AbstractPurposeThe accurate detection and quantification of hepatic steatosis using a noninvasive method are important for the management of nonalcoholic fatty liver disease. We performed a systematic review and meta-analysis of the accuracy of the ultrasound-measured attenuation coefficient (AC) in the evaluation of hepatic steatosis.
MethodsThe PubMed, Embase, and Cochrane databases were searched for prospective studies reporting the diagnostic accuracy of AC for assessing hepatic steatosis. The meta-analytic pooled sensitivity and specificity of AC for any grade of steatosis (S≥1) and advanced steatosis (S≥2) were estimated using a bivariate random-effects model. Meta-regression analysis was conducted to investigate the causes of heterogeneity among studies.
ResultsThirteen studies including 1,509 patients were identified. The pooled sensitivity and specificity of AC for S≥1 were 76% (95% confidence interval [CI], 73% to 80%; I2=43%) and 84% (95% CI, 77% to 89%; I2=74%), respectively, while for S≥2 they were 87% (95% CI, 83% to 91%; I2=0%) and 79% (95% CI, 75% to 83%; I2=59%), respectively. Study heterogeneity was associated with body mass index (BMI) and the prevalence of steatosis or significant fibrosis.
ConclusionAC can be clinically useful for assessing hepatic steatosis, with good overall diagnostic performance. The data reported in the published literature differed according to BMI and the prevalence of steatosis or significant fibrosis, and careful interpretation with consideration of these factors might be needed.
Increasing clinical attention is being paid to nonalcoholic fatty liver disease (NAFLD) because of its increasing prevalence, which has reached 16%-45% in Western countries and 9%-29% in Eastern countries [1,2]. As NAFLD can progress to cirrhosis through nonalcoholic steatohepatitis (NASH), and NASH is the most common cause of liver transplantation for women in North America [3,4], the detection and quantification of hepatic steatosis are crucial for the management of patients with NAFLD.
Liver biopsy is still considered the gold standard for the assessment of hepatic steatosis. However, as liver biopsy is subject to several limitations such as procedural invasiveness, sampling error, and interobserver variability [5-7], there have been many efforts to develop a reliable noninvasive diagnostic test for the assessment of hepatic steatosis. In this regard, the controlled attenuation parameter (CAP) obtained from transient elastography was introduced and has been widely used in clinical practice. Although it can be used to quantify hepatic steatosis, it has several limitations including suboptimal diagnostic performance for hepatic steatosis (≥S1; 69% sensitivity and 82% specificity) [8] and a large number of invalid measurements in patients with obesity [9]. Of late, the noninvasive quantification of hepatic steatosis using magnetic resonance imaging (MRI) has been considered comparable to liver biopsy in terms of accuracy [10,11]. Nonetheless, it also has drawbacks of high cost and limited availability.
In recent years, imaging methods for measurement of the attenuation coefficient (AC) have been developed and commercialized by several vendors [2,12,13]. These AC measurements are similar to CAP in that they are ultrasound-based, but AC differs from CAP in that it is incorporated with B-mode ultrasonography, which enables the simultaneous visualization of hepatic parenchyma. B-mode ultrasound-guided measurements allow the echo signal transmitted by the probe to be delivered to the liver more directly and precisely, which may result in more reliable and accurate measurements. Given the advantages of AC over CAP, several studies have reported the diagnostic performance of AC, but the reported results are limited by small study populations (<100) [2,14,15], and there are conflicting results as to whether AC has better diagnostic performance than CAP [14,15].
Therefore, we considered it timely and important to determine the diagnostic performance of AC in the assessment of hepatic steatosis, and performed a systematic review and meta-analysis of prospective studies on the accuracy of AC for noninvasive grading of hepatic steatosis.
This study was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines [16].
A comprehensive literature search of the MEDLINE, Embase, and Cochrane databases was performed to identify relevant original research investigating the performance of ultrasound attenuation imaging for evaluating hepatic steatosis. The search terms included "fatty liver," "ultrasonography," and "attenuation," and a detailed list of the search terms is provided in Supplementary Table 1. Because attenuation imaging implemented using B-mode ultrasonography was first commercialized in 2017, the literature search was performed on studies published from January 1, 2017 to October 21, 2020. The searches were limited to articles published in the English language and concerning only human subjects.
After removing duplicates, the eligibility of each article was evaluated according to predefined inclusion and exclusion criteria. The study inclusion criteria were as follows: (1) patients: adult patients (≥18 years) potentially at risk of hepatic steatosis; (2) index test: attenuation imaging on ultrasound for measurements of AC; (3) comparison: biopsy or MRI; (4) outcomes: sensitivity and specificity of AC for diagnosing any grade of steatosis (≥S1) or advanced steatosis (≥S2); and (5) study design: prospective cohort studies or clinical trials. The exclusion criteria were as follows: (1) articles that did not address the topics of interest of this study; (2) case reports, review articles, editorials, letters, conference abstracts, and proceedings; (3) articles with a patient cohort and data overlapping with another published study (in such cases, the study with the more comprehensive results for the purpose of this study was selected); and (4) articles written in languages other than English. Articles were first screened by reviewing their titles and abstracts, and were removed if any of the exclusion criteria were met. The full texts of the remaining articles were then reviewed to determine their eligibility. The reviewers selected studies independently in two sequential review sessions. Articles with any degree of ambiguity or that generated differences in opinion between the two reviewers were re-evaluated at a consensus meeting to which a third reviewer was invited.
The following data were extracted onto a predefined data form: (1) study characteristics, including authors, year of publication, institution, and subject enrollment (consecutive or selective); (2) patient characteristics including age, sex, body mass index (BMI), and the number of patients with NAFLD, significant fibrosis (≥F2) [17], and hepatic steatosis (≥S1, ≥S2) [18]; (3) ultrasound characteristics including scanner model and vendor, and AC cutoff values for ≥S1 and ≥S2; and (4) reference standard characteristics, including the type of reference standard used to evaluate hepatic steatosis and the interval between the reference standard and ultrasound measurements.
To determine the sensitivity and specificity of AC, the number of true-positive, true-negative, false-positive, and false-negative results were extracted using two-by-two diagnostic tables. When not explicitly reported, data were manually retrieved from the text, tables, and figures. Only results for at least 10 patients with hepatic steatosis (either ≥S1 or ≥S2) were extracted. If a study contained multiple AC measurements performed using different ultrasound transducers or by different operators, the results yielding the highest Youden index value were selected. Two reviewers independently performed data extraction, and any discrepancies were resolved by consensus between the two reviewers and a third reviewer.
Two independent reviewers assessed study quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) criteria [19]. The QUADAS-2 criteria consist of the four domains of patient selection, index test, reference standard, and flow of patients through the study and timing of the index test and reference standard, which were assessed using suggested questionnaires. Two reviewers determined the risk of bias and applicability of each individual study, with all discrepancies resolved by consensus between the reviewers and a third reviewer.
When results for both ≥S1 and ≥S2 were available within a single study, the two results were analyzed as separate studies. The summary sensitivity and specificity of AC for diagnosing ≥S1 and ≥S2, as well as their 95% confidence intervals (CIs), were estimated using a bivariate random-effects model [20,21]. A summary receiver operating characteristics curve was obtained using a hierarchical summary receiver operating characteristics (HSROC) model. The heterogeneity of the summary statistics was evaluated using the Higgins I2 statistic, with an I2 value exceeding 50% considered to indicate substantial heterogeneity [22]. The presence of a threshold effect was evaluated by a visual assessment of a coupled forest plot of sensitivity and specificity, and the Spearman correlation coefficient between sensitivity and 1-specificity. A correlation coefficient exceeding 0.6 was taken to indicate the presence of a significant threshold effect [20,23]. Head-to-head comparison of diagnostic accuracy between AC and CAP was also performed using available studies.
Causes of study heterogeneity were investigated using meta-regression analysis with the following covariates: (1) study location (Asia vs. others), (2) ultrasound vendor (Canon vs. others), (3) BMI (≥25 kg/m2 vs. <25 kg/m2), (4) the proportion of patients with significant fibrosis (≥F2; ≥50% vs. <50%), (5) the proportion of patients with hepatic steatosis (≥S1; ≥50% vs. <50%), (6) the type of reference standard (biopsy vs. MRI), and (7) the interval between ultrasound measurements and the reference standard (same day vs. others).
Publication bias was visually assessed with a funnel plot, and statistical significance was evaluated using the Deeks asymmetry test, with P-values <0.05 considered indicative of statistical significance. All statistical analyses were performed using Stata version 15.1 (StataCorp LP, College Station, TX, USA).
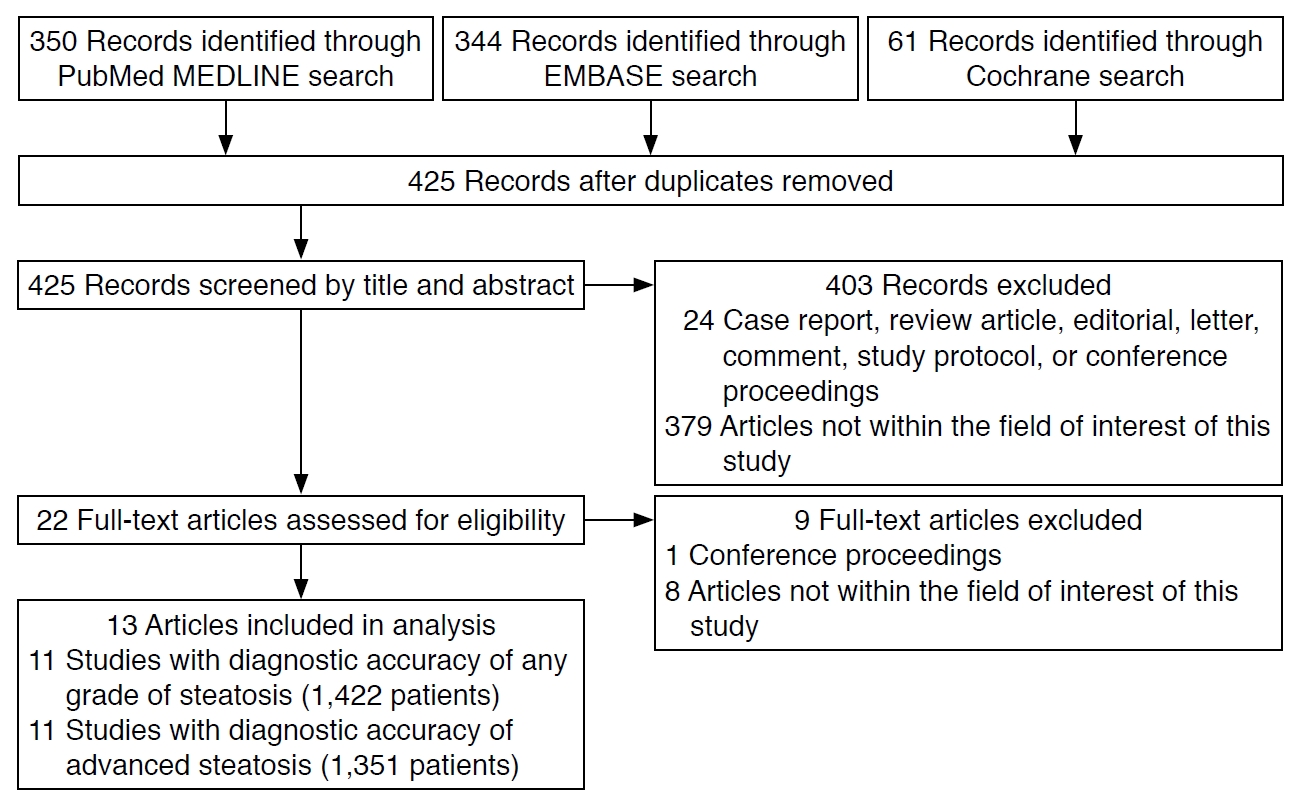
The article screening and selection process is illustrated in Fig. 1. Of the 425 articles identified after the removal of duplicates, 403 articles were excluded after screening their titles and abstracts. The remaining 22 articles were assessed for eligibility according to their full text, leading to 13 articles being finally included after the exclusion of nine articles. Nine of the included articles reported the diagnostic accuracy of AC for diagnosing both ≥S1 and ≥S2 [2,12,24-30], two articles reported it only for ≥S1 [14,15], and two reported it only for ≥S2 [13,31].
The characteristics of the included studies are shown in Table 1. Eight studies were conducted in Asia [2,12,14,24,25,28-30]. Nine studies performed AC measurements using a Canon ultrasound scanner [2,15,25-31]. Nine studies used liver biopsy as a reference standard to quantify hepatic steatosis [12-14,24,25,27-29,31], and seven performed liver biopsy and ultrasound on the same day [12,14,24,25,27-29]. Four studies used MRI with proton density fat fraction as the reference standard, with the mean/median interval between ultrasonography and MRI ranging from 7 to 32 days [2,15,26,30]. The mean BMI was over 25 kg/m2 in eight studies [2,13,15,24,26,27,29,31] and over 30 kg/m2 in two studies [13,15]. In nine studies [2,13,15,24-29] at least 50% of patients had hepatic steatosis, and in three studies at least 50% of patients had significant fibrosis [2,27,29]. The proportion of patients with NAFLD varied across studies (range, 3.4 to 100).
The study quality of the 13 articles is summarized in Supplementary Fig. 1. In the assessment of the risk of bias, one article that investigated the accuracy of AC in a biopsy-proven NAFLD cohort had an unclear risk in patient selection, as there were no details of the definition of significant alcohol consumption when excluding patients [13]. All articles had an unclear risk in index test domains because all used their own threshold of the index test determined in each cohort rather than prespecified one [2,12-15,24-31]. Two studies had an uncertain risk of bias in the reference standard domain because of the absence of information on whether the analysis was performed without knowledge of the results of the index test [27,30]. Three studies had an uncertain risk of bias in the flow and timing domain because of uncertainty in the appropriateness of the time interval between the index test and reference standard [2,13,31]. Regarding concerns over applicability, one study showed uncertainty in the patient selection domain as it included only patients with biopsy-proven NAFLD, and the full range of steatosis was not evaluated, which might have given rise to spectrum bias [13].
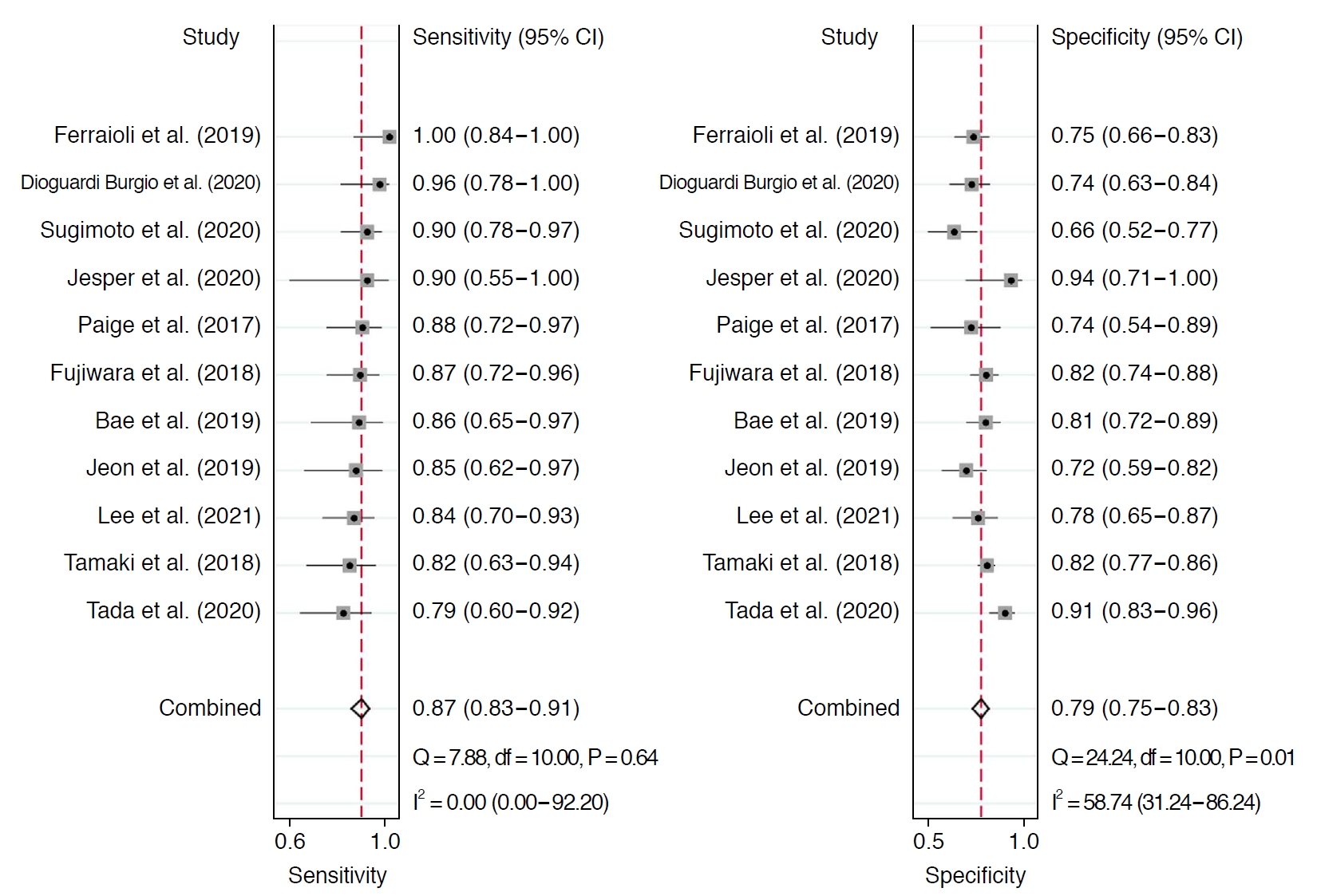
Table 2 summarizes the sensitivity and specificity of AC for diagnosing ≥S1 and ≥S2 in each individual study. In 11 studies including 1,422 patients, the pooled sensitivity and specificity of AC for the diagnosis of stages ≥S1 were 76% (95% CI, 73% to 80%) and 84% (95% CI, 77% to 89%), respectively (Fig. 2). Sensitivity did not show significant heterogeneity across studies (I2=43%), but substantial heterogeneity was noted in specificity (I2=74%). The coupled forest plots did not reveal a threshold effect, nor did the Spearman correlation (coefficient, 0.13; P=0.709). The median AC cutoff for diagnosis of ≥S1 was 0.63 (range, 0.53 to 0.69). In 11 studies including 1,351 patients, the pooled sensitivity and specificity of AC for stages ≥S2 were 87% (95% CI, 83% to 91%) and 79% (95% CI, 75% to 83%), respectively (Fig. 3). Sensitivity was not affected by study heterogeneity (I2 =0%), but marginal study heterogeneity was present in specificity (I2 =59%). No threshold effect was indicated by visual assessment of coupled forest plots or the Spearman correlation (coefficient, 0.37; P=0.258). The median AC cutoff for diagnosis of ≥S2 was 0.69 (range, 0.60 to 0.77). The areas under the HSROC curves for ≥S1 and ≥S2 were 0.83 (95% CI, 0.80 to 0.86) and 0.91 (95% CI, 0.88 to 0.93), respectively (Supplementary Fig. 2A, B).
The head-to-head comparative result of diagnostic accuracy between AC and CAP was reported in three studies for ≥S1 [15,24,26] and two studies for ≥S2 [24,26], respectively. The pooled sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC) of AC were 81% (95% CI, 76% to 86%), 88% (95% CI, 82% to 94%), and 0.91 (95% CI, 0.87 to 0.95) for ≥S1, and 92% (95% CI, 84% to 99%), 79% (95% CI, 73% to 84%), and 0.89 (95% CI, 0.82 to 0.96) for ≥S2, respectively. The pooled sensitivity, specificity, and AUROC of CAP were 74% (95% CI, 68% to 79%), 83% (95% CI, 76% to 89%), 0.85 (95% CI, 0.80 to 0.90) for ≥S1, and 84% (95% CI, 75% to 94%), 74% (95% CI, 68% to 79%), 0.87 (95% CI, 0.82 to 0.92) for ≥S2, respectively. Although AC had higher sensitivity (81% vs. 74% for ≥S1, 92% vs. 84% for ≥S2) and AUROC (0.91 vs. 0.85 for ≥S1, 0.89 vs. 0.87 for ≥S2) than CAP, the difference was not statistically significant (P>0.05).
The results of the meta-regression analysis are summarized in Table 3. Among the seven covariates, the proportion of patients with significant fibrosis, hepatic steatosis, and BMI were significant factors associated with study heterogeneity (P≤0.03). For the diagnosis of ≥S1, AC showed a higher sensitivity in studies with ≥50% of patients with hepatic steatosis (79% vs. 67%) and in studies with patients with a high BMI (≥25 kg/m2) (80% vs. 67%). For diagnosis of ≥S2, AC showed a lower specificity in studies with ≥50% of patients having significant fibrosis (71% vs. 81%) and in studies with patients with a high BMI ≥25 kg/m2 (76% vs. 86%).
There was no significant publication bias in the analyses of either ≥S1 or ≥S2 (P=0.07 and P=0.15, respectively) (Supplementary Fig. 3A, B).
In this meta-analytic study evaluating the diagnostic value of AC for hepatic steatosis, AC had good overall diagnostic accuracy for grades ≥S1 (76% sensitivity and 84% specificity) and grades ≥S2 (87% sensitivity and 79% specificity). In our head-to-head comparison of diagnostic accuracy between AC and CAP, AC showed a tendency for higher sensitivity for both ≥S1 (81% vs. 74%) and ≥S2 (92% vs. 84%) than CAP. When we consider our head-to-head comparative results between AC and CAP and the diagnostic accuracy of CAP reported in a recent meta-analysis (69% sensitivity and 82% specificity for ≥S1, and 77% sensitivity and 81% specificity for ≥S2) together [8], AC has higher sensitivity than CAP, but similar specificity. The better diagnostic performance of AC compared with CAP can be explained by the image-guided method for the measurement. The AC measurement procedure also involves grayscale B-mode ultrasound images, which can help in the accurate placement of regions of interest within the liver parenchyma. This helps to avoid structures such as large hepatic vessels or focal liver lesions that might affect the measurement results, and also helps to reduce undesirable attenuation of the echo signal by the skin and subcutaneous tissue, which may adversely affect measurements [9,27,28,32]. Therefore, AC may be a more accurate and reliable diagnostic tool than CAP for the assessment and quantification of hepatic steatosis.
In this meta-analysis, study heterogeneity ranging from marginal to substantial was noted for specificity, and the meta-regression analysis found three factors significantly associated with heterogeneity: the proportion of patients with significant fibrosis, the proportion of patients with hepatic steatosis, and BMI. Because the speckled pattern of the liver parenchyma may change from homogeneous to heterogeneous during the progression of hepatic fibrosis [33], the presence of fibrosis may change the US attenuation of the liver [34], and the reported performance of AC may differ according to the proportion of patients with significant fibrosis. The proportions of patients with hepatic steatosis and high BMI were also significantly associated with study heterogeneity. Considering that diagnostic test accuracy can vary according to patient subgroups, the spectrum of disease, the clinical setting, and the test interpreters [35], the accuracy can depend on differences in the targeted subjects. In addition, as AC is generally estimated from the slope of the ultrasound echo signal intensity obtained [2,36], it can be conjectured that patients with a higher BMI would have a longer distance between the skin and liver capsule, which could result in overestimates of the AC (i.e., high sensitivity but more false-positive cases at a given cutoff) [25,37].
The AC cutoff values for diagnosing ≥S1 and ≥S2 ranged from 0.53 to 0.69 and 0.60 to 0.77, respectively. Although the cutoff value used in each individual study was different, there was no threshold effect in the diagnosis of grades of either ≥S1 or ≥S2. However, because the optimal cutoff value was determined using the highest Youden index in the 13 included studies [2,12-15,24-31], the general applicability of the AC would be limited. In addition, the study cohort in all studies consisted of patients with various liver diseases and different spectrum of steatosis, which might also affect the different cutoffs [8,38]. Considering that hepatic steatosis requires longitudinal follow-up, a further study with a large number of homogeneous study subjects is required to determine the appropriate cutoff value for assessing hepatic steatosis.
This study has several limitations. First, the study was not preregistered before it was conducted. Second, although we summarized the median cutoff values for diagnosing ≥S1 and ≥S2, there may be limitations in distinguishing ≥S1 and ≥S2 in clinical practice. Through this study, it was not possible to obtain the exact optimal cutoff for assessing hepatic steatosis because of the different cutoff values used in the included studies and the insufficient details provided. An individual patient data meta-analysis of AC is required to investigate the optimal cutoff value. Third, marginal to substantial study heterogeneity was noted for specificity, which could preclude the creation of solid meta-analytic summary estimates. On the contrary, statistical heterogeneity was not found for sensitivity, which is generally considered to be of more importance than specificity in a screening test for the detection of hepatic steatosis. In addition, an attempt was made to minimize study heterogeneity by including only prospective studies.
In conclusion, AC can be clinically useful for assessing hepatic steatosis and showed good overall diagnostic performance. The data reported in published studies differed according to the prevalence of steatosis or significant fibrosis and BMI, and careful interpretation with consideration of these factors might be needed.
NotesAuthor Contributions Conceptualization: Jang JK, Choi SH, Kim SY, Lee SS. Data acquisition: Jang JK, Choi SH. Data analysis or interpretation: Jang JK, Lee JS, Kim KW. Drafting of the manuscript: Jang JK. Critical revision of the manuscript: Choi SH, Lee JS, Kim SY, Lee SS, Kim KW. Approval of the final version of the manuscript: all authors. Conflict of InterestSang Hyun Choi is the recipient of a grant from Bayer Healthcare. The other authors (Jong Keon Jang, Ji Sung Lee, So Yeon Kim, Seung Soo Lee, and Kyung Won Kim) have no conflicts of interest to declare. AcknowledgementsThis work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Grant No. NRF-2019R1G1A1099743) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI18C2383).
Supplementary MaterialSupplementary Table 1.Search terms for MEDLINE, Embase, and
Cochrane Library (https://doi.org/10.14366/usg.21076).
Supplementary Fig. 1.Quality assessment of the included studies based on the QUADAS-2 method (https://doi.org/10.14366/usg.21076).
Supplementary Fig. 2.Hierarchical summary receiver operating characteristics (HSROC) curves. HSROC curves for any grade of hepatic steatosis (S≥1) (A) and advanced steatosis (S≥2) (B) are shown (https://doi.org/10.14366/usg.21076).
Supplementary Fig. 3.Deek’s funnel plot asymmetry test. Deek's funnel plot asymmetery tests for any grade of hepatic steatosis (S≥1) (A) and advanced hepatic steatosis (S≥2) (B) are shown.
References1. Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 2013;58:593–608.
2. Jeon SK, Lee JM, Joo I, Yoon JH, Lee DH, Lee JY, et al. Prospective evaluation of hepatic steatosis using ultrasound attenuation imaging in patients with chronic liver disease with magnetic resonance imaging proton density fat fraction as the reference standard. Ultrasound Med Biol 2019;45:1407–1416.
3. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397.
4. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649–1659.
6. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–1906.
7. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017–1044.
8. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017;66:1022–1030.
9. de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol 2014;60:1026–1031.
10. Raptis DA, Fischer MA, Graf R, Nanz D, Weber A, Moritz W, et al. MRI: the new reference standard in quantifying hepatic steatosis? Gut 2012;61:117–127.
11. Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–1940.
12. Tamaki N, Koizumi Y, Hirooka M, Yada N, Takada H, Nakashima O, et al. Novel quantitative assessment system of liver steatosis using a newly developed attenuation measurement method. Hepatol Res 2018;48:821–828.
13. Paige JS, Bernstein GS, Heba E, Costa EA, Fereirra M, Wolfson T, et al. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol 2017;208:W168–W177.
14. Koizumi Y, Hirooka M, Tamaki N, Yada N, Nakashima O, Izumi N, et al. New diagnostic technique to evaluate hepatic steatosis using the attenuation coefficient on ultrasound B mode. PLoS One 2019;14:e0221548.
15. Ferraioli G, Maiocchi L, Savietto G, Tinelli C, Nichetti M, Rondanelli M, et al. Performance of the attenuation imaging technology in the detection of liver steatosis. J Ultrasound Med 2021;40:1325–1332.
16. McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM; PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018;319:388–396.
17. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–293.
18. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467–2474.
19. Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6:9.
20. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 2015;16:1188–1196.
21. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–990.
22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560.
23. Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9.
24. Fujiwara Y, Kuroda H, Abe T, Ishida K, Oguri T, Noguchi S, et al. The B-mode image-guided ultrasound attenuation parameter accurately detects hepatic steatosis in chronic liver disease. Ultrasound Med Biol 2018;44:2223–2232.
25. Bae JS, Lee DH, Lee JY, Kim H, Yu SJ, Lee JH, et al. Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol 2019;29:6499–6507.
26. Ferraioli G, Maiocchi L, Raciti MV, Tinelli C, De Silvestri A, Nichetti M, et al. Detection of liver steatosis with a novel ultrasound-based technique: a pilot study using MRI-derived proton density fat fraction as the gold standard. Clin Transl Gastroenterol 2019;10:e00081.
27. Dioguardi Burgio M, Ronot M, Reizine E, Rautou PE, Castera L, Paradis V, et al. Quantification of hepatic steatosis with ultrasound: promising role of attenuation imaging coefficient in a biopsy-proven cohort. Eur Radiol 2020;30:2293–2301.
28. Lee DH, Cho EJ, Bae JS, Lee JY, Yu SJ, Kim H, et al. Accuracy of two-dimensional shear wave elastography and attenuation imaging for evaluation of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2021;19:797–805.
29. Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Abe M, Yoshimasu Y, et al. The role of multiparametric US of the liver for the evaluation of nonalcoholic steatohepatitis. Radiology 2020;296:532–540.
30. Tada T, Kumada T, Toyoda H, Nakamura S, Shibata Y, Yasuda S, et al. Attenuation imaging based on ultrasound technology for assessment of hepatic steatosis: a comparison with magnetic resonance imaging-determined proton density fat fraction. Hepatol Res 2020;50:1319–1327.
31. Jesper D, Klett D, Schellhaas B, Pfeifer L, Leppkes M, Waldner M, et al. Ultrasound-based attenuation imaging for the non-invasive quantification of liver fat: a pilot study on feasibility and inter-observer variability. IEEE J Transl Eng Health Med 2020;8:1800409.
32. Lieu D. Ultrasound physics and instrumentation for pathologists. Arch Pathol Lab Med 2010;134:1541–1556.
33. Toyoda H, Kumada T, Kamiyama N, Shiraki K, Takase K, Yamaguchi T, et al. B-mode ultrasound with algorithm based on statistical analysis of signals: evaluation of liver fibrosis in patients with chronic hepatitis C. AJR Am J Roentgenol 2009;193:1037–1043.
34. Fujii Y, Taniguchi N, Itoh K, Shigeta K, Wang Y, Tsao JW, et al. A new method for attenuation coefficient measurement in the liver: comparison with the spectral shift central frequency method. J Ultrasound Med 2002;21:783–788.
35. Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM; Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149:889–897.
36. Tada T, Iijima H, Kobayashi N, Yoshida M, Nishimura T, Kumada T, et al. Usefulness of attenuation imaging with an ultrasound scanner for the evaluation of hepatic steatosis. Ultrasound Med Biol 2019;45:2679–2687.
Coupled forest plots for diagnosing any grade of hepatic steatosis.CI, confidence interval.
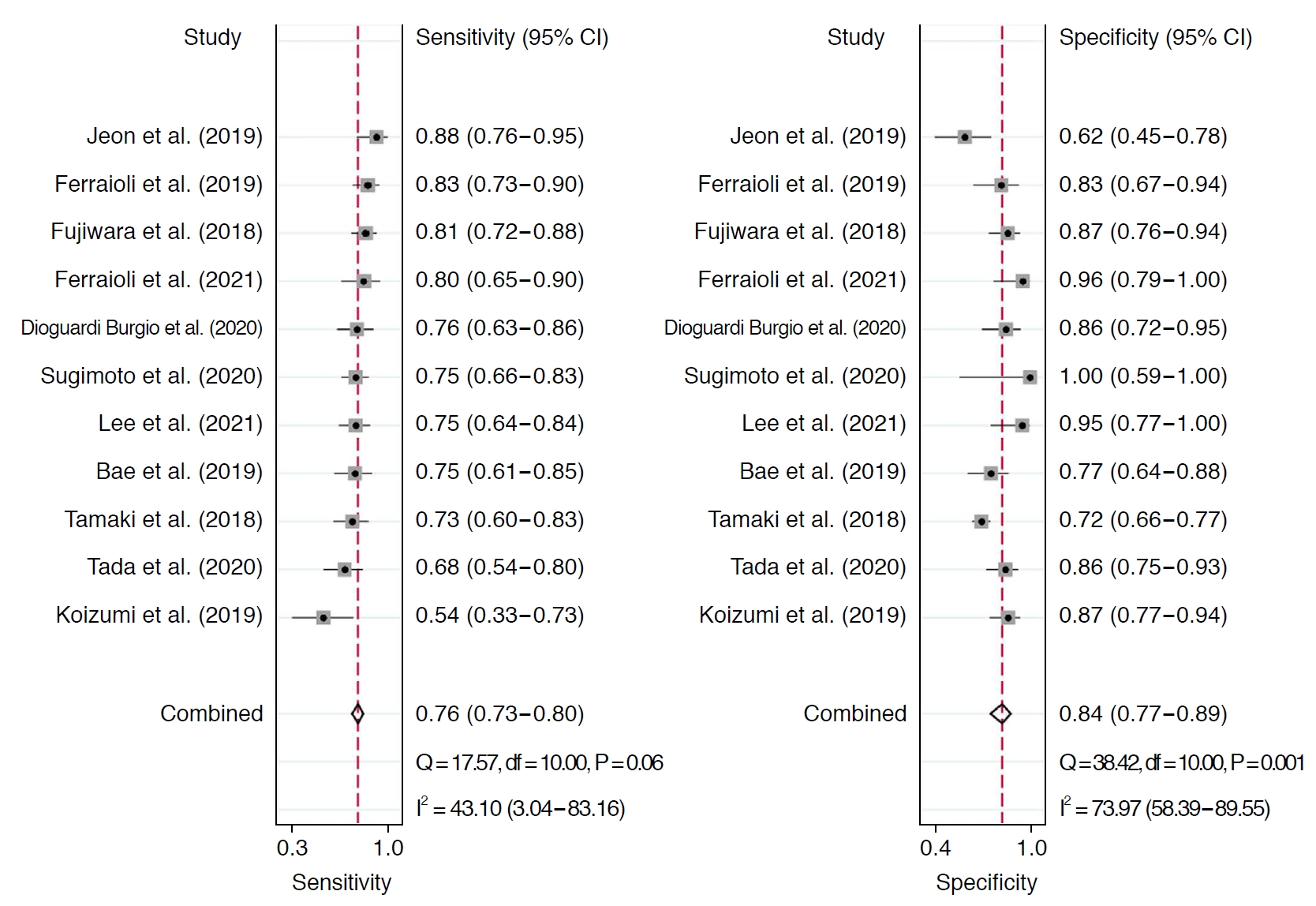 Fig. 2.Table 1.Characteristics of the included articles
Articles are listed according to year of publication and in alphabetical order of the names of the first authors for articles with the same year of publication. SD, standard deviation; BMI, body mass index; NAFLD, nonalcoholic fatty liver disease; US, ultrasonography; NA, not available; MRI, magnetic resonance imaging. Table 2.Sensitivity and specificity of the attenuation coefficient for diagnosing hepatic steatosis
Table 3.Meta-regression analysis of the accuracy of the attenuation coefficient for diagnosing hepatic steatosis |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




