AbstractPurposeThe aim of this study was to evaluate the correlations between laboratory findings and ultrasonographic measurements of renal length and cortical thickness in patients receiving follow-up for chronic kidney disease (CKD).
MethodsA total of 41 CKD patients (18 males and 23 females; mean age, 65.2 years; range, 42 to 85 years) with a low glomerular filtration rate who did not require renal replacement therapy were included in this prospective study. Patients were followed up with laboratory assays at bimonthly intervals and with ultrasonography performed twice a year. Renal cortical thickness, renal length, and estimated glomerular filtration rate (eGFR) values were compared using the paired-samples t test. Additionally, Pearson correlation analysis was conducted between renal length and cortical thickness measurements and eGFR values to assess kidney function.
ResultsAt the beginning of the study and after 24 months, mean eGFR values of the 41 patients were 35.92 mL/min and 28.38 mL/min, respectively. The mean renal length was 91.29 mm at the beginning of the study and 90.24 mm at the end of the study. The mean cortical thickness was 5.76┬▒2.05 mm at the beginning of the study and 5.28┬▒1.99 mm at the end of the study. A statistically significant positive association was found between eGFR and mean renal length (r=0.66, P<0.01) and between eGFR and mean cortical thickness (r=0.85, P<0.01), with the latter being more prominent.
Chronic kidney disease (CKD) is a common disease, with a gradually increasing incidence worldwide. Laboratory findings and clinical symptoms are utilized in the diagnosis of CKD. Radiological examinations are another crucial tool in the differential diagnosis. Plain abdominal radiography, intravenous pyelography, ultrasonography (US), and computed tomography (CT) are commonly used methods. US is a simple, cost-effective, and non-invasive method that is easy to use for renal imaging [1]. Length, volume, echogenicity, and cortical thickness are important parameters in making an ultrasonographic diagnosis [1,2]. Renal length has widely been used as a predictor of CKD, and for this reason the bipolar renal length is generally reported in US [3]. Length is feasible to measure, but is not necessarily diagnostic, as it is not always measured using a standardized approach and is related to body size [4]. In previous studies, kidney volume was used as a direct indicator of kidney size, rather than kidney length, but evaluating renal volume is difficult and requires experience. Renal cortical thickness (RCT) and echogenicity have also been used in the diagnosis of CKD. With the progression of the disease, RCT decreases and echogenicity increases. Laboratory assays play a supportive role tracking the progression of the disease during follow-up, along with the previously mentioned methods [5,6]. However, echogenicity is mainly based on the evaluation of a specialist, which in turn may yield subjective results; moreover, no established standardized normal range values currently exist for echogenicity, and a normal result for renal echogenicity does not exclude the possibility that the patient's kidney is damaged [7,8]. The aim of this study was to evaluate the correlations between laboratory findings and ultrasonographic measurements of renal length and cortical thickness in patients receiving follow-up care for CKD.
CKD patients with a low glomerular filtration rate who did not require renal replacement therapy were included in this prospective study. All participants signed a written consent form prior to the study. This study was approved by the local institutional ethics committee. The study was performed between January 2013 and November 2016. The mean follow-up time was 24 months. The exclusion criteria were as follows: having polycystic kidney disease or a serious associated condition such as active malignancy, infection, or end-stage cardiac, pulmonary, or hepatic disease. A total of 41 patients (18 males and 23 females; mean age, 65.2 years; range, 42 to 85 years) with CKD who had low glomerular filtration rates were included in this study. Patients were followed up with laboratory assays conducted at bimonthly intervals and US performed twice a year. In case of rapid progression and the presence of uremic symptoms, the follow-up period was shortened and the parameters were recorded more often than previously scheduled. In the biochemical analysis, blood urea nitrogen, creatinine, and the urinary albumin-to-creatinine ratio (ATCR) were measured. The Cockroft-Gault (CG) formula was used to calculate the estimated glomerular filtration rate (eGFR) [9]. The CG equation is defined as follows: eGFR=(140-age)├Ś(weight in kg)├Ś(0.85 if female)/(72├ŚCr), where Cr refers to creatinine. Two experienced radiologists in our radiology department who were blinded to the current renal functionality of each patient, additional imaging, or any other clinical information, performed the renal US monitoring of the patients using a standard gray-scale B-mode US apparatus with a 3.5-MHz curvilinear transducer probe (Toshiba Aplio, Tokyo, Japan). The eGFR values were calculated by nephrologists. Renal length and cortical thickness values were evaluated at the beginning of the study and after 24 months of follow-up. Bilateral renal length was measured as the maximum pole-to-pole distance in the sagittal plane. RCT was determined bilaterally by measuring the shortest distance between the renal capsule and the base of a medullary pyramid at the level of the mid-kidney (Fig. 1) [6]. When possible, these measurements were made on the kidney that was included in the study. Measurements at three different areas were made in each examination, with sufficient magnification, and then the average of these measurements was used.
Statistical analysis was performed using SPSS ver. 21 (IBM Corp., Armonk, NY, USA). RCT, renal length, and eGFR values were measured at the beginning and at the end of the study and were compared using the paired-samples t test. Additionally, Pearson correlation analysis was conducted between renal length and RCT measurements and eGFR values to assess kidney function. P-values of <0.05 were considered to indicate statistical significance.
During the follow-up of 41 patients, eight patients started hemodialysis. After hemodialysis, the relevant data for these patients were excluded from the statistical analysis, as dialysis would be expected to improve their renal function, in turn leading to biased results.
Twenty-three patients had hypertension (HT), four patients had diabetes mellitus (DM), and 12 patients had both DM and HT. In the beginning of the study, the subjectsŌĆÖ mean creatinine and eGFR levels were 2.61 mg/dL and 35.92 mL/min, respectively. Their mean ATCR was 2.41. After 24 months, the mean creatinine level increased to 3.52 mg/dL, and their eGFR decreased to 28.38 mL/min. The ATCR increased to 3.46. The mean renal length was 91.29 mm at the beginning of the study and 90.24 mm at the end of the study. The mean RCT was 5.76┬▒2.05 mm at the beginning of the study and 5.28┬▒1.99 mm at the end of the study. The mean eGFR significantly decreased over the course of the study (P<0.01). Renal length and RCT values likewise prominently decreased (P<0.01) (Table 1).
The incidence of renal parenchymal atrophy is lower in diabetic patients. In our study, the mean renal diameter and RCT values were 98.06┬▒9.69 mm and 6.95┬▒2.07 mm, respectively, in the DM patients at the beginning of the study, compared to 86.96 mm and 5.01 mm, respectively, in the non-DM patients. At the end of the study, these parameters were 97┬▒9.45 mm and 6.37┬▒1.98 mm, respectively, in the DM patients, and 85.92┬▒5.56 mm and 4.58┬▒1.86 mm, respectively, in the non-DM patients. The mean renal diameter and RCT values were significantly higher in the DM patients. Nonetheless, in both groups, these parameters decreased over the course of the study.
The mean RCT, renal length, and eGFR values of the entire study cohort were 5.52 mm, 90.77 mm, and 32.15 mL/min, respectively. A statistically significant positive relationship was found between eGFR and mean renal length (r=0.66, P<0.01) and between eGFR and mean RCT (r=0.85, P<0.01), with the latter being more prominent (Fig. 2).
The burden of CKD has dramatically increased and is consuming the resources of both developed and developing economies. For this reason, efforts to reduce the cost of managing this disease are always welcomed. This aim of this study was to identify a simpler method of determining the functional capacity of kidneys in individuals with CKD, and we attempted to determine the usefulness of RCT measurements obtained via US as an indicator of kidney function. The widely accepted consensus is that a relationship exists between renal length and functionality. In this approach, renal length measurements are generally included in US imaging reports [3]. We found that RCT exhibited a direct correlation with renal function and length measurements in patients with CKD. Based on the current study, it seems that cortical thickness measured using US may be more closely related to eGFR than renal length in patients receiving follow-up care for CKD. In a study conducted by Beland et al. [10], it was reported that cortical thickness measured using US was related to eGFR. Those authors measured the RCT and the length of the kidneys in 25 patients with CKD and found that RCT was more significantly related to eGFR than was renal length [10]. We also obtained similar results in our prospective study with more patients. In another study by Widjaja et al. [11], it was reported that renal area and cortical thickness values were better predictors of renal functionality than renal length in a series of 69 patients with renal artery stenosis.
In previous studies, kidney volume was emphasized by several authors as a direct indicator of kidney size, instead of kidney length, in both diseased and healthy patients [2,4]. In a more recent study, it was reported that kidney length and volume were correlated with eGFR in the elderly, but that kidney length had lower specificity in predicting renal impairment [12]. Another study suggested a correlation between eGFR and renal volume measured using USG in 116 healthy children [13]. However, it can be difficult to evaluate renal volume, and doing so requires experience. Volumetric estimations can be made based on the ellipsoid formula, but this method has an inherent deficiency, because the kidney has a bean-shaped structure rather than a pure 3-dimensional ellipsoid frame [14]. In addition to this fact, the ellipsoid volume would include the central sinus fat, which does not contain functioning renal tissue and may vary among patients. These factors can contribute to the finding reported in a recent study, in which a positive but weak association between sonographically determined kidney volume and various indices of eGFR was identified [15]. In contrast, in some studies conducted in the recent past, it has been reported that renal volume and cortical thickness measurements conducted using CT and magnetic resonance imaging could be beneficial for determining renal functionality levels [14,16-18].
However, radiation exposure and high costs are disadvantages of such an approach. For this reason, we think that measuring RCT is an ideal approach in patients with CKD, given adequate consideration of the advantages and disadvantages of this method. The current study has the limitations of including a small number of patients and a short follow-up period. The participating patients could have been followed up for a longer time. However, the results of our study suggest that functionality tests may show significant correlations with RCT. Another potential limitation of our study is that 24-hour urine collection was not used for glomerular filtration rate calculation and proteinuria estimation. The CG method was used for determining the eGFR, and ATCR in a spot urine sample was used for proteinuria detection. Nonetheless, these methods are universally accepted and are routinely used in clinical practice.
In conclusion, based on the data gathered in our study, RCT was correlated with renal function, and therefore, measuring RCT using US is an important method in the follow-up care of patients with CKD. Additionally, US is a cost-effective and easy-to-use way to conduct this kind of investigation. Nonetheless, the interpretation of US images generally involves relatively subjective observations made by a specialist. Thus, one should keep in mind that some discrepancies could occur in the evaluation of US image reports, which means that it would be preferable for evaluations to be made in a double-blinded manner. We believe that further, more comprehensive studies with more subjects would make a considerable contribution to the knowledge base in this field.
References1. El-Reshaid W, Abdul-Fattah H. Sonographic assessment of renal size in healthy adults. Med Princ Pract 2014;23:432ŌĆō436.
2. Jones TB, Riddick LR, Harpen MD, Dubuisson RL, Samuels D. Ultrasonographic determination of renal mass and renal volume. J Ultrasound Med 1983;2:151ŌĆō154.
3. American College of Radiology. ACR practice guideline for the performance of an ultrasound examination of the abdomen and/ or retroperitoneum (in collaboration with the American Institute of Ultrasound in Medicine AIUM) [Internet]. Reston, VA: American College of Radiology, 2007. [cited 2010 Apr 14]. Available from: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/us/us_abdomen_retro.aspx.
4. Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol 1993;160:83ŌĆō86.
5. Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, et al. Correlation of renal histopathology with sonographic findings. Kidney Int 2005;67:1515ŌĆō1520.
6. Khati NJ, Hill MC, Kimmel PL. The role of ultrasound in renal insufficiency: the essentials. Ultrasound Q 2005;21:227ŌĆō244.
7. Lamont AC, Graebe AC, Pelmore JM, Thompson JR. Ultrasound assessment of renal cortical brightness in infants: is naked eye evaluation reliable? Invest Radiol 1990;25:250ŌĆō253.
8. Eggert P, Debus F, Kreller-Laugwitz G, Oppermann HC. Densitometric measurement of renal echogenicity in infants and naked eye evaluation: a comparison. Pediatr Radiol 1991;21:111ŌĆō113.
9. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247ŌĆō254.
10. Beland MD, Walle NL, Machan JT, Cronan JJ. Renal cortical thickness measured at ultrasound: is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am J Roentgenol 2010;195:W146ŌĆōW149.
11. Widjaja E, Oxtoby JW, Hale TL, Jones PW, Harden PN, McCall IW. Ultrasound measured renal length versus low dose CT volume in predicting single kidney glomerular filtration rate. Br J Radiol 2004;77:759ŌĆō764.
12. Van Den Noortgate N, Velghe A, Petrovic M, Vandewiele C, Lameire N, Voet D, et al. The role of ultrasonography in the assessment of renal function in the elderly. J Nephrol 2003;16:658ŌĆō662.
13. Adibi A, Adibi I, Khosravi P. Do kidney sizes in ultrasonography correlate to glomerular filtration rate in healthy children? Australas Radiol 2007;51:555ŌĆō559.
14. Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, et al. Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology 1999;211:623ŌĆō628.
15. Sanusi AA, Arogundade FA, Famurewa OC, Akintomide AO, Soyinka FO, Ojo OE, et al. Relationship of ultrasonographically determined kidney volume with measured GFR, calculated creatinine clearance and other parameters in chronic kidney disease (CKD). Nephrol Dial Transplant 2009;24:1690ŌĆō1694.
16. Muto NS, Kamishima T, Harris AA, Kato F, Onodera Y, Terae S, et al. Renal cortical volume measured using automatic contouring software for computed tomography and its relationship with BMI, age and renal function. Eur J Radiol 2011;78:151ŌĆō156.
A representative longitudinal sonogram for the measurement of renal cortical thickness.A. Renal length (A) and cortical thickness (B) were measured using electric calipers. B. Three separate measurements (A, B, and C) were made with sufficient magnification to measure renal cortical thickness.
 Fig.┬Ā1.Correlations of estimated glomerular filtration rate (eGFR) values with mean renal length and mean cortical thickness.A. The correlation coefficient (r) between mean renal length and eGFR was 0.66 (P<0.01). B. The correlation coefficient (r) between mean cortical thickness and eGFR was 0.85 (P<0.01).
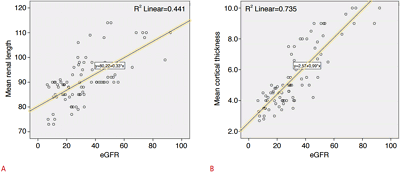 Fig.┬Ā2.Table┬Ā1.Changes in renal cortical thickness, renal length measurements, and renal function tests during the follow-up period |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




