AbstractA vascular closure device is used for hemostasis after a procedure involving an arterial puncture. The increasing frequency of endovascular arterial interventions has caused these devices to play a more important role in clinical practice. FemoSeal is a popular vascular closure device, and its safety and effectiveness have been verified. However, complications still occur in some cases, including even disastrous complications on occasion. Even with little experience, it is possible to reduce the complication rate by using real-time ultrasonography monitoring during the deployment of this device. Based on our experiences, presented herein, we suggest that complications related to FemoSeal could be reduced by using our method.
Arterial endovascular procedures are being performed with increasing frequency, and safe hemostasis of the access site is of crucial importance. For this reason, several vascular closure devices (VCDs) have been introduced.
The FemoSeal (Terumo, Tokyo, Japan) closure device is a relatively recently introduced VCD, composed of a bioabsorbable polymer blend (lactide, trimethylene carbonate, and caprolactone), made up of three components; an anchor inner disc, an outer disc, and a suture holding two discs. The discs are positioned on the inner and outer surfaces of the artery and sandwich puncture hole, leading to hemostasis.
The safety and feasibility of FemoSeal for retrograde femoral artery access have been investigated and verified in previous studies [1-8]. A recent article showed that this VCD could also be safely used even for anterograde femoral artery access [9]. Even though the incidence is low, with a range of 0.6%-2%, serious complications requiring surgical repair or transfusion have been noted in some studies [1,2,8]. According to one study [8], serious complications followed technical failure of device deployment, and in the operating field, inappropriate disc location has been reported; for instance, both discs were in the extravascular space or the outer disc did not maintain the tension of the suture.
We experienced a similar complication involving a pseudoaneurysm after a mechanical thrombectomy procedure with an anterogradely inserted 8-French (Fr) vascular sheath at our hospital in July 2018. Failure to achieve immediate hemostasis after the VCD deployment was noted and manual compression was performed for hemostasis. Even though the bleeding stopped after half an hour of manual compression, a pseudoaneurysm arose after 2 weeks. Finally, an operation revealed that both discs were in the extravascular space.
At our institutional quality improvement meeting, we hypothesized that ultrasonography (US)-guided deployment of FemoSeal could help ensure that the anchor disc is deployed in the correct location and facilitate appropriate implementation of this VCD for safe hemostasis. Since then, we have used real-time US guidance while deploying FemoSeal.
This single-center retrospective study aimed to determine whether real-time US guidance could be useful for FemoSeal. Based on our experiences, we would like to suggest that real-time US guidance could enhance the safety and the effectiveness of FemoSeal.
This article is a single-center retrospective study. From August 2018 to November 2019, we deployed FemoSeal under real-time US guide in 50 procedures, all performed by a single experienced interventional radiologist. The institutional review board approved this retrospective single-center study (2020AS0067) and waived the requirement for informed consent.
In all cases, femoral artery access was achieved with a 21-G needle using a micro-puncture set (Cook, Bloomington, IN, USA) under US guidance. All patients received approximately 4,000-6,000 IU of heparin by an intravenous bolus injection at the beginning of the procedures. The VCDs were used for femoral artery access with a vascular sheath, ranging from 6 to 8 Fr. All patients in whom FemoSeal was used in the designated period were enrolled. All devices were deployed following the instructions in the user guide supplied by the device manufacturer. For real-time US-guided deployment, only a US exam was added to the method recommended in the user guide. Because this VCD was designed for one-hand use, it was possible for real-time US guidance to be performed by the same operator. We used a 7-10 MHz linear probe with an ALT HDI400 US device (Philips, Best, The Netherlands).
FemoSeal is inserted over the guidewire into the artery through the vascular access site after removing the vascular sheath. The inner anchor disc, loaded in a wrapped manner in the FemoSeal device, is deployed from the tip of the device into the lumen of the artery. When it is deployed, the inner disc unfolds and makes an ovoid disc. Pulling the entire device backwards makes this disc attach to the inner wall of the vessel undergoing arteriotomy. The outer disc is then deployed on the outer wall of the vessel. The two discs seal the arteriotomy area like sandwiches to achieve hemostasis. We followed the user guide provided by the device manufacturer for nearly every step. The only difference between our method and that described by the manufacturer was that we deployed the inner disc when the tip of the device was pulled back close to the inner wall of the artery. An illustration of the manufacturer-supplied deployment method, a simple illustration of the hemostasis mechanism, and the US findings corresponding to each step are presented step by step in Fig. 1. Patients were placed on bed rest for about 4 hours with a simple soft dressing on the site after VCD deployment.
The basic demographic characteristics, such as age, sex, body mass index (BMI), smoking history, previous medical history, history of anti-coagulant and anti-platelet medications, of the enrolled patients were investigated in patientsŌĆÖ medical records. The size of the vascular sheaths that were used, the direction of vascular sheath insertion, and laboratory results such as the international normalized ratio, activated partial thromboplastin time, and platelet count were also examined. All laboratory examination data were collected within 2 days before the procedure.
Technical failure was defined as failure to achieve immediate hemostasis after the device deployment. A physician carefully examined each puncture site for 1-3 days after the procedure, and every patient was followed-up 4-6 weeks later using US at an outpatient clinic. Major complications were defined as follows: hematoma larger than 5 cm in diameter, pseudoaneurysm formation, blood loss needing transfusion or surgical intervention, dissection, and infection. Minor complications comprised minor bleeding requiring additional simple compression for improvement and hematoma measuring less than 5 cm. Minimal oozing manageable with a simple bandage or a small hematoma (less than 1 cm) was considered acceptable.
The incidence of technical failure, the overall complication rate, and the significant complication rate were investigated.
In total, 50 FemoSeal devices were used in 50 patients, of whom 37 were men. The patientsŌĆÖ overall mean age was 66.6┬▒6.4 years, and their mean BMI was 23.0┬▒2.1 kg/m2. Thirty-one sheaths were 6 Fr, 11 were 7 Fr, and eight were 8 Fr. Twenty-five anterograde accesses and 25 retrograde accesses were performed. PatientsŌĆÖ smoking history, underlying medical diseases, medication history, and preprocedural laboratory findings are also presented in Table 1.
Nearly all the closure procedures were completed within 1 minute after insertion of the closure device. Approximately 2-3 minutes were needed for the initial two or three cases. However, the procedure was easy to learn.
Technical success was achieved in all cases. Only one focal bruise smaller than 1 cm was noted in a patient with a 8-Fr vascular sheath.
FemoSeal is a popular VCD for arterial puncture hemostasis that has been evaluated in many studies (Table 2). The complication rate has been reported to be approximately 1%-6%, and two large-scale studies reported rates of 6% and 2.8%, respectively [1,2]. The technical failure rate ranged from 0% to 7% in previous studies. Many reviews have described the use of manual compression after technical failure [1-4,6,7]. Even though remarkable events did not occur following manual compression in some studies [4-7], large hematomas were found in 36% of cases of technical failure according to one large-scale survey, the CLOSE-UP study [2]. A clear description of the outcomes of technical failure cannot be found in the ISAR-CLOSURE study, another large-scale study [1]. Three pseudoaneurysms related to technical failure were described in another study [8]. Considering the results of previous studies, reducing technical failure could be a promising way to reduce overall complications and to enhance the effectiveness and safety of FemoSeal. Despite the small number of cases described in our study, we experienced only one insignificant complication and no technical failure. Considering that our study involved a considerable number of cases using a relatively large 8-Fr vascular sheath (8 of 50, 16%) and 50% of cases involved anterograde access, real-time US monitoring could be suggested as a useful method.
Gabrielli et al. [8] reported cases of non-engagement of the inner anchor disc to the vessel wall, resulting in the extravascular deployment of both discs, and failure to maintain suture tension between both discs, resulting in displacement of the outer disc; in these cases, surgical repair was necessary. We hypothesized that real-time US guidance could ensure intravascular deployment of the inner anchor disc and prevent deployment of the inner anchor disc to the extravascular space. Failure to maintain the suture tension could result from excessive force while pulling back the device (step 4 in Fig. 1). Real-time US guidance may enable the operator to see the engagement of the inner disc to the inner wall of the vessel and thereby avoid pulling back with excessive force. In our experience, the inner disc was well visualized on US, as described by Choo et al. [10], and the inner anchor disc could be deployed to the intravascular space under real-time monitoring with confidence (step 3 in Fig. 1). The inner disc could be clearly observed using US when the disc was engaged to the inner wall of the vessel (step 4 in Fig. 1), and it was therefore possible to avoid pulling back the device with excessive force.
Another potential strong point of our method would be related with deploying the inner disc when the tip of the closure device is near the inner wall of the artery. The manufacturer recommended that the inner disc should be deployed before starting to pull the device back. We were concerned that the inner disc might become stuck on an intra-arterial calcification, potentially leading to an unwanted embolism or incomplete sealing. Especially in cases of anterograde puncture, the disc could be potentially trapped at the common femoral artery bifurcation. However, these potential accidents could be avoided by our proposed method. Fig. 2 presents our hypothesis about FemoSeal device failure when the inner disc becomes stuck. To avoid such situations, we traced the tip of the FemoSeal catheter while pulling back. The catheter tip was well visualized, as shown in Fig. 1. Therefore, we were able to notice when the catheter tip was close to the inner wall of the vessel puncture site, and we could deploy the inner disc at a position suitable for preventing it from becoming stuck on a calcification or in an arterial bifurcation area.
Because the exact location of the tip of FemoSeal could be noted during deployment using real-time US guidance, our method made it possible to place the FemoSeal catheter in the intravascular space with certainity and to pull back the FemoSeal until it was clear that the location of inner disc deployment would be safe. It is a matter of course that puncture under US guidance is essential factor for reducing VCD-related complication. Nevertheless, adding our method could help reducing the complication more. Consequently, US guidance could be especially useful in cases such as (1) severe atherosclerotic changes and abundant intravascular calcifications, (2) an excessively deep-seated artery, and (3) the presence of an object not fully absorbed vascular closure material in the intravascular space due to recent use of a closure device. To the best of our knowledge, our preliminary study is the first attempt to implement real-time US guidance during VCD deployment, and the method proposed in this study is a potential way to enhance the effectiveness and safety of FemoSeal.
The limitations of this study are as follows: (1) This was a retrospective study, so further research and verification using a prospective design with a larger number of subjects should be conducted. (2) This was a single-arm study, with no comparison to a control group. A randomized comparison study should be conducted for further verification.
NotesAuthor Contributions Conceptualization: Lee SH, Chung HH. Data acquisition: Ha TH, Lee SH. Data analysis or interpretation: Ha TH, Lee SH, Park SJ. Drafting of the manuscript: Ha TH. Critical revision of the manuscript: Lee SH, Park SJ, Chung HH. Approval of the final version of the manuscript: all authors. References1. Schulz-Schupke S, Helde S, Gewalt S, Ibrahim T, Linhardt M, Haas K, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. JAMA 2014;312:1981ŌĆō1987.
2. Holm NR, Sindberg B, Schou M, Maeng M, Kaltoft A, Bottcher M, et al. Randomised comparison of manual compression and FemoSeal vascular closure device for closure after femoral artery access coronary angiography: the CLOSure dEvices Used in everyday Practice (CLOSE-UP) study. EuroIntervention 2014;10:183ŌĆō190.
3. Mankerious N, Mayer K, Gewalt SM, Helde SM, Ibrahim T, Bott-Flugel L, et al. Comparison of the FemoSeal vascular closure device with manual compression after femoral artery puncture: post-hoc analysis of a large-scale, randomized clinical trial. J Invasive Cardiol 2018;30:235ŌĆō239.
4. De Poli F, Leddet P, Couppie P, Daessle JM, Uhry S, Hanssen M. FemoSeal Evaluation Registry (FER). Prospective study of femoral arterial closure with a mechanical system on 100 patients who underwent angioplasty procedures. Ann Cardiol Angeiol (Paris) 2014;63:339ŌĆō344.
5. Alonzo A, Rigattieri S, Giovannelli F, Di Russo C, Sciahbasi A, Berni A, et al. Transfemoral approach with systematic use of FemoSeal closure device compared to transradial approach in primary angioplasty. Catheter Cardiovasc Interv 2016;87:849ŌĆō854.
6. Wanitschek MM, Suessenbacher A, Dorler J, Pachinger O, Moes N, Alber HF. Safety and efficacy of femoral artery closure with the FemoSeal┬« device after coronary angiography using a 7 French sheath. Perfusion 2011;26:447ŌĆō452.
7. Chivot C, Deramond H, Bouzerar R, Yzet T. Safety and efficacy of femoral artery closure with the FemoSeal device after cerebral thrombectomy using an 8 french sheath. Eur J Vasc Endovasc Surg 2018;55:730ŌĆō734.
8. Gabrielli R, Rosati MS, Millarelli M, Dante A, Maiorano M, Musilli A, et al. FemoSeal┬« device use for femoral artery closure by different techniques. Ann Vasc Surg 2018;51:18ŌĆō24.
Femoseal deployment user-guide illustrations, schematic graphics, and ultrasonography findings.Femoseal deployment user-guide illustrations, schematic graphics and ultrasonography findings are presented in upper, middle and lower rows respectively. The difference between our method and the manufacturer recommendation was illustrated in third column of the middle row. The inner disc is deployed closer to the inner wall of the artery (the lower picture) than the manufacturer method (the upper picture). Arrow pointing right, Femoseal catheter tip; double arrowheads, inner disc; arrow pointing down, outer disc.
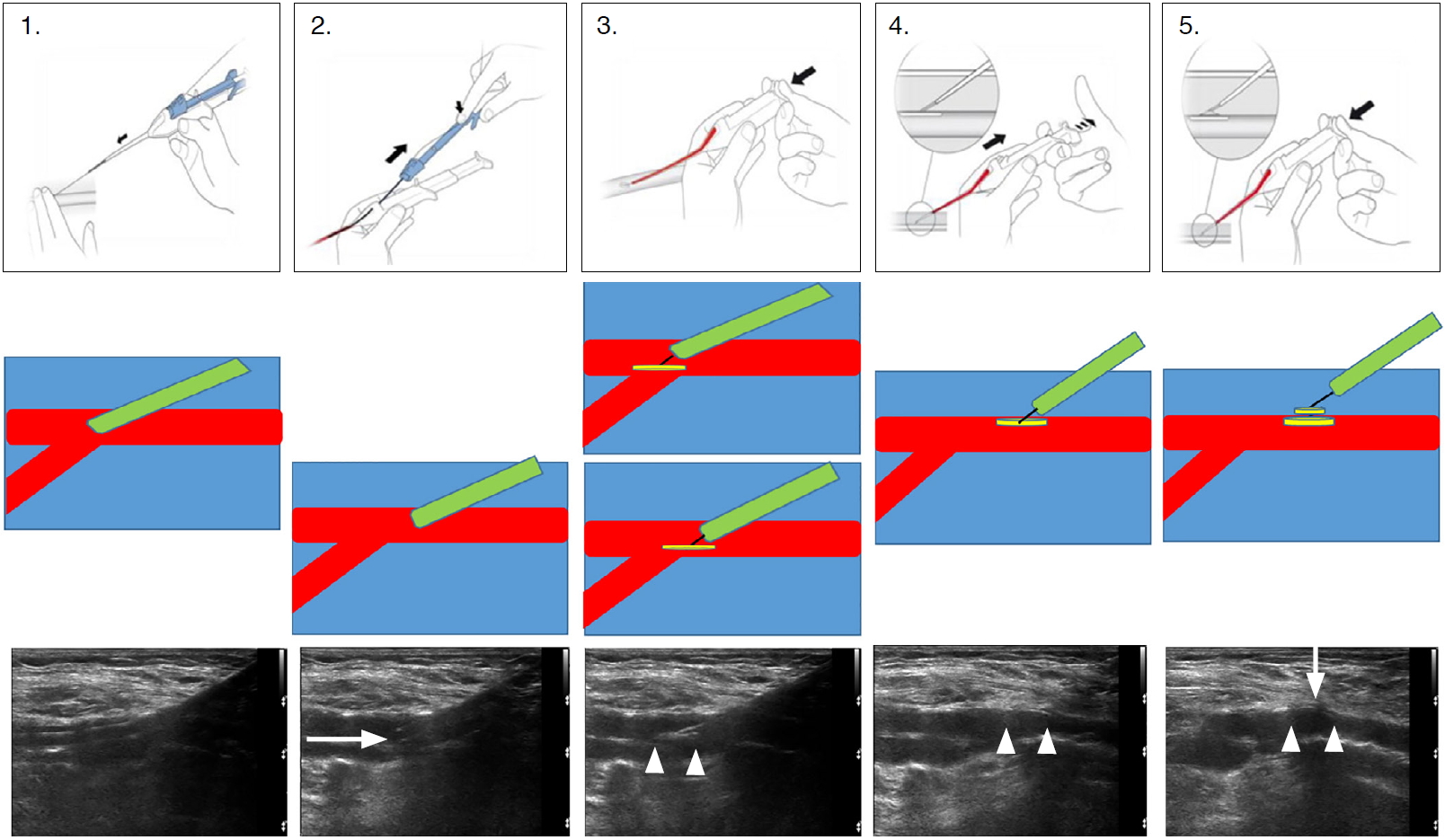 Fig.┬Ā1.Schematic graphics representing the inner disc stuck and presumed result.These illustrations show our hypothesis about FemoSeal device failure when the inner disc, becomes stuck. The inner disc could be stuck at vascular calcification (A) or femoral bifurcation (B). Hypothesized possible two complications are illustrated in other pictures. Suture between two discs is stretched (A-1-a and A-1-b) and this could make incomplete sealing due to loosening or rupture of the suture. All the two discs are deployed in the artery (A-2-a and A-2-b) and this could make embolism.
 Fig.┬Ā2.Table┬Ā1.Demographic characteristics of the patients analyzed in this study Table┬Ā2.Summary of previous studies
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




