Introduction
Contrast-enhanced ultrasound (CEUS) is a dynamic imaging technique based on intravenous injection or instillation into body cavities of an agent made up of microbubbles. During each exam, continuous insonation of the region of interest, with real-time evaluation of all phases, is performed. The microbubbles are excreted from the lung and the liver, making them safe for patients with a history of chronic renal insufficiency, dialysis, kidney transplant, and nephrectomy. Other advantages are that microbubbles do not contain iodine, have a very low rate of anaphylactoid reactions, do not use ionizing radiation, and are relatively inexpensive [1]. In the literature, several authors have stressed the important role of CEUS in the evaluation of liver disease, recommending its clinical use as a supplement to cross-sectional imaging in treatment planning, control, and monitoring of malignant liver lesions [2-4].
Unlike in Korea, the use of CEUS in Europe has only been approved for a limited number of nonhepatic indications in adults [5]; but its numerous benefits, compared to its risks, has recently made it an emergent imaging technique for non-hepatic applications as well. For this reason, the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) has recently described and recommended several possible applications of CEUS for various conditions involving the gastrointestinal and genitourinary tract, pancreatic gland, main vessels, and superficial organs, such as the thyroid, lymph nodes, salivary glands, breasts, and joints [6].
The ultrasonography (US) contrast agents currently used in diagnostic US of the liver are SonoVue, Definity/Luminity, and Sonazoid. In contrast to SonoVue, which is a pure intravascular agent without an interstitial extravascular phase, Sonazoid is an intravascular agent, licensed only in Japan and South Korea, which can also be taken up by the Kupffer cells in the sinusoidal spaces, allowing both vascular-phase and Kupffer-phase images to be obtained [7,8].
Although studies have recently demonstrated the benefits of the use of US contrast agents in children, no US contrast agent manufactured today is registered for pediatric use in Europe and CEUS is still used off-label in pediatric patients [6,9,10]. SonoVuehas recently been approved by the United States Food and Drug Administration under the name of Lumason to be used in hepatic investigations and in assessments of vesico-ureteral reflux in children [6,11].
Kidney
US is the first-line imaging modality for evaluating kidney diseases. The use of a microbubble-based contrast agent permits a better assessment of microcirculation and perfusion of the kidney than Doppler US, with no risk of nephropathy [12].
Imaging Protocol and Normal Findings
During a renal study with CEUS, the following phases are described: first the arterial pedicle and cortex enhance in the cortical phase, which occurs 15-30 seconds after the injection, and then the cortex and medulla enhance together in the parenchymal phase (25 seconds to 4 minutes after the injection), which can also be divided into early (25 seconds to 1 minute) and late (1-4 minutes) components [6,12]. Because of the high cortical perfusion, a lower dose of contrast agent is used (typically 1-1.5 mL of SonoVue, compared with 2.4 mL in a typical liver study) to avoid obscuration of the deeper parts of the kidney [12].
Role of CEUS
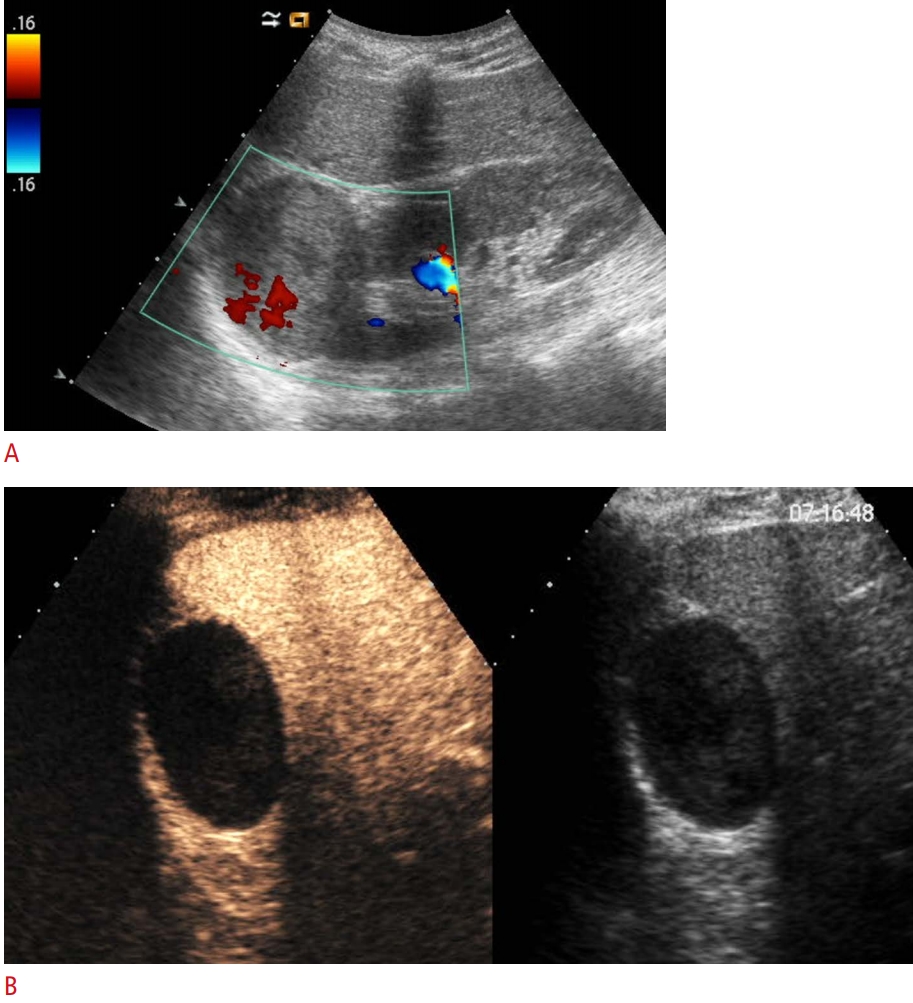
The majority of adult patients have at least 1 renal lesion, and many renal lesions are incidentally detected by imaging. Most renal lesions are benign simple cysts, but in other cases, they can be solid or mixed solid and cystic [13,14]. These masses require multiphasic contrast-enhanced renal imaging-specifically, contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) with intravenous injection of iodinated contrast or gadolinium, respectively-in order to evaluate enhancement characteristics that often differ between benign and malignant lesions [15]. In this scenario, CEUS plays an important role both in the characterization of some indeterminate masses on computed tomography (CT) and MRI and as a valuable alternative tool in selected patients in whom iodinated contrast medium or gadolinium is contraindicated or when CT or MRI scans are otherwise deemed inappropriate [16]. According to the recent update of the EFSUMB guidelines, the indications for the use of CEUS in the kidneys are suspected vascular disorders (infarction, cortical necrosis), differentiating between tumors and pseudotumors, characterization and follow-up of complex cystic masses, identification of renal abscesses, and radiofrequency ablation of non-surgical masses [6,17]. CEUS plays a very important role in distinguishing between solid lesions and hyperdense cysts. In fact, hyperdense renal lesions on unenhanced CT (20-70 Hounsfield units [HU]) require correlation with conventional sonography, where they can frequently be confidently diagnosed as cystic. In less conclusive cases, CEUS may be an alternative imaging technique to demonstrate the absence or presence of vascular flow within the lesion, avoiding both the radiation dose and potential nephrotoxicity of iodinate agents used in CT scans and the risk of false negatives for hypovascular tumors [18,19].
Renal Lesions
Solid lesions
CEUS allows a detailed assessment of the neoplastic circulation and has the potential to differentiate malignant from benign renal lesions when they appear as hypoechoic [12,20].
CEUS is more sensitive than contrast-enhanced CT and MRI in detecting vascularity in hypovascular lesions [18], and it is also sensitive in identifying unenhancing cystic areas, necrosis, debris, and hemorrhagic foci in tumors that may be hard to detect on CECT and MRI, especially in small lesions [18].
On CEUS, renal tumors show different patterns of enhancement in comparison with the renal parenchyma in at least one phase. Renal clear cell carcinoma (RCC) usually shows hyperenhancement or isoenhancement, generally inhomogeneous, in the cortical phase, washout in the late phase, and perilesional rim-like enhancement [21], whereas papillary carcinoma tends to show less hyperenhancement, usually homogeneous, during the cortical phase [21,22].
Angiomyolipoma generally shows homogeneous isoenhancement or hypoenhancement and prolonged enhancement [23], but when it presents a hypoechoic rim, cystic component, or pseudocapsule, it may be difficult to differentiate from small (<3 cm) echogenic RCC [12]. Moreover, studies have recently demonstrated that tumors smaller than 3 cm may frequently show homogeneous enhancement on CEUS, regardless of the histologic subtype [23].
Renal oncocytomas cannot be differentiated from RCC because of their similar enhancement patterns [24].
Instead, solid renal tumors may be easily distinguished from pseudotumors, abscesses, and ischemic disorders of the kidneys, as pseudotumors remain isoechoic to the normal renal parenchyma in all phases, abscesses are seen as a non-enhancing area (solitary or within areas of pyelonephritis), and infarcts appear as wedgeshaped non-enhancing areas within an otherwise enhanced kidney [6].
Concerning solid renal lesions, several studies have evaluated the diagnostic accuracy of CEUS, compared with CT and MRI, for the differential diagnosis of benign and malignant renal lesions, applying both qualitative and quantitative features. Unfortunately, according to the literature and updated guidelines, CEUS cannot be used-either with qualitative or quantitative methods-to differentiate renal solid cancerous histotypes [17,20,23,25].
Cystic lesions
Cystic renal masses are common and can vary from simple to complex; the complexity of cysts may be due to hemorrhagic, infectious, or ischemic processes inside the lesion [26]. Around 10% of renal cell carcinomas appear as complex cystic renal masses [27]. In contrast to what their malignant appearance suggests, some cystic lesions can remain stable for a long time, while malignant cysts often need timely surgery. Consequently, accurate diagnosis is essential to guide clinical management. The Bosniak classification of renal cysts based on CT was first published in 1986 and modified in 1993 [28-30] in order to predict the risk of malignancy and establish treatment recommendations [31]. This classification system distinguishes five categories (1, 2, 2F, 3, and 4) of cystic renal masses according to the number of septa; the thickness of the cyst walls or septa; the enhancement of the septa, wall, nodule, or other solid components; and the presence of calcifications [26,28,32-34]. The Bosniak classification has been applied using CEUS and a classification system similar that for CECT [15,32] has been proposed [35,36]. In clinical practice, the greatest difficulty arises for all modalities in differentiating among categories II, IIF, and III and in recommending surgery or conservative follow-up [32,37,38]. Several studies have investigated the accuracy of CEUS compared with CECT and MRI. A recent meta-analysis conducted by Lan et al. [39] revealed that the sensitivity of CEUS was higher than that of CECT, while the specificity of CEUS was slightly lower than that of CECT. The high spatial resolution of CEUS permits the detection of more septa, thickened walls and septa, and more solid components than is possible using CT [37,38,40]. Moreover, evaluation in real time can also help in the assessment of cysts with several confluent septa that may mimic a solid mass [15]. Because of the characteristic scattering property of microbubbles, the tiny capillaries that feed hair-line septa can also be revealed. This fact explains why several authors have found CEUS to show higher sensitivity than CT in depicting the cystic wall and septa vascularity [15,38,40-43]. However, minimal septal enhancement can also occur in benign renal cystic lesions, and many authors have reported that CEUS can upgrade some cystic masses compared with CT when using the Bosniak classification system [43-45]. Recently, Zhou et al. [37] performed a meta-analysis comparing the diagnostic efficacy of CEUS with that of MRI for cystic renal masses, and suggested that CEUS is equivalent to MRI in the diagnostic value for cystic renal masses, although it has higher sensitivity and lower specificity than MRI.
Summary
The most interesting field of renal CEUS applications regards the characterization of renal cysts (Fig. 1). In clinical practice, CEUS may be used to characterize and follow-up complex cysts found on conventional US and to refine the correct Bosniak category of indeterminate renal cysts on CECT. Due to its high sensitivity and low specificity, CEUS should always be used in combination with CECT in order to improve the lesion detection rate and to decrease the misdiagnosis rate. For solid lesions, CEUS has recently been recommended to evaluate low- to moderate-risk lesions in patients who have absolute or relative contraindications to CECT or MRI and as a secondary test for indeterminate lesions (small lesions or with a threshold of 15-20 HU) on CECT or MRI [25].
Spleen
The splenic parenchyma is always evaluated during abdominal US. The location, homogeneous parenchyma, and small size of the spleen make it well suited for CEUS examinations.
Imaging Protocol and Normal Findings
Due to the particular vascularization of the spleen in the early phase after the injection of microbubbles (after 12-18 seconds), an inhomogeneous opacification (a "zebra-striped" pattern) may be seen [46]. This heterogeneous appearance creates a potential pitfall to interpretation, especially for inexperienced practitioners, and makes it difficult to identify lesions that are not accurately located on gray-scale images [47]. After 60 seconds, the parenchyma enhances homogeneously with late-phase enhancement. The spleen, like the liver, has the property of retaining microbubbles, resulting in persistent late-phase enhancement that usually persists for 5-7 minutes [6,46].
Role of CEUS in Splenic Disease
According to the recent update of the EFSUMB guidelines, CEUS can be used to evaluate abnormal splenic size and to identify infarctions, ectopic splenic tissue, and splenic lesions. CEUS is particularly recommended to distinguish simple cysts from abscesses in selected cases and focal solid benign lesions from malignant ones, especially when echo-poor on US [6]. The spleen is frequently involved in patients with intra-abdominal trauma, and CEUS could play an interesting and emergent role in the detect and follow-up of splenic traumatic lesions [48,49].
In any case, knowledge of the patient's clinical presentation, past medical history, laboratory tests, and previous imaging is often essential for a differential diagnosis [50].
Splenic Tumors
An early and precise differential diagnosis between benign and malignant focal splenic lesions can provide a timely and rational treatment modality for the clinical choice between invasive and noninvasive management.
Only a few CEUS studies in the literature have investigated splenic tissue [51]. According to these studies, the sensitivity, specificity, and accuracy of CEUS are all higher than those of US. Moreover, CEUS showed comparable performance to positron emission tomography computed tomography (PET-CT) diagnosis [51]. Small echogenic lesions of the spleen are most common benign vascular tumors (hemangiomas or hamartomas). They often show isoenhancement or hyperenhancement in the arterial phase, rarely followed by slow, modest, and incomplete washout [6,47]. Instead, focal, echo-poor, splenic lesions are usually malignant and represent a diagnostic challenge [47]. They are more frequently multiple, fast-growing, and present inhomogeneous enhancement with necrotic areas in the arterial phase and rapid and complete washout in the early and late phases [47,51]. The presence of irregular intralesional vessels and a 'dotted' appearance in the parenchymal phase are other features of malignant lesions [50].
Malignant splenic lesions are almost always either lymphomas or metastases [47].
Non-lymphomatous metastases are very rare and represent a sign of advanced disease of melanoma, breast cancer, and lung cancer. They mainly show inhomogeneous hypoenhancement in the arterial phase and a rapid wash-in and rapid wash-out pattern with a contrast agent [50,51]. Late-phase imaging is very important for depicting the microcirculation in areas of viable tumors that may be mistaken for necrosis, which is essential when assessing response to chemotherapy [50]. CEUS is used to characterize these masses only when CT and/or MRI and PET are contraindicated or inconclusive. Splenic lymphoma is usually a manifestation of systemic lymphoma, mostly non-Hodgkin disease, while primitive lymphoma of the spleen is rare [46]. On US imaging, splenic lymphoma may appear as small nodules or miliary infiltrations, typical of low-grade lymphoma, or as large masses and bulky infiltrative disease, found in high-grade lymphoma [46,50]. Lesions are typically hypoechoic compared with normal splenic parenchyma; after therapy, recurrent lesions frequently show a heterogeneous or 'target' appearance [46,51,52]. In the arterial phase, they may be homogeneously isoenhancing or hypoenhancing relative to the splenic parenchyma, with internal irregular vessels, while in the parenchymal phase they become hypoenhancing, due to rapid washout [50]. In a recent prospective study, Picardi et al. [53] compared CEUS and fluorine-18 fluorodeoxyglucose (FDG)-PET in order to detect nodular infiltration in the spleen of patients with newly diagnosed Hodgkin lymphoma. They evaluated 100 adult patients indicated for pre-treatment staging according to the Ann Arbor system, and demonstrated that CEUS was more sensitive in identifying splenic nodules positive for lymphoma than CT and FDG-PET were. This was valid both for small nodules (<1 cm) and for larger ones (>1.1 cm), and this finding led to a change in Ann Arbor staging system. Therefore, even in the absence of histologic proof, CEUS should be recommended as a part of the diagnostic work-up to stage patients with Hodgkin lymphoma in order to avoid the risk of understaging and undertreatment [53].
Splenic Trauma
In a prospective multi-centric study, Catalano et al. [54] evaluated 156 adult patients with clinical and abdominal suspicion of blunt or penetrating abdominal trauma, considering CT and surgery as the reference standards. In accordance with the current literature, they found that CEUS may improve the detection and severity assessment of abdominal injuries [48,54-57]. In particular, for the spleen, they demonstrated that the sensitivity, specificity, and accuracy of CEUS were 93%, 99%, and 97% higher, respectively, than those of baseline US [54]. Comparable results were presented by Sessa et al. in a study of 256 patients with low-energy isolated abdominal trauma. They evaluated the accuracy of CEUS and demonstrated that, in these patients, US should be replaced by CEUS as the firstline approach, because it shows a high sensitivity in both lesion detection and grading [58].
The venous phase is the most accurate for detecting traumatic lesions of the spleen [48].
On the basis of the distribution of contrast material (homogeneous, heterogeneous, or absent) CEUS can identify parenchymal injuries, including contusions, lacerations, hematomas, bleeding, infarcts, and arteriovenous fistulas [57]. It should be used after the Focused Assessment with Sonography in Trauma (FAST) protocol or US, especially when the baseline US detects peritoneal/retroperitoneal fluid but fails to identify organ injury and/or demonstrates subtle parenchyma changes, or in cases of negative US findings with persistent laboratory suspicion in hemodynamically stable patients with a history of low-energy trauma [48,54,57,59].
Pseudoaneurysm is a rare complication of splenic trauma, but its identification is essential to avoid delayed splenic rupture. It is seen as well-delineated anechoic lesion on US and as an area of hyperenhancement in arterial-phase lesions on CEUS [51].
Summary
CEUS may significantly improve the accuracy of the detection and characterization of focal splenic lesions, especially malignant ones (Fig. 2). Concerning malignant lesions, the differential diagnosis between lymphoma and metastases is impossible on the basis of splenic CEUS appearance alone, although necrosis is more commonly seen in metastatic disease [50].
CEUS could play a valuable role in selected patients with abdominal trauma for the diagnosis and follow-up of splenic injuries; for unstable trauma patients; for cases of abdominal trauma in which CECT is contraindicated, failed, or unclear; and in monitoring of conservatively treated injuries [49,54].
CT should instead be reserved for cases of severe trauma with clinical suspicion of multiorgan lesions and cases with inconclusive CEUS findings [59].
Pancreas
CEUS of the pancreas leads to a major improvement in the diagnostic accuracy of US, which is usually applied in the initial evaluation of pancreatic diseases. When a pancreatic lesion, frequently incidental, is detected on US, an immediate differential diagnosis is essential for appropriate management.
Imaging Protocol and Normal Findings
The blood supply of the pancreas is entirely arterial, so that enhancement of the gland begins almost together with aortic enhancement. In a pancreatic CEUS study, it is possible to distinguish an arterial phase (15-20 seconds after the microbubble injection), in which parenchymal enhancement may be seen, and a portal venous phase (from 30 to 120 seconds after injection), characterized by enhancement of the portal vein. During the late phase (about 120 seconds after injection) a complete evaluation of the liver should be performed, in order to identify possible metastatic lesions [60,61].
Role of CEUS
CEUS is a safe and accurate imaging method for differentiating solid from cystic pancreatic tumors and solid tumors from pseudo-tumor masses; doing so influences the choice of further examinations and allows a faster diagnosis to be obtained [62].
According to the most recent EFSUMB guidelines, CEUS can be used to distinguish between ductal pancreatic adenocarcinoma, which represents the most common primary malignancy of the pancreas [6,63], and pancreatic neuroendocrine tumor [6].
Among cystic pancreatic lesions, CEUS can distinguish cystic tumors from pseudocysts and detect the presence of vascular or avascular (necrotic) components in cystic lesions. Furthermore, CEUS is recommended for the follow-up of indeterminate pancreatic cysts and necrotizing pancreatitis [6].
Solid Pancreatic Tumors
On CEUS, pancreatic adenocarcinoma typically appears as a hypoenhancing mass compared to the surrounding parenchyma [64-68] and shows minimal enhancement in all the dynamic phases, because of its intense desmoplastic reaction with relatively poor mean vascular density and perfusion [69]. In the Pancreatic Multicenter Ultrasound Study (PAMUS) meta-analysis, a high accuracy of CEUS in characterizing pancreatic masses was reported, as solid pancreatic lesions were correctly characterized in respect to pathology with an accuracy of 91.7% and pancreatic ductal adenocarcinoma was correctly characterized with an accuracy of 87.8% [70]. In the literature, a specificity close to 100% for transabdominal CEUS diagnosis of ductal adenocarcinoma, with a sensitivity of 90%, has been reported [71,72]. Recently, a metaanalysis was conducted with the aim of evaluating the role of CEUS in differentiating among pancreatic lesions. The authors reported a sensitivity of 89% and specificity of 84% for CEUS in the differentiation between ductal adenocarcinoma from non-ductal adenocarcinoma and a sensitivity of 95% and a specificity of 72% of CEUS in distinguishing neoplastic from non-neoplastic lesions [73]. According to the European Neuroendocrine Tumor Society (ENETS) Consensus Guidelines from 2012, CEUS is considered an alternative imaging method to diagnose pancreatic neuroendocrine tumors and to accurately assess the liver staging of the disease [74].
Cystic Pancreatic Lesions
Cystic lesions of the pancreas include a large variety of lesions, with different etiology and biology, each requiring a different management strategy [75,76]. Therefore, the evaluation of a pancreatic cystic tumor should enable differentiation between benign and malignant lesions [77].
Mucinous cystic neoplasms (MCNs) represent about 10% of all cystic pancreatic lesions [78] and include mucinous neoplasms with low-grade dysplasia (MCN) and mucinous cystadenocarcinoma. MCN is a benign lesion with a high malignant potential [79], and surgical resection is required in all cases. As stressed by the authors of new EFSUMB guidelines, thanks to the microbubble-specific software that deletes all background signals and only shows the signal of the blood-pool contrast agent, CEUS can clearly demonstrate the micro-vascularization of septa and solid components of cystic pancreatic lesions, improving the differential diagnosis between mucinous cystic tumors and pseudocysts [64,80]. Pancreatic pseudocysts are persistent, late, peri-pancreatic collections that often develop after the onset of acute pancreatitis. They present a thin encapsulating wall, contain only fluid or fluid mixed with non-vascularized debris, and characteristically do not enhance in any phase on CEUS [70,80,81]. The reported sensitivity and specificity of CEUS in characterizing pseudocysts is very high [82].
Intraductal papillary mucinous neoplasms (IPMNs) are a group of exocrine mucin-producing tumors that spread into the main pancreatic duct and/or its collateral branches. They often show benign behavior, but they sometimes present solid components (perfused nodules) and a high malignant potential. Unfortunately, CEUS is not able to demonstrate communication with the ductal system, which is essential for the final diagnosis of IPMN, but it may be helpful for differentiating between perfused (nodules) and nonperfused (mucin plugs) areas [81,83,84].
Serous cystadenoma (SCA) is a benign cystic tumor, with a typical lobulated microcystic honeycomb architecture, thin wall, and thin multiple septa orientated toward the center [77], that never communicates with the main pancreatic duct [85], and is well demonstrated on magnetic resonance cholangiopancreatography [86]. The absence of communication with the main pancreatic duct is not demonstrable by US or CEUS [81]. SCAs are typically hypervascular and, therefore, hyperenhancing on CEUS, since the septa are composed of abundant sub-epithelial micro- and macrovessels, and especially when the cysts are small, they may mimic a solid lesion [76,87,88].
Acute and Chronic Pancreatitis
In acute pancreatitis, CEUS is an alternative imaging method for diagnosing mild and severe forms that, due to hyperemia, appear hypervascularized [64] and for delineating parenchymal necrosis in cases of severe acute pancreatitis, which appears as non-enhanced lesions, with a high accuracy [89]. Due to the lack of a panoramic view, CEUS may not substitute for the first CT examination for staging acute pancreatitis. However, it may be used in follow-up of acute pancreatitis, reducing the number of CT examinations [89].
Within the category of chronic pancreatitis, autoimmune pancreatitis is a rare disorder of presumed autoimmune etiology that accounts for up to 10% of chronic pancreatitis cases [90]. This inflammatory process shows three patterns of distribution: focal, multifocal, and diffuse. The focal pattern is less common than diffuse one, but it often involves the pancreatic head and may be confused with ductal adenocarcinoma. In clinical practice, CEUS may play an important role in differentiating these 2 entities. A recent retrospective study of Dong et al. [91] reported that on CEUS, autoimmune pancreatitis displayed focal or diffuse isoenhancement (86.6%), in comparison to the surrounding parenchyma in the arterial phase and hyperenhancement (65%) or isoenhancement (35%) in the late phase, in contrast to ductal adenocarcinoma, which appeared hypoenhancing (93.7%) in all phases of the study.
Summary
CEUS is very useful for diagnosing pancreatic tumors and for characterizing and differentiating ductal adenocarcinoma (Fig. 3) from other pancreatic lesions, such as neuroendocrine tumors or focal pancreatitis [73]. It may help to define the size and the margins of pancreatic lesions, and therefore may be useful for guiding pancreatic biopsies [92]. Furthermore, with the use of CEUS, changes in pancreatic tumor vascularization during chemotherapy can be documented [93].
CEUS can be used to characterize cystic pancreatic lesions, and it is recommended for the follow-up of borderline cystic lesions, if well visualized on US, in order to reduce the use of MRI [94].



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+


 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




