AbstractPurposeThe aim of this study was to analyze the imaging findings and clinical characteristics of extratendinous migration of calcific tendinitis of the shoulder with temporal changes.
MethodsSeventy-six patients with extratendinous calcific tendinitis of the shoulder diagnosed by ultrasonography (US) or magnetic resonance imaging (MRI) were enrolled in this retrospective study. Clinical symptoms and imaging findings (on radiography, US, and MRI) of extratendinous calcific tendinitis during an acute painful attack were analyzed. Temporal changes were analyzed in 28 patients before an acute attack and 40 patients after an acute attack. For comparison, 65 patients with intratendinous calcific tendinitis were included.
ResultsPatients with extratendinous calcific tendinitis had a significantly higher average visual analogue scale (VAS) score (8.8┬▒1.6) than the intratendinous group (6.4┬▒2.2) (P<0.001). The fragmented type (80.5%) was the most common shape on US; sonographic black hole appearance (14.6%) and echogenic fluid (9.8%) were characteristic findings of intrabursal calcifications. In 28 patients with previous radiographs, radiographic type III (78.6%) was dominant and the location of calcific deposits changed (82.1%) during the acute painful attack, which was also perceivable in 12 patients with previous US or MRI. In follow-up radiographs of 40 patients, calcifications shrunk by more than 50% or became invisible in 82.5% of patients, with symptom improvement (VAS score, 8.9┬▒1.5 to 1.9┬▒1.2). Follow-up US and MRI of 16 patients also showed decreased size (56.3%) or disappearance (43.7%) of calcific deposits.
Calcific tendinitis of the shoulder is a common, self-limiting disease caused by deposition of calcium into the rotator cuff tendons. The calcific phase of this disease can be subdivided into the formative, resting, and resorptive stages [1]. Following a variable period of the asymptomatic resting stage, the calcifications progress to the resorptive stage [2]. In the resorptive stage, phagocytosis of the calcific deposits with increased intratendinous pressure may cause extrusion of calcifications from the tendon [3,4]. Although some authors described uncommon migration of calcific tendinitis of the rotator cuff [3,5-8], the most up-to-date articles on this topic have reported migration of calcific tendinitis to a single location, especially bone involvement [9-18]. Moreover, there is a lack of information on sequential changes in the calcification associated with calcific tendinitis of the rotator cuff throughout the course of the disease and the clinical significance of those changes. The purpose of the present study was to evaluate the imaging findings and clinical characteristics of extratendinous calcific tendinitis of the rotator cuff with their temporal changes.
This retrospective study was approved by the Institutional Review Board of the Catholic University of Korea, Bucheon St. MaryŌĆÖs Hospital (HC20RISI0052). The requirement for informed consent was waived because the study retrospectively used data available from an electronic database.
Our radiology database search for the term "calcific tendinitis" from April 2004 to September 2020 yielded 407 patients with calcific tendinitis diagnosed by ultrasonography (US) or magnetic resonance imaging (MRI) of the shoulder. US and MRI were used to determine the location of calcific deposits. Of those 407 patients, 106 patients with extratendinous calcific tendinitis were initially selected. Among them, 30 patients were excluded due to the following exclusion criteria: (1) lack of information in the medical records on the severity of symptoms at the time of imaging examination using US or MRI and (2) any conservative management or intervention before visiting our hospital. Finally, 76 patients (76 shoulders) with extratendinous calcific tendinitis were included in this study. Using the same exclusion criteria, 65 patients (68 shoulders) with the diagnosis of intratendinous calcific tendinitis were enrolled for a comparison between extratendinous and intratendinous calcific tendinitis of the shoulder. A flow diagram of this study is shown in Fig. 1. Radiography was performed as a routine study with US and/or MRI in all patients from both groups.
PatientsŌĆÖ medical records were reviewed for the following clinical characteristics: pain intensity, represented by a visual analogue scale (VAS), and patientsŌĆÖ experience in the emergency room (ER), hospitalization, intervention, or surgical treatment for calcific tendinitis.
A standardized set of shoulder radiographs consisted of an anteroposterior radiograph with or without rotation, a supraspinatus outlet view, and an axillary view. US of the shoulder was performed by an experienced musculoskeletal radiologist using an HDI 5000 or an IU 22 (ATL/Philips, Bothell, WA, USA) with a 17.5-MHz linear array transducer. MRI was performed with a 1.5 T system (Gyroscan NT, Philips, Eindhoven, The Netherlands) or a 3 T system (Achieva, Philips, Eindhoven, The Netherlands) using a shoulder coil. The MRI parameters were variable due to the relatively long inclusion period. The MRI protocol consisted of the following pulse sequences: axial T2-weighted fast spin echo (TR [repetition time]/TE [time to echo], 1,800-4,300/90) images or proton density (PD)-weighted images with fat suppression (TR/TE, 2,400/25) images; oblique coronal T2-weighted fast spin echo images, fat-suppressed T2-weighted (TR/TE, 1,800-4,300/70) images, and PD-weighted images without fat suppression; and oblique sagittal T2-weighted fast spin echo or fat-suppressed T2-weighted images. Contrast-enhanced MRI after administration of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist, Shering, Germany) was obtained with the following sequences: contrast-enhanced axial, oblique coronal, and sagittal T1-weighted images with fat suppression (TR/TE, 450-700/20). The imaging parameters were a 14- to 16-cm field of view, a 3-mm slice thickness with a 0.3- to 1-mm gap, a 256├Ś196 or 560├Ś560 acquisition matrix, and 2-4 excitations.
Radiographs of the shoulder were evaluated for the location, shape, size, margin, and density of the calcific deposits. The calcific deposits were classified according to the classification system proposed by Gartner and Simons [19]. Herein, we added type 0 to define calcific deposits that were radiographically nonvisible but visible on US. US and/or MRI was used to determine the location of the calcific deposits. In particular, MRI was used to localize the intraosseous calcific deposits. US was used to evaluate the location, shape, size, echogenicity, and degree of posterior acoustic shadowing of the calcific deposits. The sonographic shape of the calcific deposits was classified into five types: arc-like, nodular (an echogenic nodule without shadowing), fragmented, black hole (central low echogenicity with hyperechoic rim), and an echogenic fluid (colloidal suspension of calcium salts). When two or more shapes coexisted, each was counted separately. Similarly, MRI findings were evaluated for the location, morphology, size, and signal intensity of the calcific deposits. Accompanying bone marrow edema, pericalcific soft tissue edema, bursitis, rotator cuff tear, and tenosynovitis in the long head of the biceps tendon were evaluated on US and MRI as ancillary findings.
To investigate sequential changes of calcific deposits, radiologic images of the shoulder (including radiography, US, and MRI) obtained before an acute attack in 28 out of 76 patients were analyzed for changes in the location, morphology, and size of the calcific deposits to compare with those obtained during the acute attack. In addition, follow-up images after an acute attack in 40 out of 76 patients were evaluated for changes in morphology and size of the calcific deposits, with the exclusion of the other 36 patients, who had undergone surgery or intervention for treatment of the shoulder pain during the acute attack or who did not undergo additional imaging studies. Sixteen patients underwent both prior and follow-up imaging studies. Temporal changes in VAS scores were used to correlate the temporal imaging changes with clinical symptoms.
Two radiologists who had 3 and 30 years of experience in musculoskeletal imaging retrospectively reviewed the aforementioned imaging findings and reached a consensus.
The study subjects were categorized into two groups according to the presence of extratendinous calcifications. In order to evaluate symptom severity in patients with extratendinous calcific tendinitis, the mean VAS score was obtained from both the extratendinous and the intratendinous group, and was then compared between the two groups using the t-test. Similarly, to evaluate the imaging characteristics of extratendinous calcifications, imaging features on US and MRI were evaluated in both groups and were then compared using the Pearson chi-square test or the Fisher exact test. All statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA), and a P-value < 0.05 was considered to indicate a statistically significant result.
There was a significant difference in clinical findings between extratendinous calcific tendinitis and intratendinous calcific tendinitis (Table 1). The 76 patients with extratendinous calcific tendinitis had higher VAS scores (mean, 8.8┬▒1.6) than the 65 patients with intratendinous calcific tendinitis (mean, 6.4┬▒2.2) (P<0.001). The patients with intratendinous calcific tendinitis tended to have an even distribution of pain scores. Of the patients with extratendinous calcific tendinitis, 18.4% visited the ER for pain, 34.2% were hospitalized, and 15.8% received either interventional or surgical treatment. Only one (1.5%) of the patients in the intratendinous group visited the ER for pain.
The locations of calcific deposits in 76 patients with extratendinous calcific tendinitis were: sub-bursal (n=74), intrabursal (n=44), intraosseous (n=19), intramuscular (n=1), and the potential space between the deltoid muscle and subdeltoid bursa (n=2). Calcific deposits were seen in two or more extratendinous locations in 50 of 76 patients (65.8%) with extratendinous calcific tendinitis. The locations of calcific deposits in 76 patients with extratendinous calcific tendinitis are presented in Table 2.
Detailed imaging features on US are given in Table 2. Two or more sonographic shapes were combined in 24 cases. The fragmented type of calcification was more common in the extratendinous calcific tendinitis group than in the intratendinous group (P<0.001) (Figs. 2, 3). Arc-like calcifications seen in eight patients in the extratendinous group were accompanied by nodular or fragmented calcific deposits. The black hole appearance and echogenic fluid were found only in extratendinous calcifications. The anechoic or hypoechoic core of the black hole calcifications corresponded to the intermediate to high signal core on MRI and to radiolucent calcific deposits on radiographs (Figs. 4, 5).
Calcific deposits revealed a variable signal intensity, ranging from a signal void to intermediate to high signal intensity on T2-weighted fast spin echo or fat-suppressed T2-weighted images. Intraosseous calcific deposits were seen in various forms with different MRI signal intensities: isolated subcortical low signal intensity or a signal void (9 of 19, 47.3%), intramedullary disseminated tiny low signal intensities (4 of 19, 21.1%), variable-sized cystic lesions with tiny internal low signal intensity (5 of 19, 26.3%), and a form of bone marrow edema (1 of 19, 5.3%). On MRI, pericalcific soft tissue edema was strongly associated with extratendinous instead of intratendinous calcific tendinitis (100% vs. 25%, P<0.001).
Bursitis was more frequently associated with extratendinous calcifications than intratendinous calcifications (86.8% vs. 36.8%, P<0.001) as shown on US and MRI. There was no statistically significant difference in rotator cuff tear (23.7% vs. 41.2%, P=0.025) and tenosynovitis of the long head of the biceps tendon between the two groups (23.7% vs. 19.1%, P=0.506).
A detailed analysis of the demographic and imaging features of 28 patients with extratendinous calcific tendinitis with previous imaging studies is shown in Table 3. The most prevalent radiographic type changed from type I (50.0%) to type III (78.6%) during the acute attack. In 23 of the 28 patients with extratendinous calcific tendinitis, follow-up radiographs showed changes in the location of calcific deposits (Figs. 2, 3, 6). The temporal changes in the location of calcific deposits consisted of three patterns: (1) the emergence of new calcific deposits outside the expected tendon boundary (8 of 23, 34.8%), (2) changes in the location of the existing calcifications (12 of 23, 52.2%), and (3) changes in the size of existing calcifications with lesser density (3 of 23, 13.0%). In all 12 patients with previous US and MRI, there were perceivable extratendinous migrations of calcific deposits in the acute attack (Fig. 6). US and MRI also showed morphological changes in the calcific deposits (6 of 12, 50%). Along with these radiologic changes, the patientsŌĆÖ mean VAS score increased from 4.53┬▒1.3 to 9.6┬▒0.8 during the acute attack.
Detailed clinical and imaging features in 40 patients with follow-up imaging studies after the acute painful attack are presented in Table 4. Follow-up radiographs of the calcific deposits in 40 patients revealed a size reduction by more than 50% or disappearance of the calcific deposits in 33 patients (82.5%) (Figs. 4-6). US and MRI showed changes in morphology (50%) and size (decreased in 56.3% and disappeared in 43.7%) of the calcific deposits (Fig. 5). The average VAS score significantly decreased from 8.9┬▒1.5 to 1.9┬▒1.2.
This study showed that extratendinous calcifications of the rotator cuff were associated with higher levels of shoulder pain than intratendinous calcifications. The majority of patients who visited the ER for shoulder pain associated with calcific tendinitis were found to have extratendinous calcific tendinitis. Furthermore, patients with extratendinous calcifications often required aggressive pain management, inpatient care, surgical removal of calcific deposits, or interventions including extracorporeal shock wave therapy and percutaneous needle aspiration. Interestingly, there were many patients with extratendinous calcifications whose excruciating pain was triggered at some point and resolved spontaneously without any intervention. Uhthoff suggested that acute pain during resorptive phase may be induced by an increased intratendinous pressure [20]. In addition to that, it is suggested that rupture and migration of the calcifications may cause severe pain due to mechanical irritation and an inflammatory reaction along the path of migration. The higher incidence of pericalcific soft tissue edema and bursitis in extratendinous calcific tendinitis in the present study supports an ongoing inflammatory response to migration of calcific deposits. It appears that subsequent absorption of the calcific deposits with regression of the inflammatory response will eventually help to relieve shoulder pain.
In the present study, US played an important role in localizing extratendinous calcifications and evaluating their properties. The morphology of calcifications can change with focal breaks during the resorptive stage, developing into a complete fragmentation of the deposits [21]. The fragmentation can lead to the disappearance of calcific deposits by disruption of the fibrous tissue and activation of an inflammatory reaction [22]. Interestingly, in our study, some intrabursal calcifications showed the appearance of a black hole, which disappeared rapidly on follow-up radiographs and US images. One possible explanation is that the dissolved calcified materials containing fluid may exhibit a black hole appearance. Sconfienza et al. [23] referred to this type of calcifications as fluid calcifications and also reported that there was almost no resistance to the plunger during US-guided procedures. Meanwhile, when soft calcifications enter the bursa, they can be seen as hyperechoic fluid. Mechanical irritation and an inflammatory reaction during migration can cause subacromial-subdeltoid bursitis containing calcium and debris, which can be identified well on US.
In this study, the sequential analysis of radiography, US, and MRI revealed the natural course of calcific tendinitis, with extratendinous migration and eventual regression of calcific deposits with concurrent symptom improvement. US and MRI performed before and during an acute attack revealed the extratendinous migration of calcific deposits. The imaging findings could also be identified on radiographs, which showed three types of migration patterns of calcific deposits. Meanwhile, those migrated calcifications shared similar imaging features in the remaining cases of extratendinous calcifications on radiography, US, and MRI. As in our study, the migrated intrabursal calcific deposits may be so small or subtle that they can be easily overlooked on radiographs. Therefore, in patients with calcific tendinitis suffering from aggravating shoulder pain, it is important to sufficiently adjust the images to take a closer look. Pereira et al. [16] demonstrated that calcifications disappear immediately after the resorptive phase and the granulation tissue fills the space instead of calcific deposits, which represents the post-calcific stage. As reported by a previous study [24], spontaneous regression of calcific deposits was observed on follow-up radiography in this study. In addition, these changes were further clarified by observing the follow-up US and MRI, which coincided with symptom improvement. In particular, the black hole appearance and echogenic fluid on US disappeared shortly after acute attacks. Since the clinical symptoms and treatment outcomes are known to correspond to the radiologic findings over time, rather than the baseline imaging findings [24,25], follow-up radiography and US after an acute attack are important.
As shown in the present study, the intramuscular migration of calcification is uncommon. It is not known whether the calcific deposits follow pre-existing tendon tears to the myotendinous junction or cause intrasubstance delaminating-type tendon tears during migration [8,16]. The low frequency of intramuscular migration of the calcifications and its unusual appearance with a diffuse muscular edema on MRI can hinder its differentiation from other conditions such as traumatic injury, hematoma, denervation edema, or pyogenic myositis [16,26]. As in our study, demonstrating the continuity between the intramuscular calcifications and calcifications moving out from the tendon is important for the correct diagnosis of the origin of calcification at an unusual location.
This study shows the role of MRI in assessing bone involvement and surrounding inflammatory changes during the resorptive stage of calcific tendinitis. However, MRI is limited in identifying small calcifications and there are limitations in applying a calcification-sensitive MRI protocol in daily practice. Therefore, in cases of calcifications in unusual locations, US plays a key role in identifying migration with tiny calcifications left in the path of migration and remaining intratendinous components. Furthermore, while not presented in this study, the usefulness of US findings of bone involvement has been reported [3]. Therefore, an examiner should keep this in mind to find clues about the intraosseous migration of the calcifications in patients with calcific tendinitis.
The present study has three main limitations. First, because of its retrospective design, only patients in whom the degree of clinical symptoms was recorded in the chart were enrolled in the study. Second, possible selection bias was unavoidable as more severely symptomatic patients underwent additional imaging studies in addition to standard radiography under usual clinical circumstances. Third, the time interval between the acute attack and the follow-up studies was variable, as imaging examinations were performed according to the severity of the symptoms rather than at regular intervals.
In conclusion, patients who had extratendinous migration of calcific tendinitis presented with severe clinical manifestations. Changes in the location, morphology, and size of the calcific deposits were perceivable on radiography and to a greater extent on US and MRI at the time of acute symptomatic attacks. The fragmented type on US was the most common type of extratendinous calcification, and the sonographic black hole appearance and echogenic fluid were the characteristic findings of intrabursal calcifications. Followup imaging studies revealed spontaneous regression of calcific deposits with concurrent symptom improvement.
NotesAuthor Contributions Conceptualization: Sung MS. Data acquisition: Kim MJ, Sung MS, Jeong CH. Data analysis or interpretation: Kim MJ, Sung MS, Jeong CH, Park HI. Drafting of the manuscript: Kim MJ, Sung MS. Critical revision of the manuscript: Sung MS, Jeong CH, Park HI. Approval of the final version of the manuscript: all authors. References2. Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg 1997;5:183ŌĆō191.
3. Della Valle V, Bassi EM, Calliada F. Migration of calcium deposits into subacromial-subdeltoid bursa and into humeral head as a rare complication of calcifying tendinitis: sonography and imaging. J Ultrasound 2015;18:259ŌĆō263.
4. Uhthoff HK, Sarkar K, Maynard JA. Calcifying tendinitis: a new concept of its pathogenesis. Clin Orthop Relat Res 1976;118:164ŌĆō168.
5. Lawande MA, Daftary AR. MRI findings in calcific deposits in and around shoulder: atypical locations beyond supraspinatus. Clin Radiol 2020;75:579ŌĆō585.
6. Kalayci CB, Kizilkaya E. Calcific tendinitis: intramuscular and intraosseous migration. Diagn Interv Radiol 2019;25:480ŌĆō484.
7. Bianchi S, Becciolini M. Ultrasound appearance of the migration of tendon calcifications. J Ultrasound Med 2019;38:2493ŌĆō2506.
8. Becciolini M, Bonacchi G, Galletti S. Intramuscular migration of calcific tendinopathy in the rotator cuff: ultrasound appearance and a review of the literature. J Ultrasound 2016;19:175ŌĆō181.
9. Chan R, Kim DH, Millett PJ, Weissman BN. Calcifying tendinitis of the rotator cuff with cortical bone erosion. Skeletal Radiol 2004;33:596ŌĆō599.
10. Flemming DJ, Murphey MD, Shekitka KM, Temple HT, Jelinek JJ, Kransdorf MJ. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol 2003;181:965ŌĆō972.
11. Kim YS, Lee HM, Kim JP. Acute calcific tendinitis of the rectus femoris associated with intraosseous involvement: a case report with serial CT and MRI findings. Eur J Orthop Surg Traumatol 2013;23 Suppl 2:S233ŌĆōS239.
12. Kraemer EJ, El-Khoury GY. Atypical calcific tendinitis with cortical erosions. Skeletal Radiol 2000;29:690ŌĆō696.
13. Malghem J, Omoumi P, Lecouvet F, Vande Berg B. Intraosseous migration of tendinous calcifications: cortical erosions, subcortical migration and extensive intramedullary diffusion, a SIMS series. Skeletal Radiol 2015;44:1403ŌĆō1412.
14. Marinetti A, Sessa M, Falzone A, Della Sala SW. Intraosseous migration of tendinous calcifications: two case reports. Skeletal Radiol 2018;47:131ŌĆō136.
15. Martin S, Rapariz JM. Intraosseous calcium migration in calcifying tendinitis: a rare cause of single sclerotic injury in the humeral head (2010: 2b). Eur Radiol 2010;20:1284ŌĆō1286.
16. Pereira BP, Chang EY, Resnick DL, Pathria MN. Intramuscular migration of calcium hydroxyapatite crystal deposits involving the rotator cuff tendons of the shoulder: report of 11 patients. Skeletal Radiol 2016;45:97ŌĆō103.
17. Porcellini G, Paladini P, Campi F, Pegreffi F. Osteolytic lesion of greater tuberosity in calcific tendinitis of the shoulder. J Shoulder Elbow Surg 2009;18:210ŌĆō215.
18. Sola WC Jr, Drake GN, Ramos CH, Gomes A, Gartsman GM. Calcific tendinitis of the rotator cuff associated with intraosseous loculation: two case reports. J Shoulder Elbow Surg 2009;18:e6ŌĆōe8.
19. Gartner J, Simons B. Analysis of calcific deposits in calcifying tendinitis. Clin Orthop Relat Res 1990;254:111ŌĆō120.
21. Albano D, Coppola A, Gitto S, Rapisarda S, Messina C, Sconfienza LM. Imaging of calcific tendinopathy around the shoulder: usual and unusual presentations and common pitfalls. Radiol Med 2021;126:608ŌĆō619.
22. Darrieutort-Laffite C, Blanchard F, Le Goff B. Calcific tendonitis of the rotator cuff: from formation to resorption. Joint Bone Spine 2018;85:687ŌĆō692.
23. Sconfienza LM, Bandirali M, Serafini G, Lacelli F, Aliprandi A, Di Leo G, et al. Rotator cuff calcific tendinitis: does warm saline solution improve the short-term outcome of double-needle US-guided treatment? Radiology 2012;262:560ŌĆō566.
24. Cho NS, Lee BG, Rhee YG. Radiologic course of the calcific deposits in calcific tendinitis of the shoulder: does the initial radiologic aspect affect the final results? J Shoulder Elbow Surg 2010;19:267ŌĆō272.
Flowchart of patient inclusion and exclusion.US, ultrasonography; MRI, magnetic resonance imaging. a)Conservative management included recent oral analgesics or steroid injections. b)Interventions included extracorporeal shock wave therapy, percutaneous needle aspiration, or shoulder surgery.
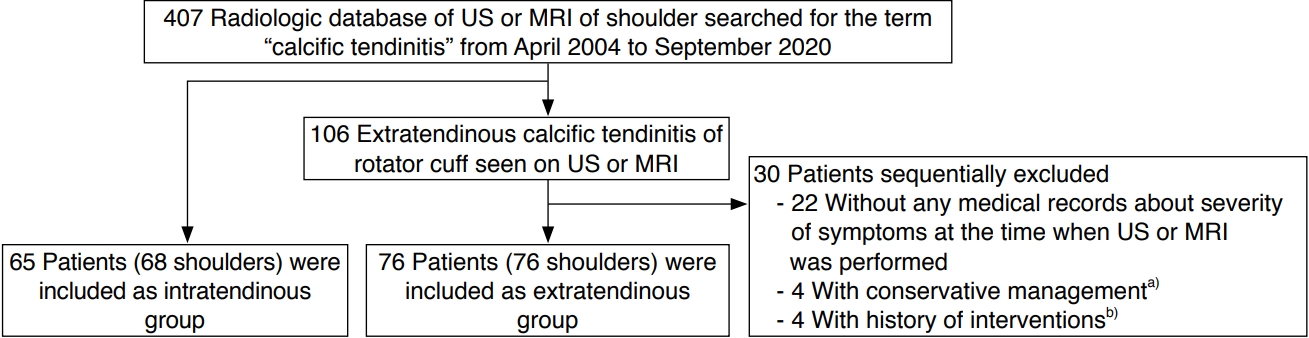 Fig.┬Ā1.Extratendinous migration of calcific tendinitis in a 46-year-old woman who presented with aggravating shoulder pain (visual analogue scale 9).A-C. When compared to the serial radiographs taken 1 year ago (A) and 6 months ago (B), the calcifications have become ill-defined and less dense (type III), among which some show extratendinous migration (arrows) (C). D. After 10 days, the calcifications decreased, with only fine, faint calcifications remaining (arrows). E, F. Ultrasonography on the same day demonstrates migrated intrabursal calcific deposits, which appear as echogenic fragmented small calcifications (arrow) and homogeneous hyperechoic calcific deposits (open arrows). Note the intratendinous and sub-bursal calcifications indenting the floor of the bursa (arrowheads). G, H. Coronal fat-suppressed T2-weighted (G), and contrast-enhanced fat-suppressed T1-weighted (H) magnetic resonance images show subacromial-subdeltoid bursitis with heterogeneous internal signal intensities (arrows), corresponding to intrabursal calcific tendinitis.
 Fig.┬Ā2.Intramuscular migration of calcific tendinitis in a 61-year-old woman with excruciating shoulder pain (visual analogue scale 9) visiting emergency department.A, B. A radiograph taken 2 years before an attack of acute pain (A) shows a dense well-defined calcification (type I) in the supraspinatus tendon. A radiograph obtained in the emergency room (B) reveals calcifications that have increased in size and became less dense and fragmented (type II). C. After 3 days, the calcifications spontaneously reduced in size, with only a small remaining calcification (type III). D, E. Ultrasonography taken on the same day shows small calcifications from the intratendinous to the sub-bursal, intrabursal, and potential spaces (arrowheads), which extend to a large calcification in the supraspinatus muscle with a black hole appearance (arrows). F-H. Coronal (F) and sagittal (G) T2-weighted, and contrast-enhanced fat-suppressed sagittal T1-weighted (H) magnetic resonance images confirm the intramuscular calcification (arrows) with pericalcific edema and enhancement.
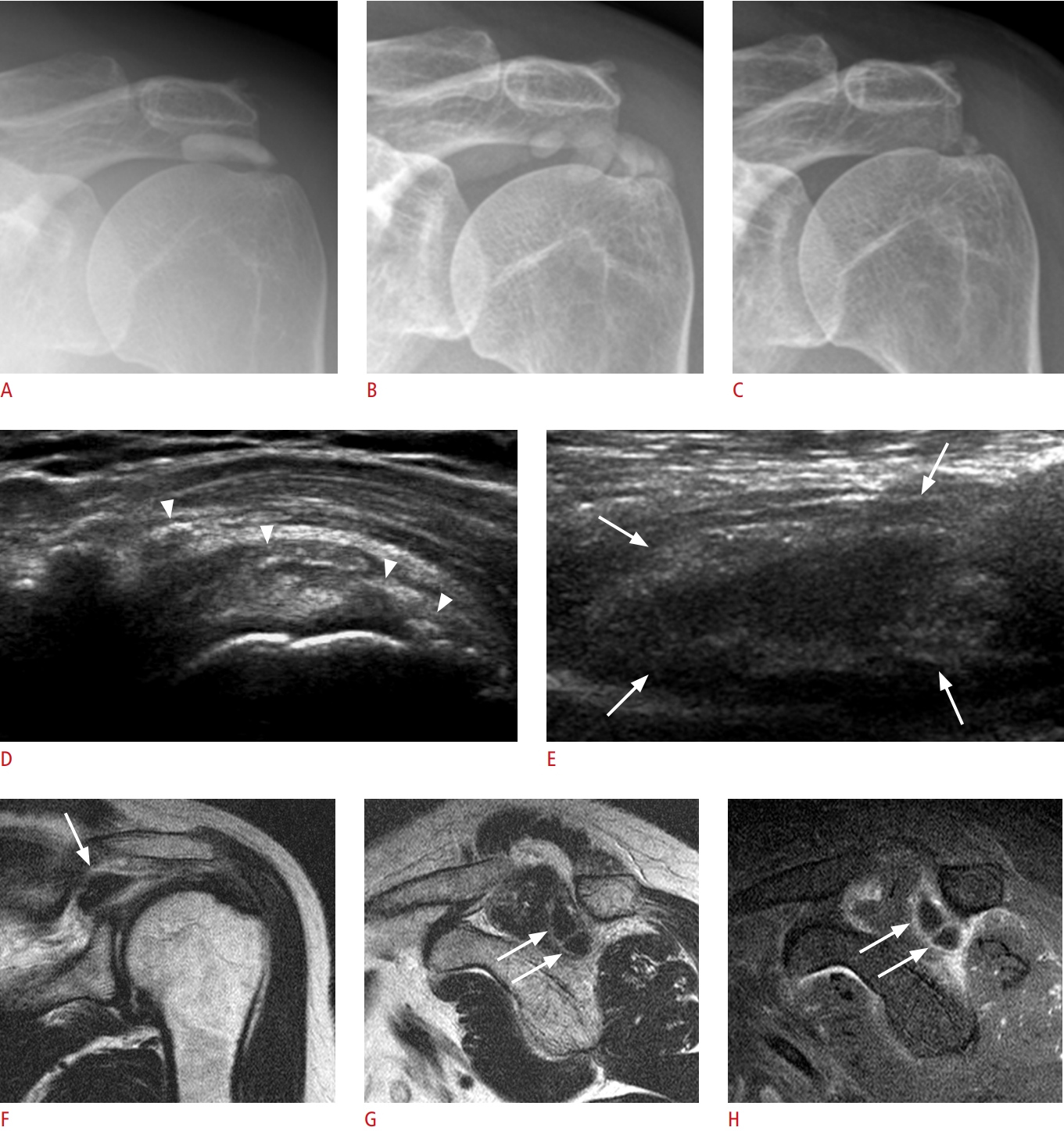 Fig.┬Ā3.Temporal changes in extratendinous calcific tendinitis in a 57-year-old woman with sudden onset of severe shoulder pain (visual analogue scale 9).A, B. Initial radiographs with a supraspinatus outlet view (A) and axillary view (B) show faint calcifications (arrows), extending to the subdeltoid bursa. C, D. After 9 days, Ultrasonography depicts various intrabursal calcifications, manifesting as nodular, echogenic fluid (open arrows), and a black hole appearance with anechoic core (arrow). E-I. Corresponding sagittal fat-suppressed T2-weighted (E), axial T2-weighted (F, G), enhanced axial (H) and sagittal (I) fat-suppressed T1-weighted magnetic resonance images show homogeneously enhanced sandy calcifications with an intermediate signal intensity (open arrows) and a rim-enhanced calcification with intermediate signal intensity (arrows). J, K. Follow-up radiograph after 3 weeks (J) shows markedly decreased calcific deposits (arrowhead), and a 1-year follow-up radiograph (K) shows complete regression.
 Fig.┬Ā4.Sequential changes in extratendinous calcific tendinitis with a black hole appearance in a 58-year-old woman with sudden shoulder pain (visual analogue scale 10).A-H. The radiograph, US, and magnetic resonance imaging findings of calcific deposits are consistent with each other. A. On an axillary view of a shoulder radiograph, proximal calcifications (arrow) appear less dense than the distal portion (open arrow). B-D. US on the same day demonstrates calcifications of various properties inside the bursa: proximal calcifications appear as black holes with anechoic cores (arrows), while distal calcifications show hyperechoic nodules with partial posterior acoustic shadowing (open arrow). Nodular calcifications were also present (not shown here). E-H. Corresponding enhanced fat-suppressed T1-weighted coronal (E) and axial fat-suppressed T2-weighted (F-H) Magnetic resonance images show central high signal intensity of the proximal calcific deposits (arrows) and signal void of the distal calcific deposits (open arrows). Note perilesional soft tissue edema with adjacent deltoid muscle compression. I, J. Follow-up radiograph and US conducted 2 weeks later show extensive regression and decreased size of the calcific deposits (arrowheads). K, L. After 5 months, only a small linear calcification (arrowheads) remains on radiography and US. US, ultrasonography.
 Fig.┬Ā5.Sequential changes in calcific tendinitis with extratendinous migration in a 58-year-old woman with intense shoulder pain (visual analogue scale 10).A. A radiograph taken 1 year and 6 months before an acute attack shows a well-defined nodular calcification (type I) in the supraspinatus tendon. B, C. After 1 year, the calcification has slightly increased in size on radiography. Note the arc-like intratendinous calcification in the supraspinatus tendon (arrows) on ultrasonography. D. Six months later, when the patient visited the emergency room for excruciating pain, the calcification has become larger and less dense (arrow). Note the faint radiolucent calcific deposits nearby (type III) (open arrow). E-G. Corresponding fat-suppressed T2-weighted (E, F) and contrast-enhanced T1-weighted (G) coronal magnetic resonance images demonstrate the intrabursal location of the calcifications with combined subdeltoid bursitis and soft tissue edema. H-J. The calcific deposits markedly decreased after 9 days (H), with gradual regression on follow-up studies at 3 weeks (I) and 2 months (J).
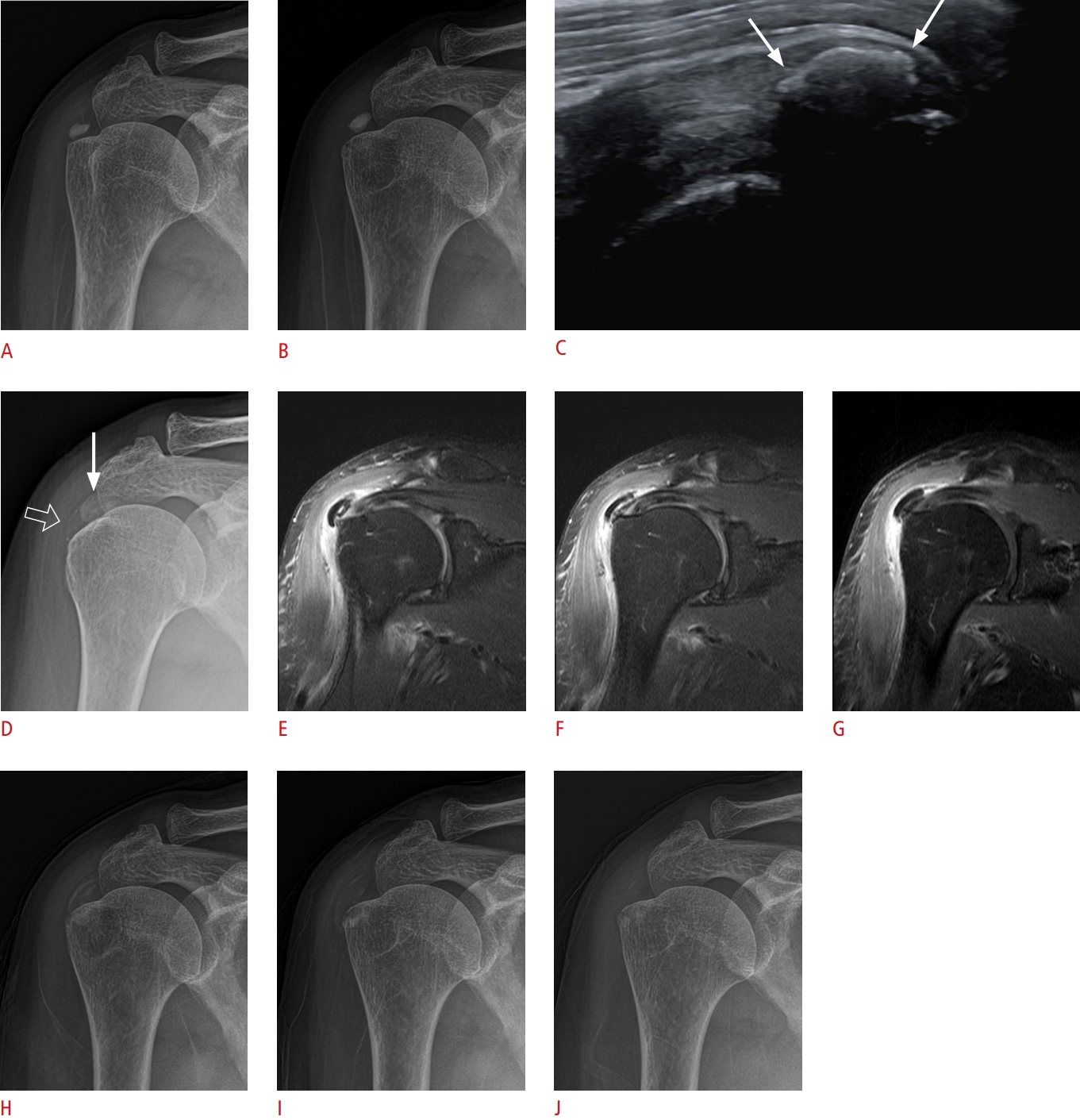 Fig.┬Ā6.Table┬Ā1.Comparison of demographic and clinical characteristics between patients with extratendinous and intratendinous calcific tendinitis
Table┬Ā2.Locations and imaging features of extratendinous calcific tendinitis
Table┬Ā3.Temporal changes in the clinical and imaging characteristics of extratendinous calcific tendinitis before and during acute attacks
Table┬Ā4.Temporal changes in the clinical and imaging characteristics of extratendinous calcific tendinitis in acute attacks and subsequent follow-up
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI




