Introduction
Musculoskeletal ultrasonography plays an important role after orthopedic surgery. After surgery using metal hardware, susceptibility artifacts due to the ferromagnetic material on magnetic resonance imaging (MRI) and beam-hardening streak artifacts on computed tomography (CT) often prevent an accurate evaluation of abnormal lesions in the bone and soft tissues around the metal hardware. In contrast, artifacts from metal hardware on ultrasound are less distinct [1,2]. Ultrasonography cannot see within the joint or into the bone around the metal hardware. However, an added advantage of ultrasonography is its ability to perform dynamic imaging to permit the evaluation of joint, muscle, and tendon motion. Further, its real-time nature can guide an interventional procedure. Ultrasonography can be used for accurately diagnosing various abnormalities including tendon tear, joint effusion, and infection in a postoperative orthopedic patient. Ultrasonography is also a crucial tool for detecting the recurrence of soft tissue tumors, bone unions, and complications after amputation surgery [2]. This article discusses the frequent applications of musculoskeletal ultrasonography in various postoperative situations. In particular, the ultrasonographic findings of postoperative lesions and pitfalls are discussed.
Soft Tissues around Metal Hardware
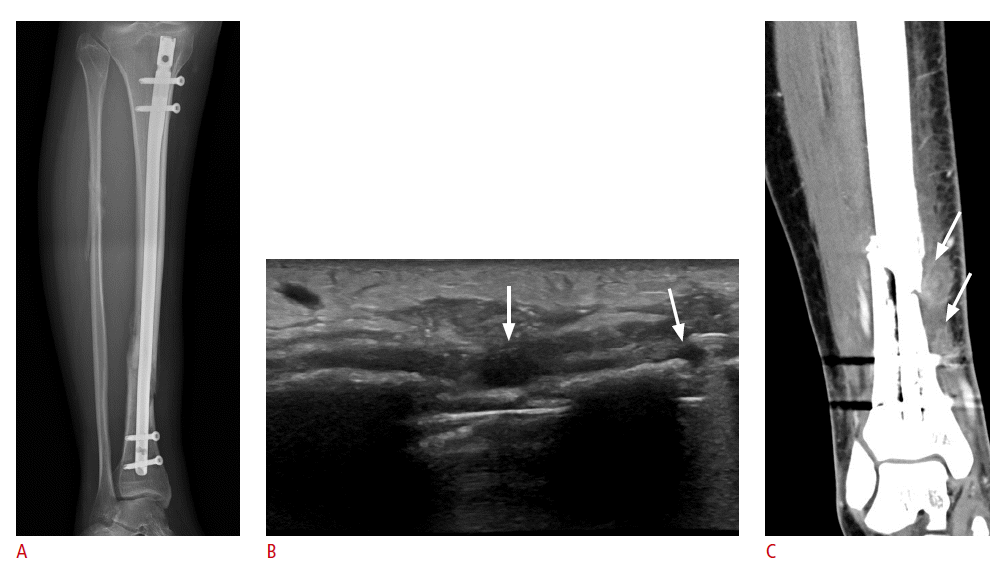
After orthopedic surgery, an evaluation of the soft tissues around the metal hardware is often necessary. Ultrasonography is an excellent modality that can diagnose conditions that may occur postoperatively, such as joint effusion, soft tissue infection (Fig. 1), hematoma (Fig. 2), and bursitis [1-5]. Tendon or ligament abnormalities can also be examined easily with ultrasonography. When a tendon is found adjacent to the metal hardware, it is important to note that the condition may develop into tendon rupture or tenosynovitis. In such cases, a dynamic study can be used for accurately evaluating whether tendon impingement is causing the symptoms or not.
Ultrasonography may be useful for detecting joint effusion, indicating joint abnormality. Superficial joints including those of the knee, ankle, elbow, and wrist can be visualized easily. Joint recesses filled with an anechoic fluid can be identified. However, larger joints such as the shoulder joint or the pelvic girdle are located further from the ultrasonography transducer; therefore, the detection of a joint effusion becomes more difficult in these cases. This dilemma becomes more complex after surgery as the normal anatomic guideline and the tissue echogenicity may be changed. Further, following total hip arthroplasty, a pseudocapsule with soft tissues may look like a hypoechoic area along the femoral neck and can be confused for a joint effusion [5,6]. In general, a significant joint effusion and irregular distension of the pseudocapsule along with an extra-articular fluid or abscess are sufficient for diagnosing joint infections (Fig. 3). Additional color or power Doppler imaging may be useful for increasing diagnostic confidence in the case of infections. If needed, joint aspiration may be attempted under ultrasonography or fluoroscopy guidance.
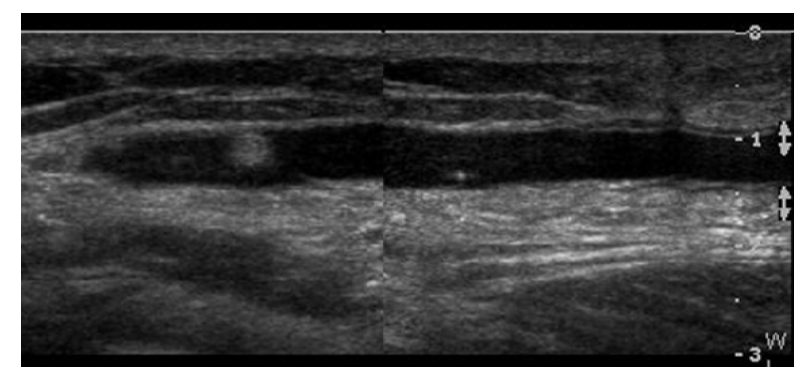
Ultrasonography can evaluate for soft tissue infection around the metal hardware outside of the joints. Fluid collection and hyperemia of the soft tissue around the metal hardware might be a sign of infection (Fig. 4). Occasionally, hypoechoic periprosthetic soft tissue edema may present immediately after surgery and can appear similar to an infection. In such a case, the patient’s clinical history as well as symptoms must be carefully observed, and serial follow-up ultrasonography could be helpful in differentiating between an infection and reactive edema. If necessary, ultrasound-guided aspiration should be considered [2].
Arthroplasty
An evaluation of imaging can be problematic in patients with symptomatic arthroplasty and should be tailored to the clinical request. Ultrasonography can aid in the detection of the pathology of an arthroplasty joint and the adjacent soft tissue structures.
A common application of ultrasonography after shoulder arthrography is to evaluate the status of the rotator cuff that suffers from postoperative pain, weakness, and limited motion [4,7]. Ultrasonography is carried out using a method similar to the ultrasonography of a native shoulder. The prosthetic humeral head can be seen deep past the rotator cuff as a hyperechogenicity with a posterior reverberation artifact. Several signs that indicate a rotator cuff tear are the same as those in the case of a native shoulder. Findings of a full-thickness tear include a focal discontinuity with an interposed hypoechoic or anechoic fluid, nonvisualization of the tendon, or deltoid muscle into the vacant tendon. The criteria for determining partial cuff tears are somewhat controversial and may include a thinned or thickened relatively heterogeneous-appearing tendon and a hypoechoic defect in the tendon. Atrophy of the rotator cuff muscles associated with a chronic tear usually demonstrates fatty infiltration, leading to decreased size and increased echogenicity [7,8].
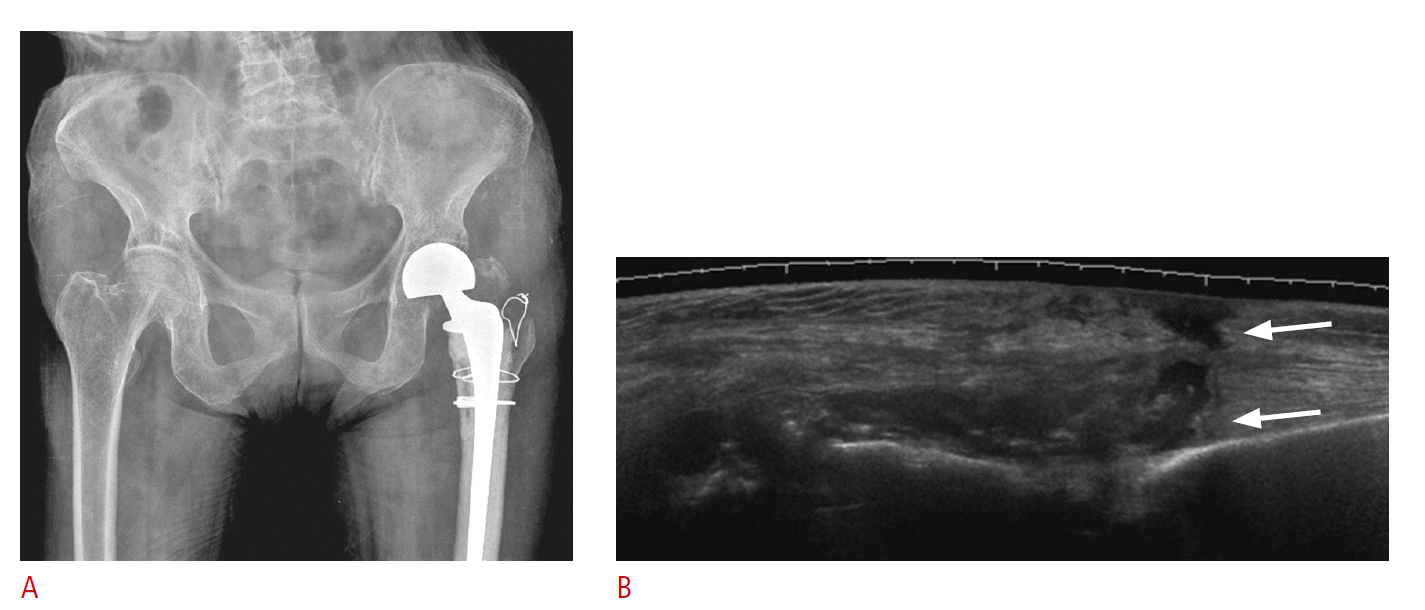
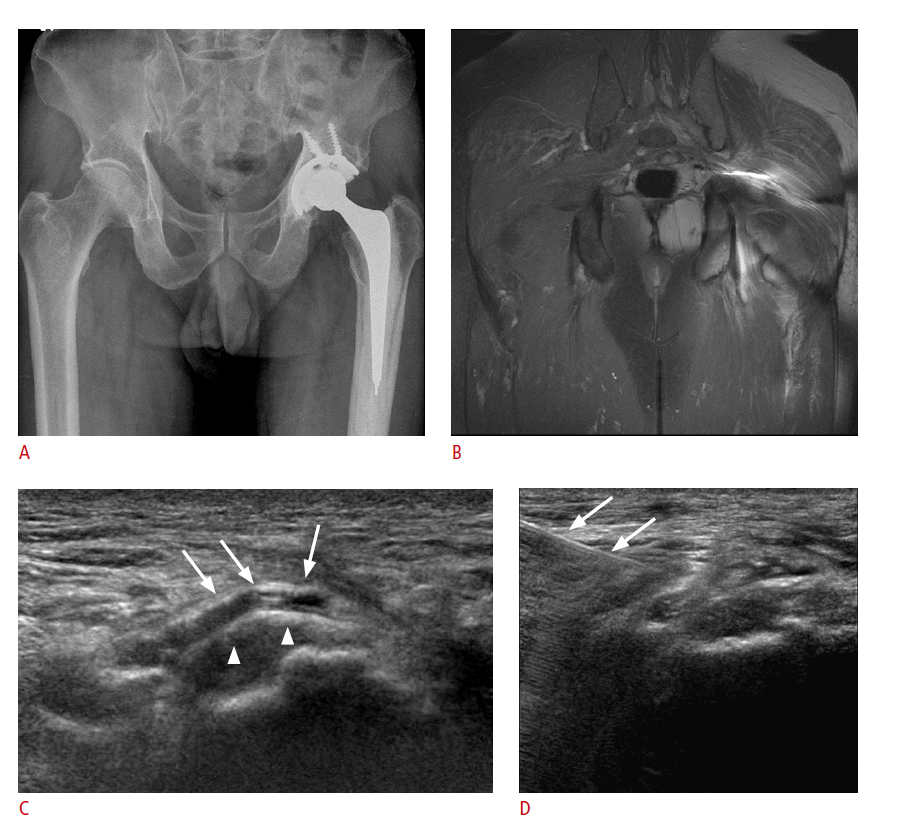
The detection of a hip effusion, which is indicative of the joint pathology including an infection, is probably a major indication of ultrasonography of the symptomatic hip after arthroplasty. Other indications include the evaluation of an iliopsoas tendon, a periprosthetic hematoma in patients with persistent wound drainage, an acetabular element, or bursitis (Fig. 5) appearing as a septated cystic mass [6].
Anterior knee pain is common after total knee arthroplasty. Abnormalities of the patellar tendon are even more common. Ultrasonography can identify these abnormalities in the tendon without being affected by metal artifacts. Flexion and extension during the examination can be helpful in distinguishing a complete rupture with no retraction from a partial tear. Ultrasonography can also be used to identify capsular thickening, which can be a cause of stiffness after arthroplasty. Joint space narrowing after arthroplasty is due to wear of the polyethylene. This is identifiable most effectively by serial radiographs, but ultrasonography is an alternative modality [2,7].
Postoperative Tendon
In comparison to research dealing with an ultrasonographic diagnosis of tendon abnormalities prior to surgery, there is little literature available that deals with postoperative or repaired tendons [9-13]. In general, for repaired tendons, the echogenicity varies from hyperechoic to hypoechoic. A normal fibrillar pattern may or may not be seen completely. These changes indicate varying degrees of granulation tissue or scar remodeling. A postoperative tendon may be thickened or thinned. The criteria for a tendon tear before an operation include abnormal hypoechoic area, loss of the fibrillar pattern, and tendon thinning. These criteria cannot be directly applied to an assessment of the postoperative tendon. Further, ultrasonography may be used for evaluating tendon integrity and recognizing a peritendinous abnormality [9,10]. A dynamic study using ultrasonography is helpful for the diagnosis of a tendon tear. In this situation, tendon movement can be evaluated by moving the extremity or palpating the muscle. Retraction of the tendon end without the tendon sliding motion crossing the repair area is indicative of a full-thickness tear. This dynamic study is most effective in the evaluation of a single tendon [11].
Rotator Cuff Tendon
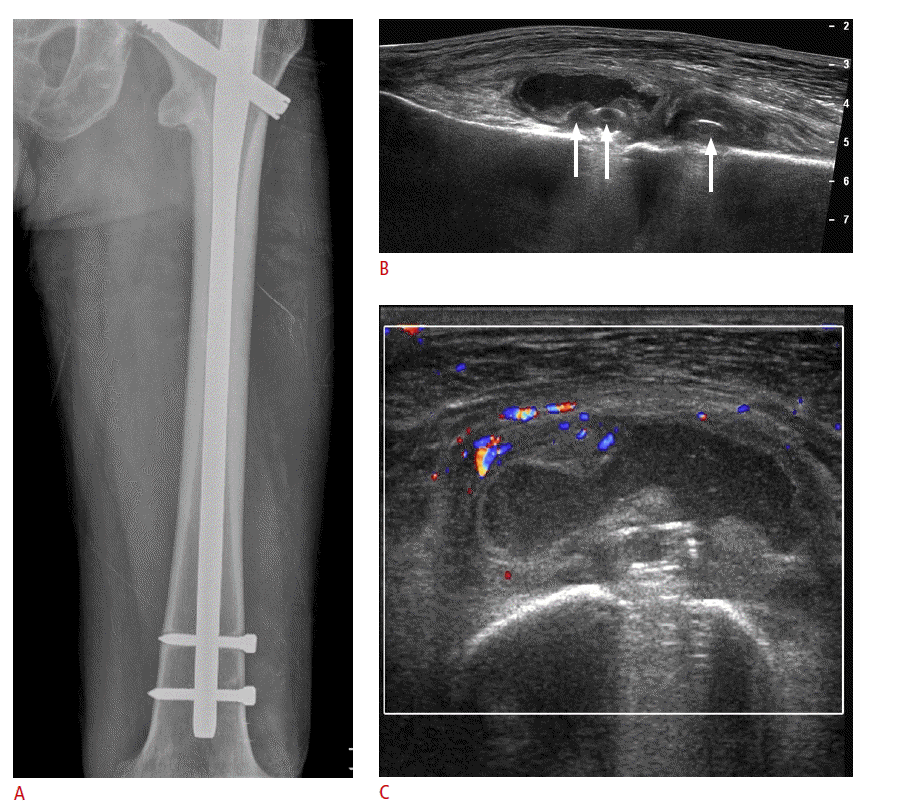
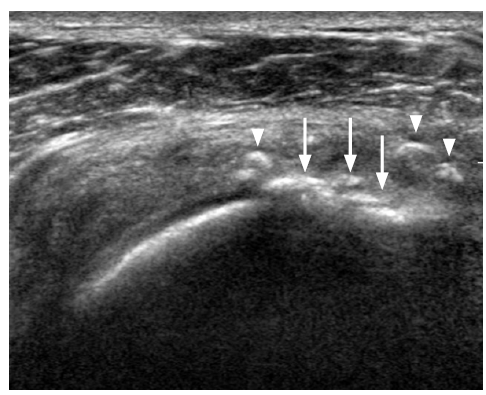
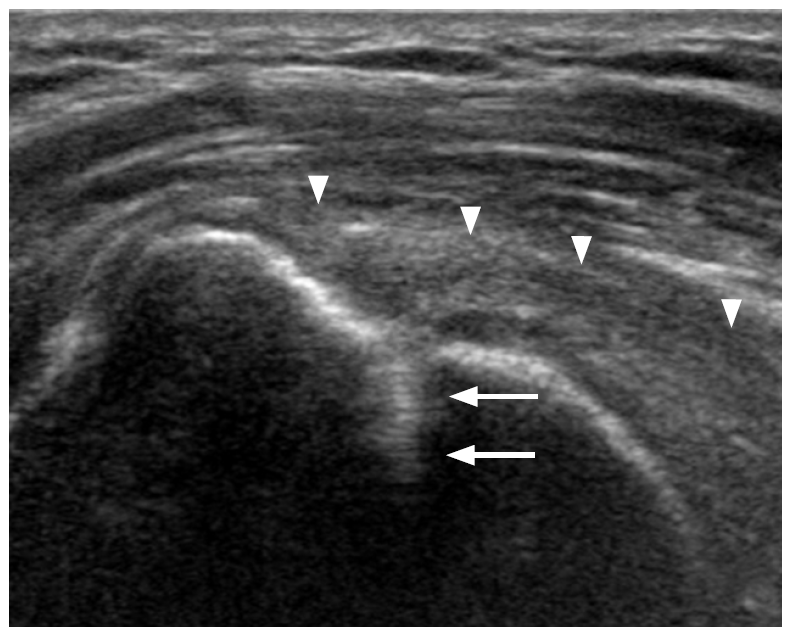
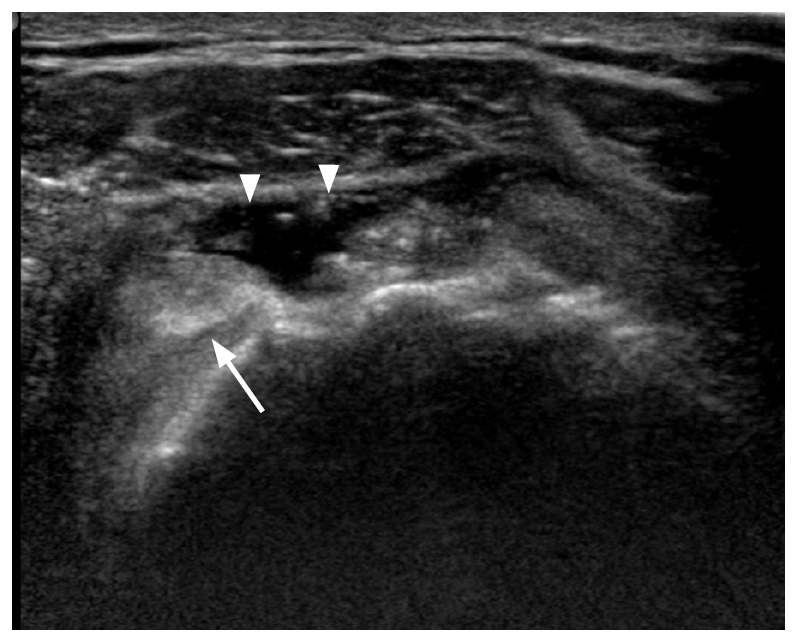
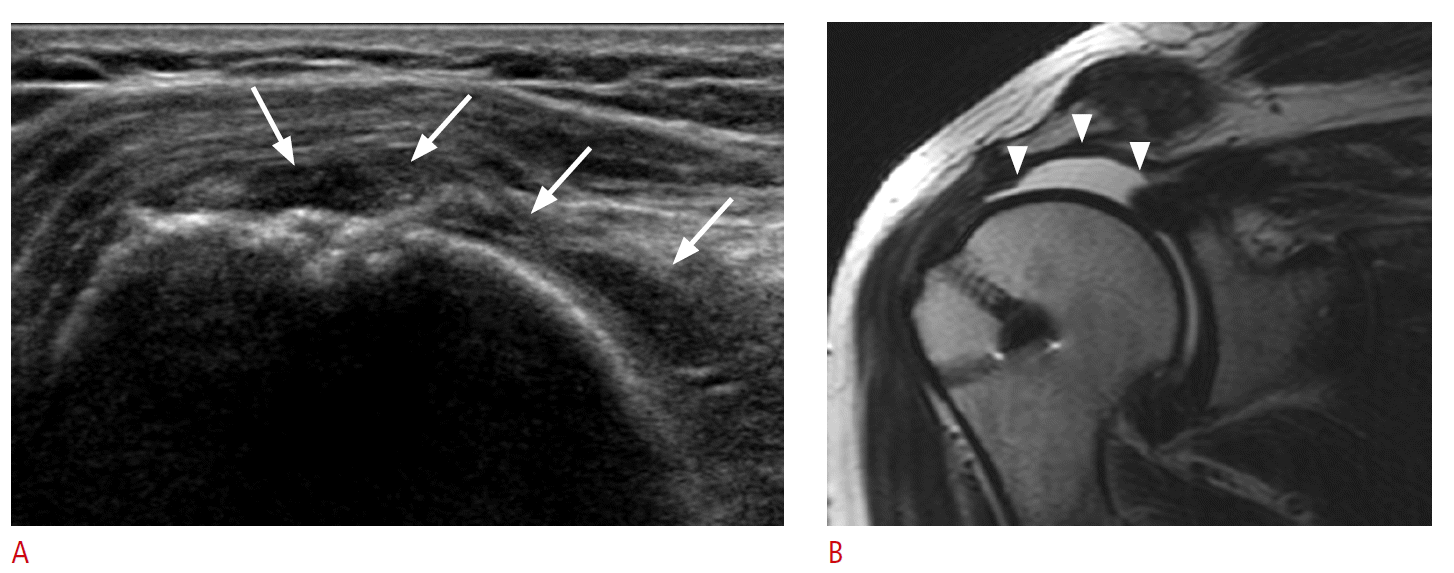
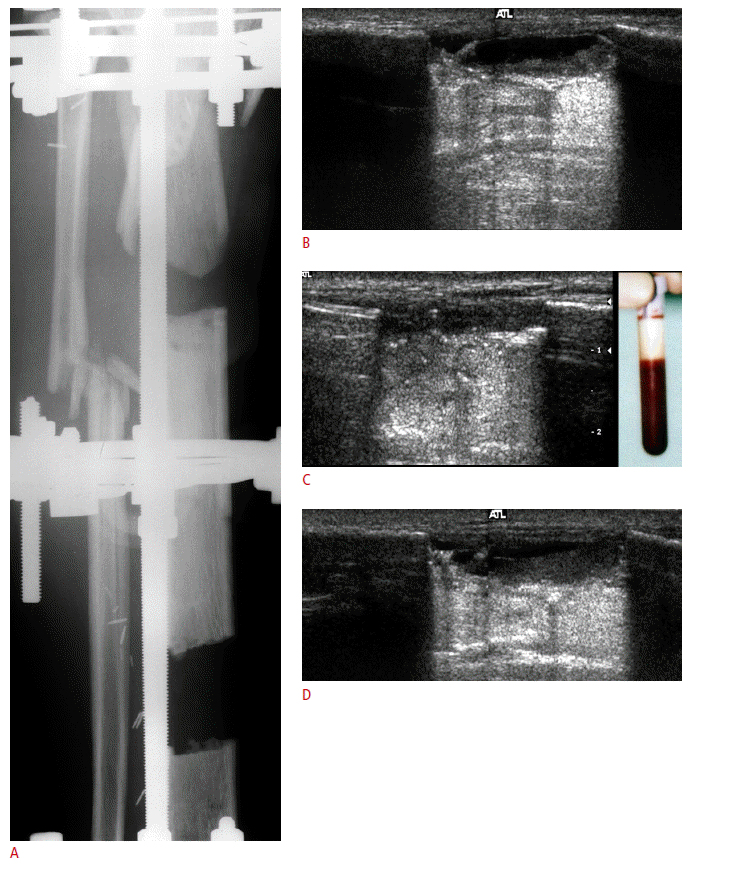
A majority of postoperative tendon research focuses on rotator cuff repair. A repaired rotator cuff tendon shows a heterogeneous echogenicity with peribursal soft tissue thickening. Normal fibrillar architecture may or may not be present. There may be hyperechoic suture material within the repaired tendon. A cortical irregularity along the greater tuberosity is often seen at the suture anchor surface (Fig. 6). The suture material may create posterior acoustic shadowing or reverberation artifacts (Fig. 7). Reverberation is the persistence of sound in an enclosed space after its generation. This is due to continuously repeated echoes between anatomic interfaces, which are strong reflectors [9,10,13]. Hypoechoic defects within a repair may persist for several years. This is suggestive of a granulation tissue rather than a true retear within the repair (Fig. 8). Doppler imaging may be helpful in differentiation because the presence of vascularity within the defect indicates granulation [13]. A true tear can be definitely diagnosed with the identification of the intervening fluid that redistributes when the transducer is pressed against the repaired tendon (Fig. 9). Other evidence of a retear includes a dislodged suture anchor, broken suture, or retracted tendon ends. If the retear is massive, the tendon may not be visible completely (Fig. 10). Extensive joint effusion or peribursal fluid collection with a coexisting inflammatory response can be seen [10].
Achilles Tendon
The Achilles tendon is the most commonly injured of all the ankle tendons. The repaired Achilles tendon retains increased thickness for at least 2 years, and shows decreased echogenicity without a retear. A pathologic specimen of a repaired Achilles tendon shows loss of the fibrillar tendon structure, decreased collagen, increased non-collagenous extracellular matrix, and focal variations in cellularity [11,14]. In general, the vascularity within the tendon is not increased, but there might be a subtle increase during the initial healing process. Vascular regression continues as the scar matures, and a fibrous, avascular scar is eventually formed. If there is increased vascularity 2 years after surgery, a re-injury might be indicated. The area of the retear appears as an anechoic or hypoechoic defect (Fig. 11) [11,12].
Postoperative complications include infection, ossification, suture granuloma, and peritendinous adhesion. If there is an infection, an anechoic abscess may be seen within the tendon and the vascularity at the periphery of the abscess is sharply increased. Ossification appears as a hyperechogenicity with posterior acoustic shadowing. A suture granuloma appears as a hypoechoic lesion. Sometimes, it is difficult to differentiate it from a small abscess. Peritendinous adhesion, formed by the accumulated scar tissue around the repair site, interferes with tendon gliding. Scar formation provides the initial physical continuity at the repair site, but a scar proliferation attachment to the surrounding tissue is not expected. The border of the tendon is indistinct and there are hypoechoic areas around the repaired tendon [11].
Recurrent Soft Tissue Tumors
Ultrasonography can be applied to an assessment of a recurrent or remaining lesion after the treatment of soft tissue tumors [15,16]. A solid soft tissue mass is typically hypoechoic with a well-defined margin, while postoperative soft tissue fluid collection is anechoic with an uneven shape and poorly-defined margins. If the fluid collection is round and mass-like, it will be compressed easily by using the transducer. If there is posterior enhancement, it is likely to be fluid collection rather than a solid mass (Fig. 12). However, tumor necrosis or hemorrhage can occur, and the tumor may contain both solid and cystic internal components. If it is difficult to differentiate between a localized fluid collection and a recurrent or remaining tumor, ultrasound-guided aspiration and biopsy may be necessary. Ultrasonography performs better than CT and is comparable to MRI in the identification of soft tissue tumor recurrence. MRI should still be considered if the ultrasonography is inconclusive [17,18].
Bone Union
Ultrasonography may be used for a clinical evaluation of a malunion or a nonunion after internal fixation using an intramedullary nail. The fracture area is systematically assessed in the longitudinal and transverse scans. The formation of continuous echogenic callus with no identification of the intramedullary nail at the fracture sites represents a union. On the other hand, the interruption of an echoic cortical outline with a posterior reverberation artifact due to an intramedullary nail represents a nonunion [19]. Further, ultrasonography can be used to accurately evaluate a limb lengthening procedure by using the Ilizarov technique. New bone formation can be detected with ultrasonography before its radiographic recognition. The ultrasonographic appearance of a new bone includes echogenic foci within the distraction site, which become aligned in the longitudinal plane [20]. A cyst or fluid that is present in the distraction gap signifies the slow formation of the new bone compared with the rate of limb lengthening. Ultrasoundguided fluid aspiration is beneficial because fluid collection may interfere with the new bone formation at the distraction site (Fig. 13).
Amputation Surgery
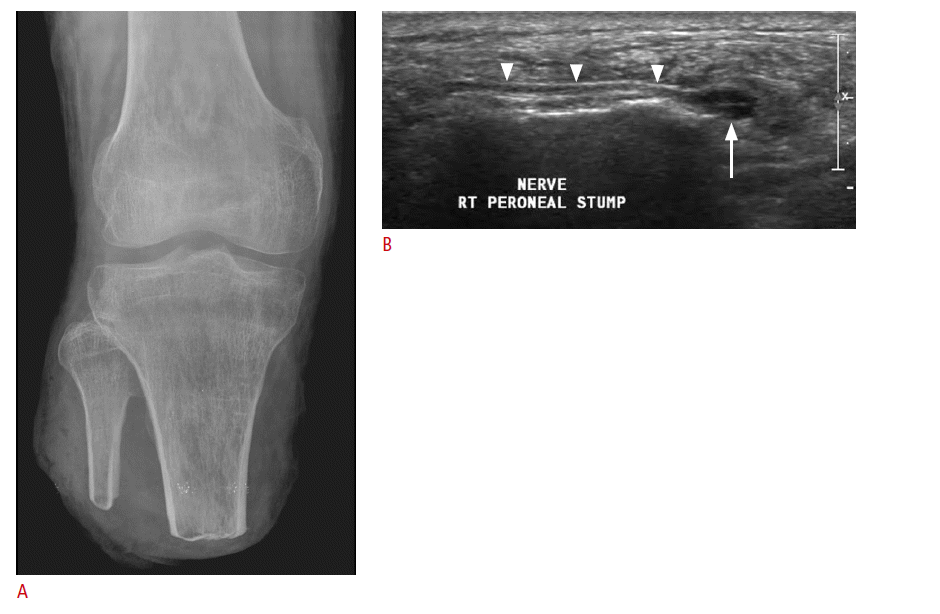
Ultrasonography can be used for finding the causes of limb stump pain after amputation surgery. Postoperative complications causing stump pain include infections, neuromas, venous thrombosis, popliteal artery aneurysms, or phantom limb pain. Ultrasonography can reveal an inflammation of the stump as well as fluid collection. Postoperative fluid collections may appear as anechoic lesions with posterior enhancement. After amputation surgery, development of a neuroma at the severed end of a major nerve is a concern. It is a tumor-like proliferation of regenerated nerve axons and is usually seen 1-12 months after amputation surgery. The pain associated with a neuroma is often difficult to distinguish from other types of stump pain. Ultrasonography is useful for the confirmation and location of a painful neuroma [21]. Its findings include a well-defined hypoechoic mass with continuity between the neuroma and a peripheral nerve (Fig. 14). Ultrasonography is effective in assessing whether the symptoms originate from the neuroma by compressing the mass with the transducer [22]. Vascular complications such as venous thrombosis and popliteal artery aneurysms can also be diagnosed by using ultrasonography along with color Doppler imaging.
Summary
Ultrasonography of the postoperative musculoskeletal system plays an important role in the accurate diagnosis of abnormal lesions in the bone and soft tissues. Musculoskeletal ultrasonography is particularly helpful for the evaluation of soft tissues around the metal hardware, postoperative tendons, guidance for interventional procedures and aspiration of the joint fluid, assessment of recurrent or residual soft tissue tumors and bone unions, and identification of the causes of limb stump pain after amputation surgery. Ultrasonography is a fast and reliable method with no harmful irradiation for the evaluation of postoperative musculoskeletal complications. In particular, it is not affected by the excessive metal artifacts that appear on CT or MRI.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+



 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




