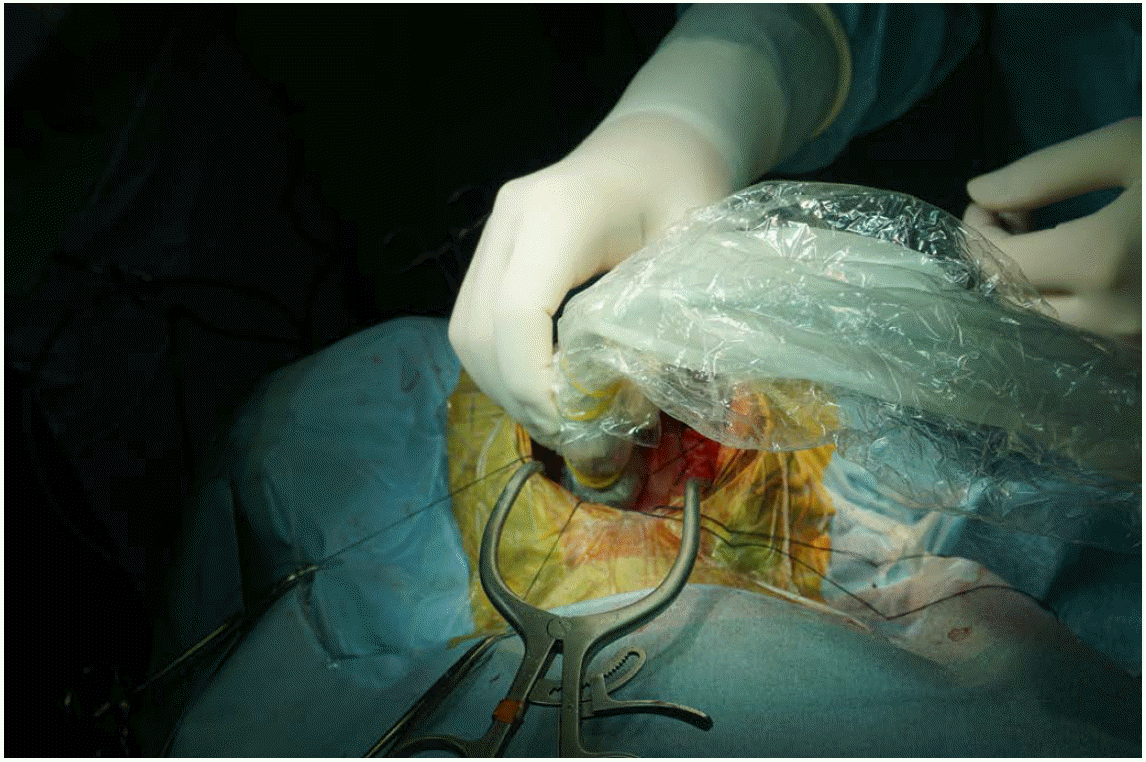 Fig. 1.
Juxtacortical anatomy.
A. Cortical gray matter shows hypoechogenicity (arrows) as compared to subcortical white matter on ultrasonography (US). Anechoic intraventricular cerebrospinal fluid (asterisk) is a useful landmark for intraoperative US neuronavigation. B. Color Doppler US can differentiate subdural fluid collection (asterisk) from subarachnoid fluid collection. Note the thin echogenic line suggesting the arachnoid membrane (arrowheads).
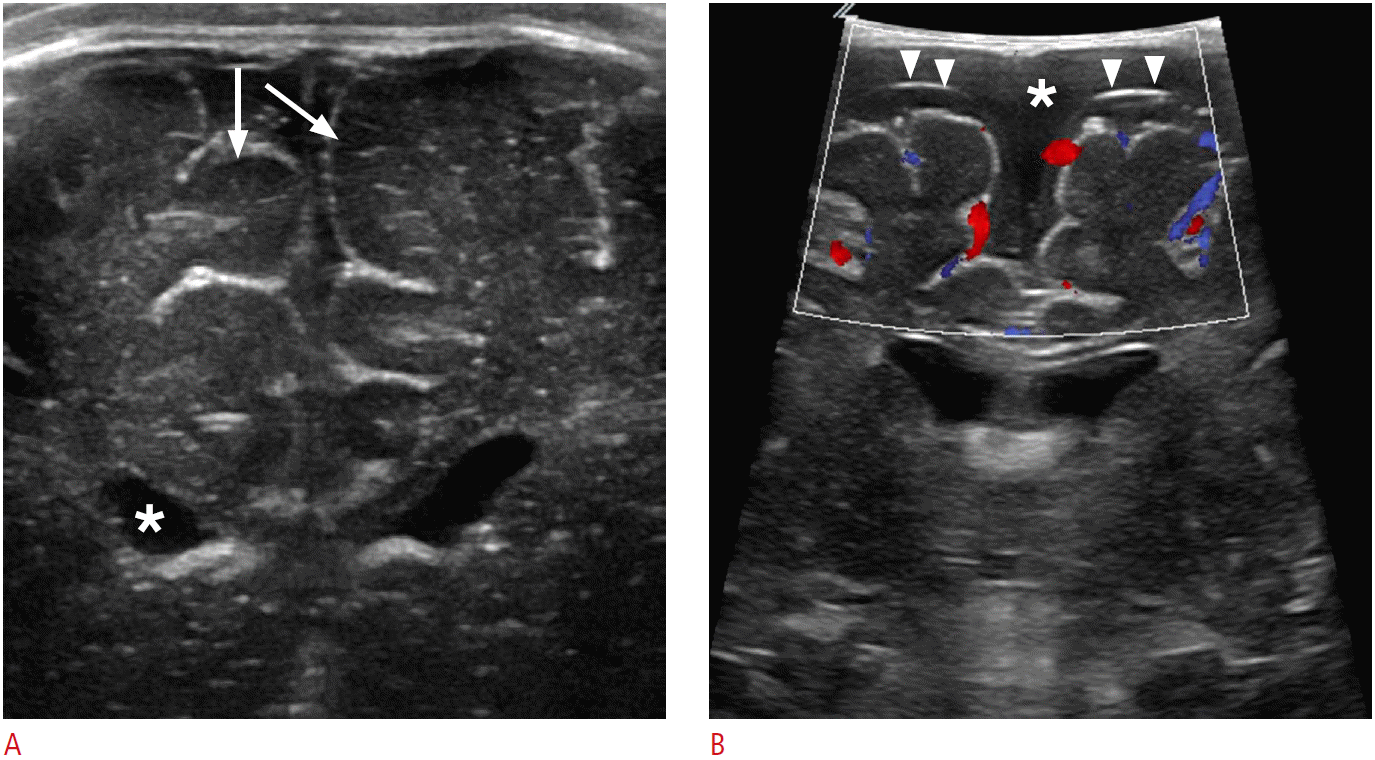 Fig. 2.
A 9-year-old girl with frontal lobe epilepsy.
A. An axial T2-weighted image shows a well-defined high signal intensity lesion in the right frontal subcortical white matter (arrow). B, C. This lesion shows high signal intensity on a T1-weighted image (B), and additional contrast enhancement (C) is not obvious (arrows). D, E. Intraoperative ultrasonography (US) shows a well-defined hyperechoic lesion in the right frontal subcortical white matter (D, arrows). Color Doppler US (E) shows prominent peripheral vascularity. Pathology revealed a ganglioglioma.
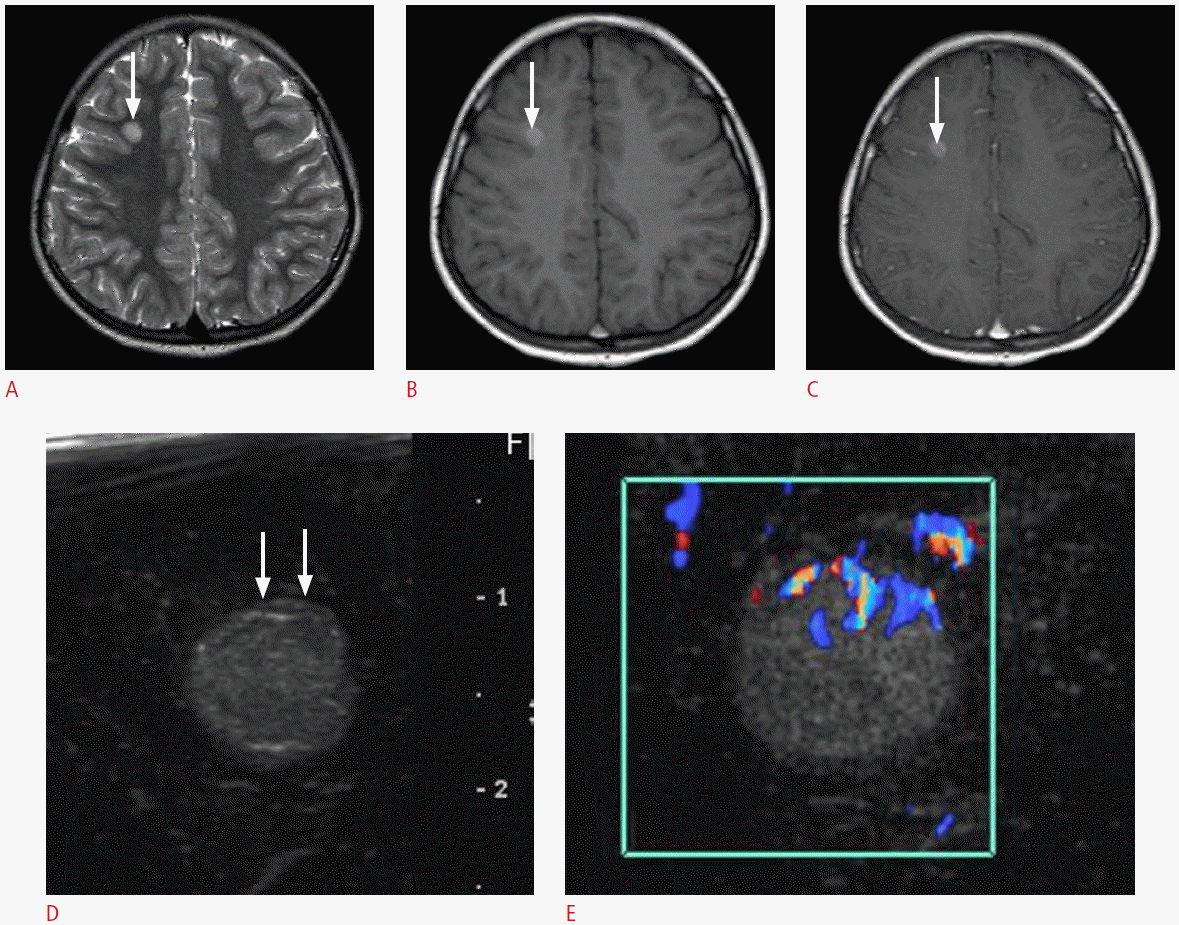 Fig. 3.
A 10-year-old boy with intractable left temporal lobe epilepsy.
A, B. Preoperative axial (A) and coronal (B) T2-weighted images show localized cortical thickening with subcortical hyperintensity in the left temporal lobe (arrows). C. Intraoperative ultrasonography through left temporal craniostomy reveals gyriform hyperechogenicity in the left superior temporal gyrus (arrows). Pathologic diagnosis was a ganglioglioma.
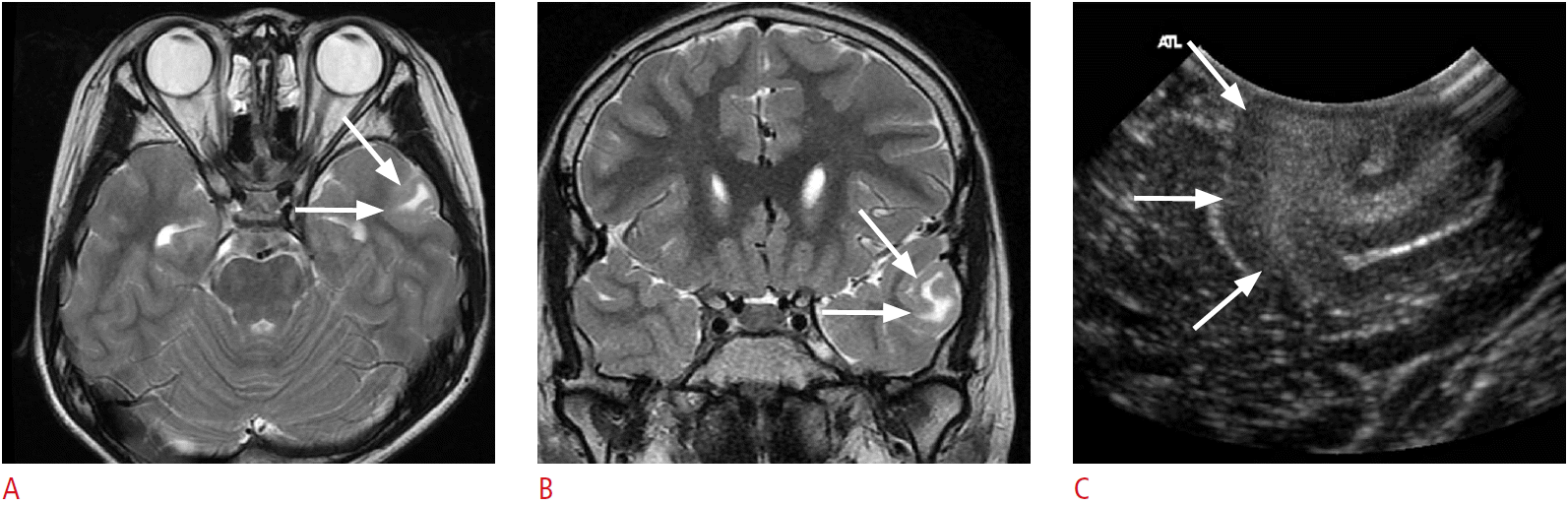 Fig. 4.
A 13-year-old boy with a pathologically proven primitive neuroectodermal tumor.
A. Postoperative axial T2-weighted image shows an ill-defined hyperintense mass in the right parietal lobe with the surrounding edema. Note the central bright signal intensity area, which suggests intratumoral necrosis (arrow). B. Intraoperative ultrasonography demonstrates an ill-defined hyperechoic mass with a central low echoic portion (arrow) that corresponds to the central hyperintense area on the T2-weighted image. The mass and the surrounding edema are poorly differentiated. The pathologic report revealed a recurrent primitive neuroectodermal tumor.
 Fig. 5.
A 16-year-old girl with intractable left frontal lobe epilepsy.
A. An axial T2-weighted image shows a lobulated contour, high signal intensity lesion in the left frontal precentral cortex (arrows). B. Intraoperative ultrasonography shows a well-defined hyperechoic lesion confined to the precentral cortex (arrows). Note the arc-like dense hyperechogenicity within the lesion (arrowheads). C. The hyperechoic arc corresponds to calcification on the preoperative brain computed tomography (arrow). Pathology revealed a ganglioglioma.
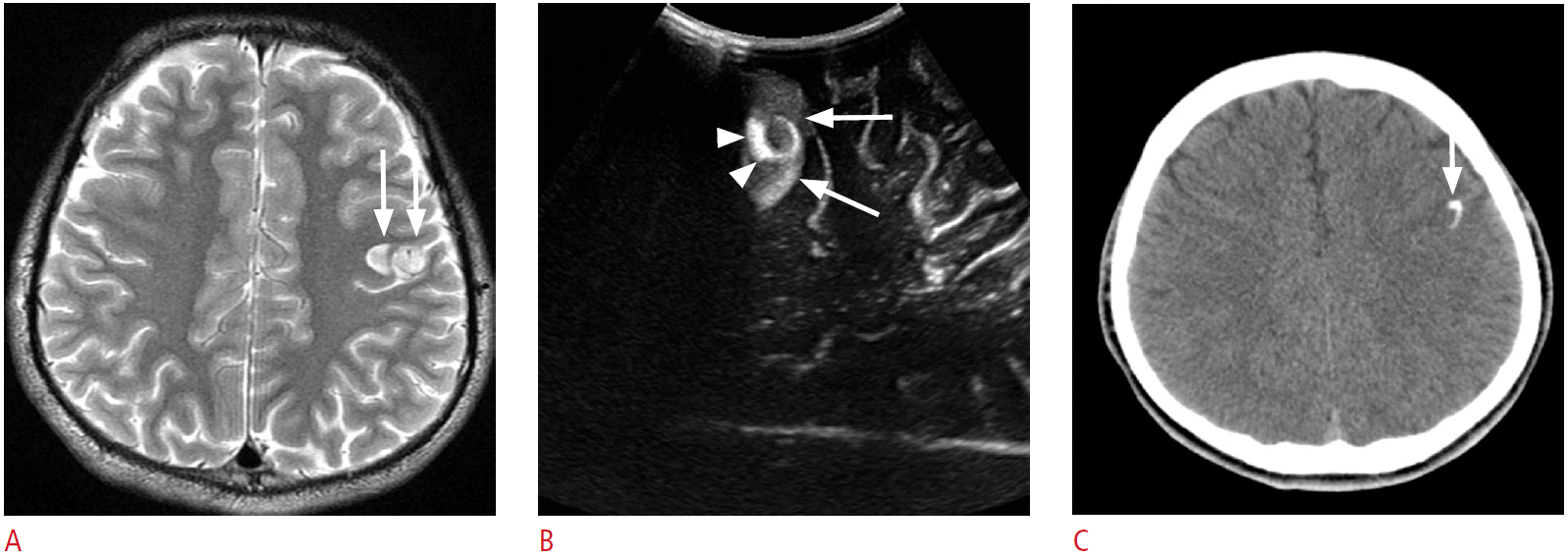 Fig. 6.
A 4-year-old girl with right temporal lobe epilepsy.
A, B. Preoperative magnetic resonance images reveal a focal cystic lesion in the right temporal lobe (arrows). Note the ill-defined hyperintensities in the surrounding cortex and the subcortical white matter (arrowheads, B). C. Intraoperative ultrasonography (US) through the right temporal craniostomy shows a well-defined cystic lesion in the periventricular white matter (arrow). Note the illdefined hyperechogenic area at the caudal aspect of the cystic lesion (asterisk). D. After the resection of the cystic lesion in the temporal periventricular white matter with the adjacent cortex and the subcortical white matter, fluid-filled surgical defects (arrows) are clearly seen on US. However, the ill-defined hyperechoic area in the caudal part of the surgical defect remains (asterisk). Anechoic cerebrospinal fluid and hyperechoic choroid plexus (arrowheads) can be used as anatomic indicators. The pathologic diagnosis was a ganglioglioma. E. A methionine positron emission tomography-computed tomography image obtained 1 year later demonstrates residual tumors in the right temporal lobe (arrow).
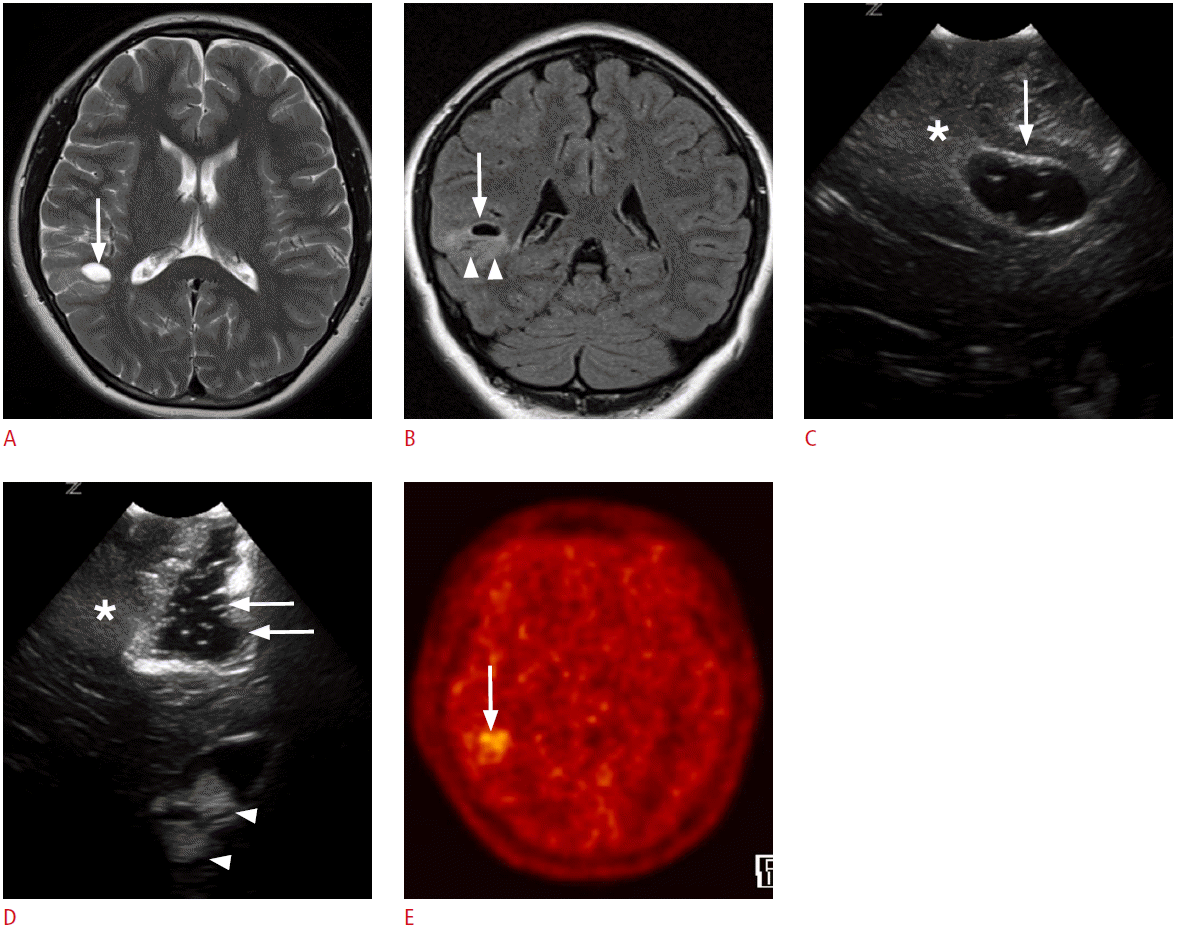 Fig. 7.
A 2-month-old girl with intractable left frontal lobe epilepsy.
A, B. Preoperative axial (A) and coronal (B) T2-weighted images show a localized thickening of the left frontal cortex (arrows). C. Ictal single photon emission computed tomography shows a hypermetabolic lesion in the corresponding left frontal lobe (arrows). D. Intraoperative ultrasonography (US) through a frontal craniostomy reveals a well-defined hyperechoic lesion in the left frontal cortex extending into the subcortical white matter (arrows). Note the band-like cortical hyperechogenicities (arrowheads), which are connected to the focal hyperechoic cortical lesion (arrows) in the left frontal lobe. E. Intraoperative US reveals complete surgical resection of the left frontal lobe lesions (arrows). The frontal horn of the left lateral ventricle serves as an anatomic indicator (asterisk). The pathologic diagnosis was type-II focal cortical dysplasia.
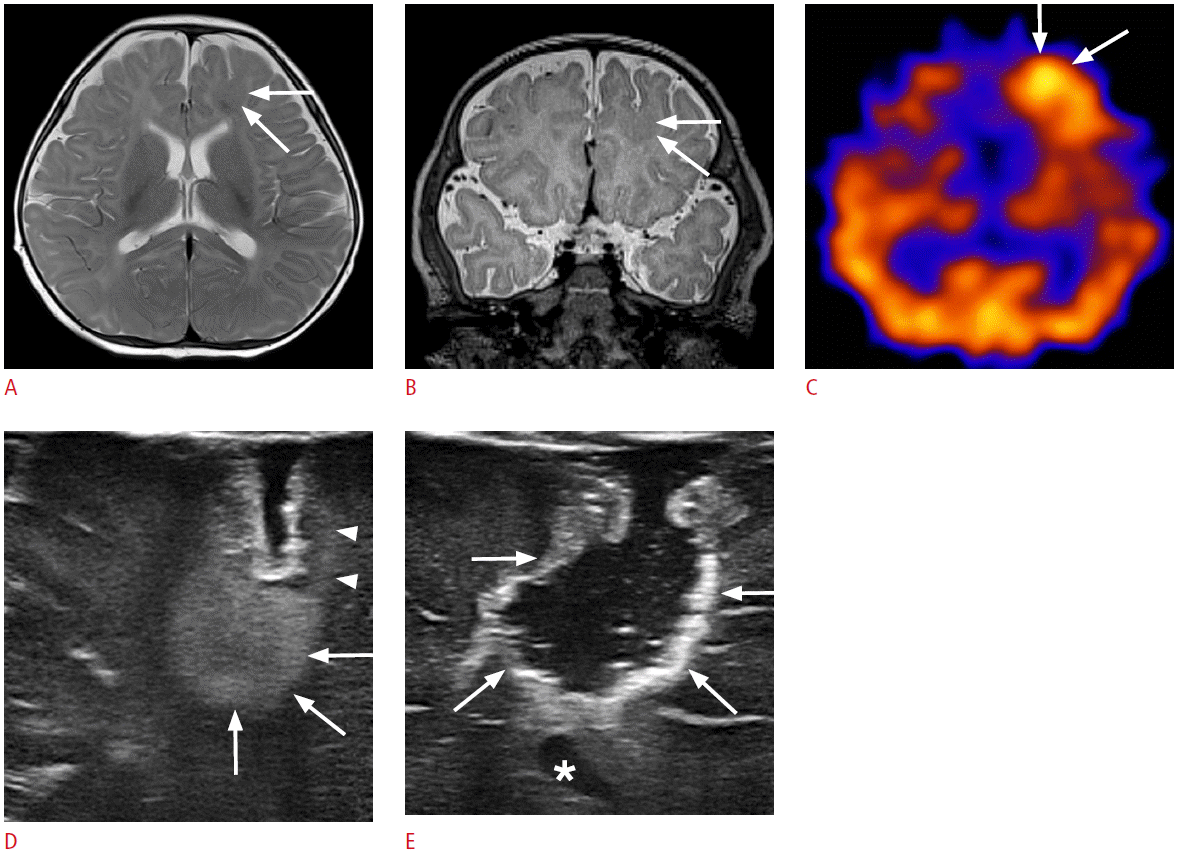 Fig. 8.
A 3-month-old boy with hydrocephalus.
A. Preoperative ultrasonography (US) through anterior fontanelle shows dilated lateral and third ventricles suggesting aqueductal stenosis. B, C. Intraoperative US though anterior fontanelle reveals intraventricular location of the catheter tip (arrows) on the coronal (B) and sagittal (C) planes. D. Cranial US through anterior fontanelle obtained 3 days after the ventriculoperitoneal shunt demonstrates the decreased size of the lateral and the third ventricles.
 Fig. 9.
|
|
| TOOLS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
METRICS

|
|
|
|
|
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+









 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




