AbstractPurposeThis study investigated the value of Doppler ultrasonography in predicting clinical outcomes after antirejection treatment for patients with acute cellular rejection (ACR) following liver transplantation (LT).
MethodsThis retrospective study included 84 patients who were pathologically diagnosed with ACR and received antirejection treatment within 90 days following LT. Two radiologists searched for abnormal Doppler parameters at ACR diagnosis and within 7 days after antirejection treatment initiation, including portal blood velocity (PBV) <20 cm/s, hepatic artery resistive index <0.5, and a monophasic hepatic vein flow pattern. Interval PBV changes were also evaluated. The frequencies of abnormal Doppler parameters and PBV changes were compared by treatment outcome.
ResultsThe frequency of abnormal PBV in the early post-treatment phase (PBVearly post-treatment) was significantly higher among poor responders (50.0% [10/20]) than among good responders (7.8% [5/64]) (P<0.001). The sensitivity, specificity, and accuracy of abnormal PBVearly post-treatment as a predictor of poor response to antirejection treatment were 50.0% (10/20), 92.2% (59/64), and 82.1% (69/84), respectively. A decrease (>10%) from the PBV at event (PBVevent) to PBVearly post-treatment was significantly more common among poor responders (50.0% [10/20]) than among good responders (20.3% [13/64]) (P=0.019). The sensitivity, specificity, and accuracy of this PBV decrease in predicting poor treatment response were 50.0% (10/20), 79.7% (51/64), and 72.6% (61/84), respectively.
IntroductionAcute cellular rejection (ACR) is a common complication of liver transplantation (LT), affecting up to 40% of patients [1]. This condition typically arises within the first 3 months after surgery [2]. Most ACR episodes are asymptomatic, and cases are usually suspected based on an elevation in liver enzymes [3]. These episodes generally respond well to steroid boluses or increased immune suppression treatment and usually do not impact graft or patient survival [4,5]. However, an estimated 10%-30% of patients with ACR may exhibit resistance to conservative antirejection therapy [6,7]. Patients who experience repeated episodes of severe ACR may progress to chronic rejection, which is generally characterized by gradual graft dysfunction and can lead to graft loss [8,9]. Early identification of indicators of poor graft survival is vital to prepare for potential re-transplantation. Currently, however, no noninvasive method has been established to predict clinical outcomes following antirejection treatment in patients with ACR. Liver function tests are commonly used for post-transplantation monitoring, but they lack the sensitivity and specificity needed for ACR detection and exhibit a weak correlation with severity [10,11]. Gouw et al. [12] proposed that the pathological shift from portal-based graft damage to a lobular-based process signifies the early transition of ACR to chronic rejection. However, liver biopsy is not suitable for routine monitoring due to its invasive nature.
Doppler ultrasonography serves as a useful, noninvasive imaging modality for postoperative follow-up in LT cases and can help rule out early vascular complications in patients suspected to have ACR [13]. The pathophysiological mechanism of ACR suggests that severe periportal inflammation can progress to centrilobular venule obstruction and increased intrasinusoidal pressure, ultimately resulting in portal hypertension [14]. This condition can subsequently alter Doppler parameters. Several of these parameters, including a decrease in portal blood velocity (PBV) and a monophasic hepatic vein flow pattern (HVP), are instrumental in diagnosing ACR [13,15,16]. These parameters may be influenced by changes in intrasinusoidal pressure due to antirejection treatment. However, the effectiveness of Doppler ultrasonography in assessing treatment response for ACR patients remains unestablished. Consequently, this study aimed to evaluate the potential of Doppler ultrasonography in predicting clinical outcomes after antirejection treatment in patients with ACR after LT.
Materials and MethodsCompliance with Ethical StandardsThe study protocol received approval from the institutional review board of the Asan Medical Center (IRB No. 2023-0617). Given the retrospective nature of this study, the board waived the requirement for informed consent.
PatientsBetween January 2012 and June 2017, at the authors’ affiliated institution, 2,222 patients over the age of 18 years underwent LT for the treatment of various liver diseases. Among these individuals, 117 were pathologically diagnosed with ACR via transjugular or percutaneous liver biopsy within 90 days of LT. Of those patients, 101 underwent Doppler ultrasonography within 3 days of ACR diagnosis (termed event Doppler ultrasonography) and again during the 7 days following initiation of antirejection treatment (referred to as early post-treatment Doppler ultrasonography). However, 17 of these patients were excluded due to the presence of concurrent liver disease identified through pathologic examination. These included 10 patients with ischemic liver damage, four with recurrent viral hepatitis, two with hemodynamic derangement, and one with hemorrhagic necrosis. The study thus included the remaining 84 patients, comprising 57 (67.9%) men and 27 (32.1%) women (mean age, 53.3±10.3 years; range, 20 to 73 years). The mean interval from LT to ACR diagnosis was 22.0±17.7 days (range, 4 to 83 days). Electronic medical records were reviewed to identify the levels of liver enzymes, specifically aspartate transaminase (AST) and alanine aminotransferase (ALT), on the day of ACR diagnosis. The median levels of these enzymes were also calculated during the 7 days following the start of treatment. The AST and ALT ratios were then calculated by dividing the median post-treatment enzyme level by the level at the time of ACR diagnosis.
Doppler Ultrasonography TechniqueAll Doppler ultrasonography examinations were performed by board-certified abdominal radiologists using a scanner equipped with a 1- to 4-MHz transducer. The standard Doppler scan parameters were adjusted to achieve maximum gain without background noise, the lowest pulse repetition frequency without aliasing artifacts, and a Doppler sample gate between 2 and 5 mm for optimal signal detection. To quantify the portal flow to the graft, Doppler spectrograms of each recipient’s portal vein were obtained at a point less than 1 cm caudal to the anastomosis. This is because measurements taken at the anastomosis or post-anastomotic portal vein can be either overestimations or underestimations due to stenosis or turbulent flow. The PBVs (measured in cm per second) were determined using angle correction, with the angle maintained at 60° or less. If the PBV displayed a wavy pattern during measurement, the peak velocity was taken as the PBV. Doppler spectrograms of the hepatic artery were obtained at the graft hepatic artery. The hepatic artery resistive index (HARI) was then automatically calculated using the following formula: (peak systolic velocity-end diastolic velocity)/peak systolic velocity. Finally, Doppler spectrograms of the hepatic vein were obtained within 2 cm of its junction with the inferior vena cava, over several cardiac cycles.
Analysis of Doppler ParametersThe Doppler ultrasonography images were retrospectively reviewed by two radiologists (K.W.K., who had 20 years of experience in LT imaging, and J.Y.C., who had 1 year of experience in LT imaging). Both radiologists were blinded to the pathologic information. Any disagreements between the two readers were settled through consensus evaluations.
The PBV measured at the event Doppler ultrasonography was defined as PBVevent, while the median PBV value on early post-treatment Doppler ultrasonography was defined as PBVearly post-treatment. A PBV lower than 20 cm/s was deemed abnormal, as per previous research [15]. The difference between PBVevent and PBVearly post-treatment was further analyzed and categorized as no change, a decrease, or an increase. A change exceeding 10% in PBVearly post-treatment relative to PBVevent was interpreted as either a decrease or an increase. The HARI values on the event and early post-treatment Doppler ultrasonography were defined as HARIevent and HARIearly post-treatment, respectively. A HARI lower than 0.5 was considered abnormal [17]. The HVP values on the event and early post-treatment Doppler ultrasonography were defined as HVPevent and HVPearly post-treatment, respectively. Monophasic or continuous wave patterns were identified as abnormal HVP [16].
Histologic Assessment of ACRTransjugular or percutaneous liver biopsy samples were obtained from patients suspected of having clinically relevant ACR. The diagnosis of ACR was made histologically using the Banff method [18]. The rejection activity index (RAI) was determined by summing the scores for the following criteria: (1) portal inflammation, (2) damage due to bile duct inflammation, and (3) venous endothelial inflammation. The severity of each component was scored on a scale from 0 to 3. A diagnosis of relevant ACR was made if the RAI exceeded 4.
Clinical OutcomesThe clinical outcomes of patients with relevant ACR were investigated by examining their electronic medical records. Patients were categorized as either good or poor responders, depending on their reaction to the antirejection treatment. Good responders were defined as those who recovered without the necessity for an additional liver biopsy, or those for whom follow-up biopsy indicated a decrease in RAI. All other patients were classified as poor responders. This group exhibited either histological progression, as evidenced by either no interval change or an increase in RAI on follow-up biopsy, or graft failure, which included re-transplantation and graft-related death.
Statistical AnalysisThe demographic and clinical characteristics of the good and poor responders were compared using Student t-tests for continuous variables and the chi-square or Fisher exact test for categorical variables. The differences in laboratory and Doppler findings between the good and poor responders were compared using the chi-square or Fisher exact test. Within the subgroups categorized by event Doppler ultrasonography findings, relationships between post-treatment Doppler parameters and treatment response were analyzed using the Fisher exact test. All statistical analyses were performed using SPSS Statistics for Windows (version 23.0, IBM Corp., Armonk, NY, USA). A two-sided P-value of less than 0.05 was considered to indicate statistical significance.
Results
Table 1 presents the characteristics of all patients. Most patients were diagnosed with ACR due to abnormal laboratory findings during regular outpatient follow-up. Overall, 64 patients (76.2%) were good responders, while 20 (23.8%) were poor responders. The poor responders included 10 (50.0%) who died of graft failure, five (25.0%) who underwent re-transplantation due to graft failure, and five (25.0%) who exhibited histological progression on follow-up biopsy. No significant differences were observed in age, sex, ACR onset, RAI at the time of diagnosis, or graft type between the good and poor responders. Furthermore, no significant differences were noted in liver enzyme levels on the day of ACR diagnosis, median values during the subsequent 7 days post-treatment, or their ratios between the two groups.
The frequency of abnormal PBVevent was 33.3% (28 of 84 patients) overall, with no significant difference between the good (29.7% [19/64]) and poor (45.0% [9/20]) responders (P=0.205). However, abnormal PBVearly post-treatment (17.9% [15/84]) was significantly more common among poor responders (50.0% [10/20]) compared to good responders (7.8% [5/64]) (P<0.001). One patient exhibited hepatofugal flow on event Doppler ultrasonography, while two patients displayed hepatofugal flow on early post-treatment Doppler ultrasonography. All three were poor responders. The diagnostic performance of abnormal PBVearly post-treatment in predicting poor clinical outcomes following antirejection treatment was as follows: sensitivity of 50.0% (10/20), specificity of 92.2% (59/64), positive predictive value of 66.7% (5/15), negative predictive value of 85.5% (59/69), and accuracy of 82.1% (69/84). Representative cases are illustrated in Figs. 1 and 2. In the subgroup of 28 patients with abnormal PBVevent, the median PBVearly post-treatment remained persistently abnormal in 10 patients and normalized (to ≥20 cm/s) in the remaining 18. In this subgroup, the frequency of persistently abnormal PBVearly post-treatment was significantly higher in poor responders (77.8% [7/9]) than in good responders (15.8% [3/19]) (P=0.003). The diagnostic values of abnormal PBVearly post-treatment in predicting poor clinical outcomes after antirejection treatment were as follows: sensitivity of 77.8% (7/9), specificity of 84.2% (16/19), positive predictive value of 70% (7/10), negative predictive value of 88.9% (16/18), and accuracy of 82.1% (23/28). Among the 56 patients with normal PBVevent (≥20 cm/s), the median PBVearly post-treatment remained normal in 51 patients but deteriorated to <20 cm/s in the remaining 5. In this normal PBVevent subgroup, the frequency of abnormal PBVearly post-treatment was also significantly higher in poor responders (27.3% [3/11]) than in good responders (4.4% [2/45]) (P=0.047). The diagnostic values of abnormal PBVearly post-treatment in predicting poor clinical outcomes following antirejection treatment were as follows: sensitivity of 27.3% (3/11), specificity of 95.6% (43/45), positive predictive value of 60% (3/5), negative predictive value of 84.3% (43/51), and accuracy of 82.1% (46/56) (Table 2). In terms of the change between PBVevent and PBVearly post-treatment, a significant difference was observed between the outcome groups (P=0.019), with a significantly higher frequency of decrease in poor responders (50.0% [10/20]) than in good responders (20.3% [13/64]). Among the 23 patients with a PBV decrease, 15 exhibited normal PBV on both event and early post-treatment Doppler ultrasonography. The diagnostic values of the decrease between PBVevent and PBVearly post-treatment in predicting poor clinical outcomes after antirejection treatment were as follows: sensitivity of 50.0% (10/20), specificity of 79.7% (51/64), positive predictive value of 43.4% (10/23), negative predictive value of 83.6% (51/61), and accuracy of 72.6% (61/84). Changes between PBVevent and PBVearly post-treatment according to treatment response are illustrated in Fig. 3. The frequency of HARIevent was 9.5%, impacting eight of 84 patients. Among these eight patients, HARIearly post-treatment was normalized in seven, while one patient continued to exhibit abnormalities. Among the 76 patients who initially presented with a normal HARIevent, three subsequently displayed abnormal HARIearly post-treatment. Consequently, the frequency of abnormal HARIearly post-treatment was 4.8% (4 of 84 patients). No significant difference was present in the frequency of HARIevent or HARIearly post-treatment between the good and poor responders.
The frequency of abnormal HVPevent was 14.3%, affecting 12 of 84 patients. Among the 12 patients with abnormal HVPevent, nine continued to show abnormal HVPearly post-treatment, while the condition normalized in the remaining three. In contrast, among the 72 patients with normal HVPevent, HVPearly post-treatment became abnormal in one patient and remained normal in the other 71. Consequently, the frequency of abnormal HVPearly post-treatment was 11.9% (10 of 84 patients). Neither HVPevent nor HVPearly post-treatment (either overall or in the subgroups categorized by HVPevent) differed significantly between the good and poor responders.
DiscussionThis study examined patients who had been pathologically diagnosed with early ACR following LT. The frequency of abnormal PBVevent did not significantly differ between good and poor responders to antirejection treatment. However, abnormal PBVearly post-treatment was significantly more common in poor responders than good responders. The decrease between PBVevent and PBVearly post-treatment was also significantly greater in poor responders. However, other Doppler parameters, such as HARI or HVP, liver enzyme levels, and RAI, showed no significant relationship with treatment response.
Previous studies have demonstrated that ACR triggers an acute transient increase in portal pressure. This increase is significantly associated with the severity of ACR [19,20]. Such a shift in portal pressure results in abnormal PBV as detected by Doppler ultrasonography [13,15]. However, in the present study, abnormal PBVevent was observed in only about one-third of patients diagnosed with ACR. Multiple studies have indicated that the absolute PBV values at the time of the event did not significantly differ between patients with and without ACR [13,16]. This could be partially attributed to the broad individual variation in PBV during the early postoperative period. This variation depends on factors such as the severity of preoperative portal hypertension and graft size [21], as well as patterns of portosystemic collaterals [22,23]. Furthermore, the selection process of patients with clinically relevant ACR, which required a biopsy, may have influenced these results. In this study, no significant relationship was found between abnormal PBVevent and treatment response. Similarly, the RAI at the time of ACR diagnosis was not significantly associated with the treatment response. This aligns with the findings of Horoldt et al. [24], who reported no correlation between the RAI (or any of its individual components) and treatment response or graft survival. These results suggest that the severity of increased intrasinusoidal pressure and portal hypertension caused by ACR at the time of diagnosis does not predict treatment response or prognosis. The reversibility of these conditions may not correspond with the severity of ACR.
A significant association was observed between PBVearly post-treatment and treatment response in the patient subgroups categorized based on PBVevent (abnormal or normal). In the subgroup with abnormal PBVevent, most patients who exhibited normal recovery of PBVearly post-treatment (88.9%) demonstrated a good response to antirejection treatment and favorable clinical outcomes. Conversely, a high proportion (70.0%) of those with persistently abnormal PBVearly post-treatment exhibited poor response to treatment. Thus, a persistently abnormal PBVearly post-treatment was predictive of unfavorable clinical outcomes, with relatively high sensitivity (77.8%) and specificity (84.2%). In the subgroup with normal PBVevent, patients with consistently normal PBVearly post-treatment generally (84.3%) exhibited a good response, while most (60.0%) of those whose PBVearly post-treatment deteriorated to an abnormal value showed poor outcomes. Although the sensitivity was low (27.3%) in this subgroup, the specificity was relatively high (95.6%). Furthermore, a decrease of more than 10% between the PBVevent and PBVearly post-treatment, even if the value remained within the normal range, could be associated with a poor response to treatment. Therefore, understanding the value of an abnormal PBVearly post-treatment and a decrease between PBVevent and PBVearly post-treatment in predicting poor response to antirejection treatment could aid in managing patients with ACR in clinical settings and facilitate timely preparation for potential re-transplantation.
Although patients with ACR typically present with elevated liver enzyme levels, in this study, no significant differences were observed in liver enzyme profiles between the good and poor responders to antirejection treatment. The mean levels of both AST and ALT were slightly higher in poor responders at the time of ACR diagnosis, but these differences were not statistically significant. This observation aligns with a previous report suggesting that serum biochemical profile is not a reliable indicator of the presence or severity of rejection [10]. Furthermore, other hepatic vasculature Doppler parameters did not demonstrate a significant correlation with treatment response in this study. The use of HARI is known to be unreliable in assessing ACR after LT [25,26]. In contrast, while the presence of a monophasic HVP has been suggested to be useful in diagnosing ACR, this finding was uncommon in this series, both at the time of ACR diagnosis and during the early post-treatment period. This low incidence may be due in part to the selection process of clinically relevant ACR, which involved a biopsy. Neither HVPevent nor HVPearly post-treatment differed significantly between good and poor responders in this study. Therefore, the prognostic value of HVP may be considered inadequate for predicting clinical outcomes following antirejection treatment. Further studies with more refined selection criteria are necessary.
This study had several limitations. First, the retrospective design may have introduced selection bias into the study population. The inclusion criteria were patients pathologically diagnosed with ACR in the early postoperative period, for whom Doppler ultrasonography studies were available at the time of ACR diagnosis and throughout the early post-treatment period. Given the absence of definitive guidelines for performing a liver biopsy, patients with mild ACR could not be included in the study population. Second, the frequency of follow-up Doppler ultrasonography among eligible patients varied, contingent on each patient’s condition and the discretion of the clinician. This variability could influence the characteristics observed in early post-treatment Doppler ultrasonography. To address these limitations, a well-designed prospective study may be necessary. Additionally, interobserver variability may have been present in the measurement of Doppler parameters. To mitigate this, the measurement protocol of PBV was standardized, and the HVP examinations were conducted by trained, board-certified abdominal radiologists using a single ultrasound machine, which should alleviate these concerns.
In conclusion, both abnormal PBVearly post-treatment and a decrease between PBVevent and PBVearly post-treatment were significantly more common among those who displayed a poor treatment response within the study population of patients with clinically relevant ACR shortly after LT. Thus, Doppler ultrasonography can serve as a valuable noninvasive method for predicting treatment outcomes and prognosis in patients with early ACR after LT.
NotesAuthor Contributions Conceptualization: Choi JY, Kim KW, Jang JK, Lee SG. Data acquisition: Choi JY, Kim KW. Data analysis or interpretation: Choi JY, Kim KW, Choi SH, Kwon HJ, Yoon YI, Song GW. Drafting of the manuscript: Choi JY, Kim KW. Critical revision of the manuscript: Kim KW, Jang JK, Choi SH, Kwon HJ, Yoon YI, Song GW, Lee SG. Approval of the final version of the manuscript: all authors. AcknowledgementsThis research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (No. 2017R1E1A1A03070961).
References1. Bartlett AS, Ramadas R, Furness S, Gane E, McCall JL. The natural history of acute histologic rejection without biochemical graft dysfunction in orthotopic liver transplantation: a systematic review. Liver Transpl 2002;8:1147–1153.
2. Neil DA, Hubscher SG. Current views on rejection pathology in liver transplantation. Transpl Int 2010;23:971–983.
3. Choudhary NS, Saigal S, Bansal RK, Saraf N, Gautam D, Soin AS. Acute and chronic rejection after liver transplantation: what a clinician needs to know. J Clin Exp Hepatol 2017;7:358–366.
4. Fisher LR, Henley KS, Lucey MR. Acute cellular rejection after liver transplantation: variability, morbidity, and mortality. Liver Transpl Surg 1995;1:10–15.
5. Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology 1998;28:638–645.
6. Aydogan C, Sevmis S, Aktas S, Karakayali H, Demirhan B, Haberal M. Steroid-resistant acute rejections after liver transplant. Exp Clin Transplant 2010;8:172–177.
7. Andreu H, Rimola A, Bruguera M, Navasa M, Cirera I, Grande L, et al. Acute cellular rejection in liver transplant recipients under cyclosporine immunosuppression: predictive factors of response to antirejection therapy. Transplantation 2002;73:1936–1943.
8. Uemura T, Ikegami T, Sanchez EQ, Jennings LW, Narasimhan G, McKenna GJ, et al. Late acute rejection after liver transplantation impacts patient survival. Clin Transplant 2008;22:316–323.
9. Thurairajah PH, Carbone M, Bridgestock H, Thomas P, Hebbar S, Gunson BK, et al. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation 2013;95:955–959.
10. Abraham SC, Furth EE. Receiver operating characteristic analysis of serum chemical parameters as tests of liver transplant rejection and correlation with histology. Transplantation 1995;59:740–746.
11. Henley KS, Lucey MR, Appelman HD, Baliga P, Brown KA, Burtch GD, et al. Biochemical and histopathological correlation in liver transplant: the first 180 days. Hepatology 1992;16:688–693.
12. Gouw AS, van den Heuvel MC, van den Berg AP, Slooff MJ, de Jong KP, Poppema S. The significance of parenchymal changes of acute cellular rejection in predicting chronic liver graft rejection. Transplantation 2002;73:243–247.
13. Bolognesi M, Sacerdoti D, Mescoli C, Nava V, Bombonato G, Merkel C, et al. Acute liver rejection: accuracy and predictive values of doppler US measurements: initial experience. Radiology 2005;235:651–658.
14. Jeong WK, Kim KW, Lee SJ, Shin YM, Kim J, Song GW, et al. Hepatofugal portal venous flow on Doppler sonography after liver transplantation: analysis of presumed causes based on radiologic and pathologic features. J Ultrasound Med 2012;31:1069–1079.
15. Sugimoto H, Kato K, Hirota M, Takeda S, Kamei H, Nakamura T, et al. Serial measurement of Doppler hepatic hemodynamic parameters for the diagnosis of acute rejection after live donor liver transplantation. Liver Transpl 2009;15:1119–1125.
16. Lee SJ, Kim KW, Kim JH, Kim SY, Lee JS, Kim HJ, et al. Doppler sonography of patients with and without acute cellular rejection after right-lobe living donor liver transplantation. J Ultrasound Med 2012;31:845–851.
17. Dodd GD 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 1994;192:657–661.
18. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997;25:658–663.
19. Hadengue A, Lebrec D, Moreau R, Sogni P, Durand F, Gaudin C, et al. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology 1993;17:175–178.
20. Gadano A, Hadengue A, Widmann JJ, Vachiery F, Moreau R, Yang S, et al. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology 1995;22:458–465.
21. Jang YJ, Kim KW, Jeong WK, Shin YM, Song GW, Hwang S, et al. Influence of preoperative portal hypertension and graft size on portal blood flow velocity in recipient after living donor liver transplantation with right-lobe graft. AJR Am J Roentgenol 2010;194:W165–W170.
22. Baik SK. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int 2010;30:1403–1413.
23. Merkel C, Sacerdoti D, Bolognesi M, Bombonato G, Gatta A. Doppler sonography and hepatic vein catheterization in portal hypertension: assessment of agreement in evaluating severity and response to treatment. J Hepatol 1998;28:622–630.
24. Horoldt BS, Burattin M, Gunson BK, Bramhall SR, Nightingale P, Hubscher SG, et al. Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation? Liver Transpl 2006;12:1144–1151.
A 50-year-old woman diagnosed with acute cellular rejection (with a rejection activity index of 8) 8 days after receiving a living donor liver transplantation.The transplantation, which involved a right hemiliver graft, was performed to treat toxic hepatitis. A. An event Doppler ultrasound (US) image, taken on the day of acute cellular rejection diagnosis, reveals an abnormal portal blood velocity of 12.4 cm/s. B. A post-treatment Doppler US image, captured 2 days after the event Doppler US, displays a consistently abnormal portal blood velocity of 16.7 cm/s. Despite receiving antirejection treatment, the patient died of graft failure 21 days after transplantation.
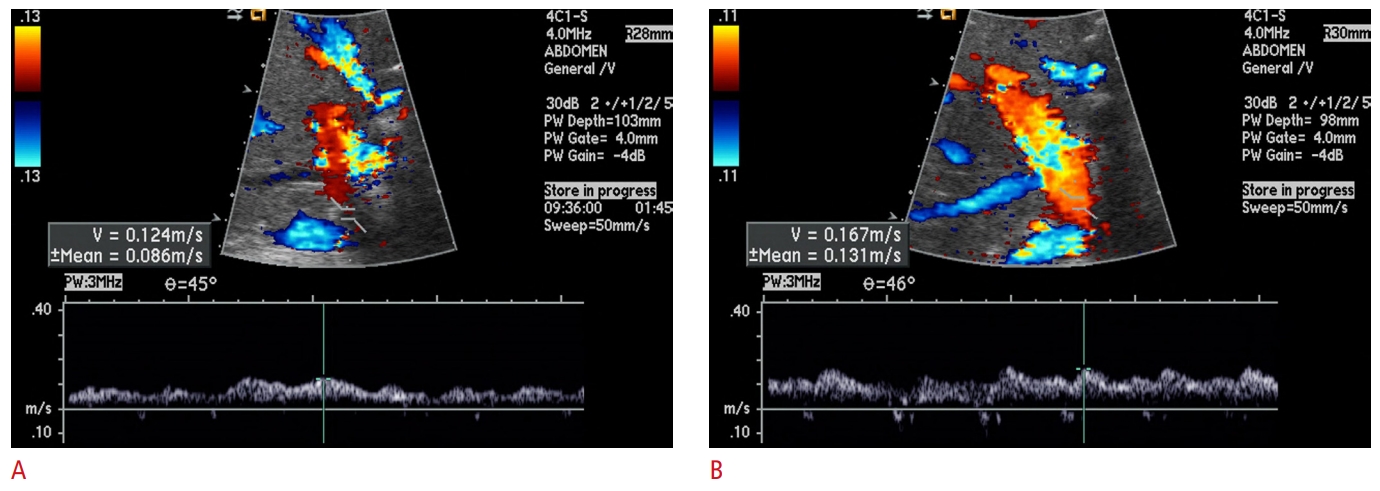 Fig. 1.A 45-year-old man diagnosed with acute cellular rejection (with a rejection activity index of 5) 9 days after receiving a deceased donor liver transplantation due to alcoholic cirrhosis.A. An event Doppler ultrasound (US) image, taken on the day of acute cellular rejection diagnosis, reveals an abnormal portal blood velocity of 15.3 cm/s. B. A post-treatment Doppler US image, taken 7 days after the event Doppler US, displays a portal blood velocity that has normalized to a value of 32.2 cm/s. Following antirejection treatment, the patient’s clinical findings improved, and he was subsequently discharged.
 Fig. 2.Changes in portal blood velocity between event Doppler ultrasonography (PBVevent) and early post-treatment Doppler ultrasonography (PBVearly post-treatment) among poor responders (A) and good responders to treatment (B).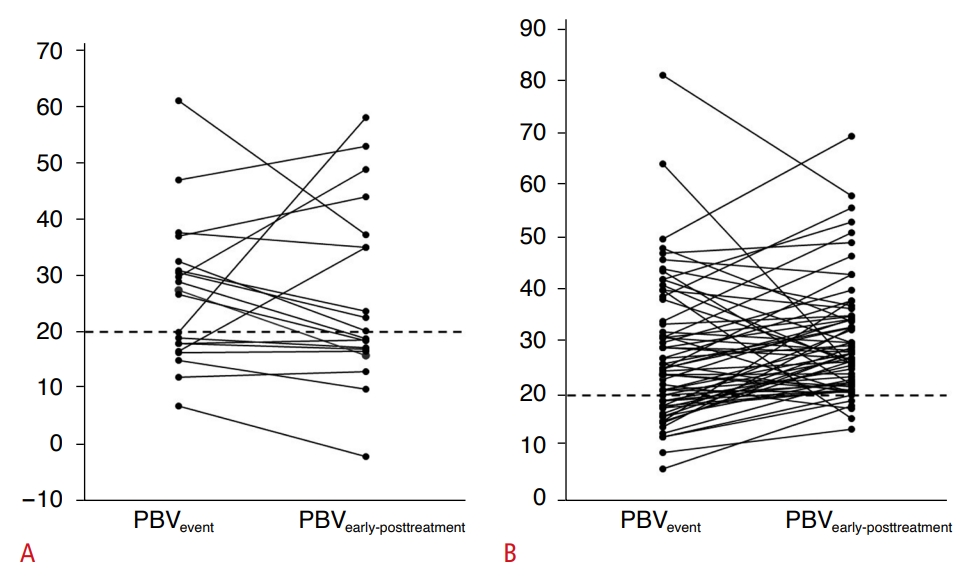 Fig. 3.Table 1.Clinicopathologic characteristics, along with laboratory and Doppler findings, of good and poor responders to antirejection treatment Values are presented as mean±SD or number (%). ACR, acute cellular rejection; DDLT, deceased donor liver transplantation; LDLT, living donor liver transplantation; AST, aspartate transaminase; ALT, alanine aminotransferase; PBV, portal blood velocity; HARI, hepatic artery resistive index; HVP, hepatic vein flow pattern; SD, standard deviation. Table 2.Relationships between post-treatment Doppler parameters and antirejection treatment response in patient subgroups categorized by findings on event Doppler ultrasonography |