Introduction
Mammography is the only imaging modality that has been proven to reduce mortality from breast cancer [1]. However, the sensitivity of mammography in dense breasts is poor [2]. In the United States, state-level legislation requires radiologists to inform patients of the aforementioned disadvantage of mammography and the possible need for supplemental screening modalities, such as ultrasonography, in patients with dense breasts [3].
Three-dimensional automated breast ultrasonography (ABUS) has been approved in the United States and Europe to serve as a screening tool for breast cancer. ABUS is used as an adjunct to mammography and for screening asymptomatic women with dense breasts [4].
ABUS is designed to overcome the shortfalls associated with hand-held US (HHUS), such as operator dependency and lack of reproducibility. It provides proper orientation and documentation, resulting in better reproducibility, and is also reliable for follow-up studies. For technologists, the operation of ABUS is user-friendly, with no need for prolonged training. In addition to this, it is timeefficient for radiologists [5-8].
Optimal image quality is essential for a proper diagnosis, and an imaging system should ensure high-quality images before approval for use in clinical practice [9]. Image quality can be impacted by the image acquisition process [9]. Artifacts can interfere with the visibility of abnormalities, reduce overall image quality, and adversely affect the reliability of an imaging system [10]. Radiologists should be aware of common artifacts in order to ensure the accuracy of the diagnosis.
This article describes issues related to the quality of ABUS images and illustrates common artifacts encountered when interpreting ABUS images.
Image Acquisition and Image Quality
According to guidelines on standards of mammography practice, mammography must meet the highest possible quality standards. However, no comparable guidelines and regulations exist for ABUS. The United States Food and Drug Administration recommends 8 hours of training before an operator can use ABUS in clinical settings [11].
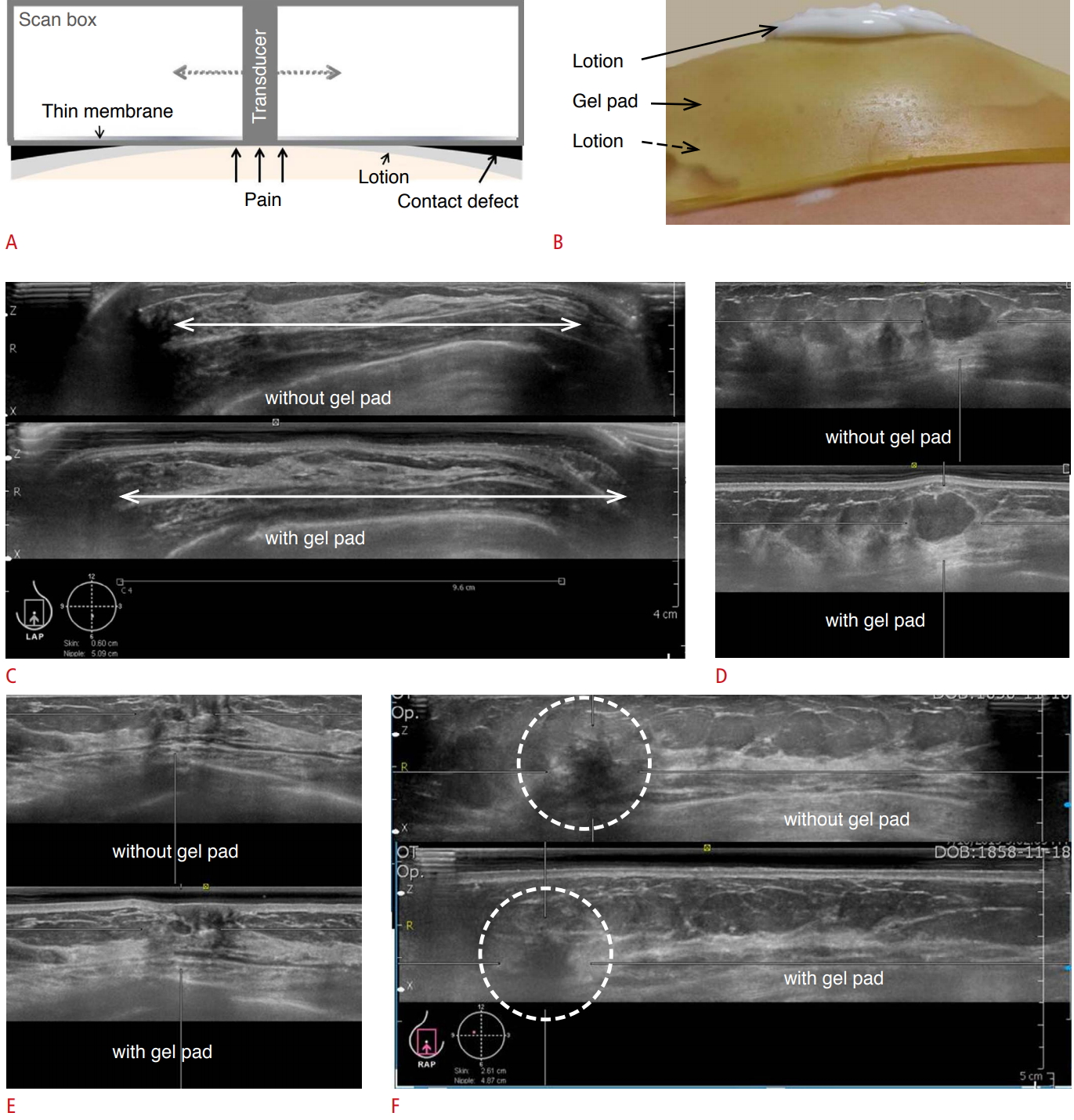
In ABUS, the patient assumes the same position as in HHUS. An ABUS-exclusive lotion that serves as a coupling agent is applied to the skin instead of the usual gel (Fig. 1A). The transducer is positioned on the breast with slight pressure and locked prior to scanning (Fig. 1B).
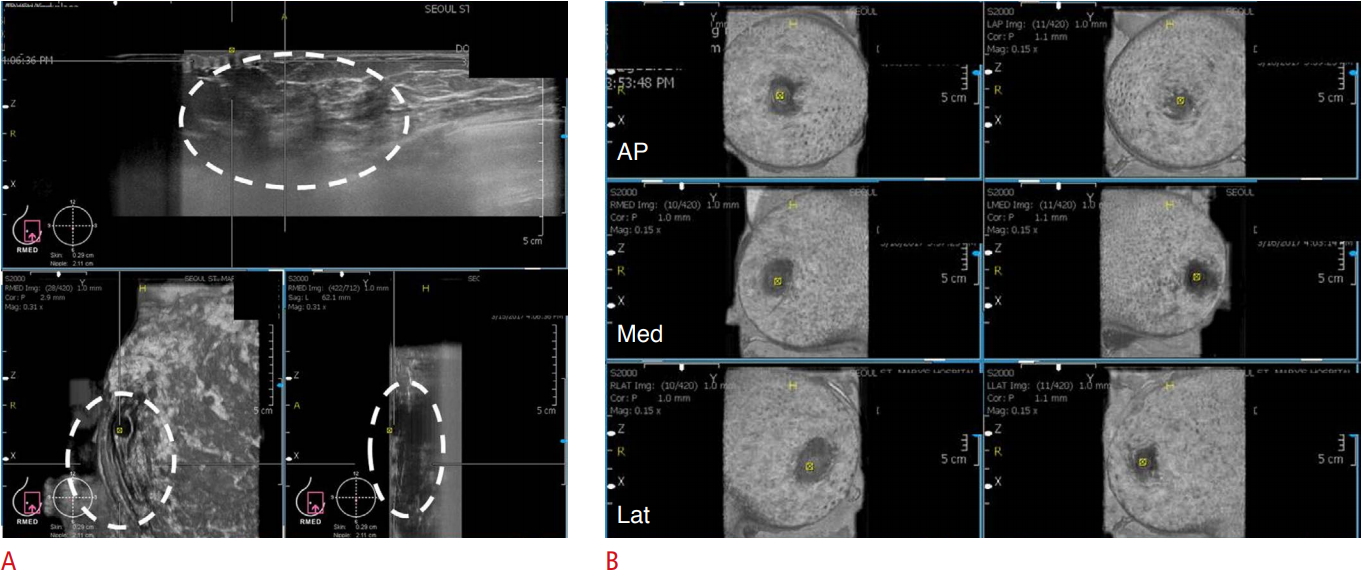
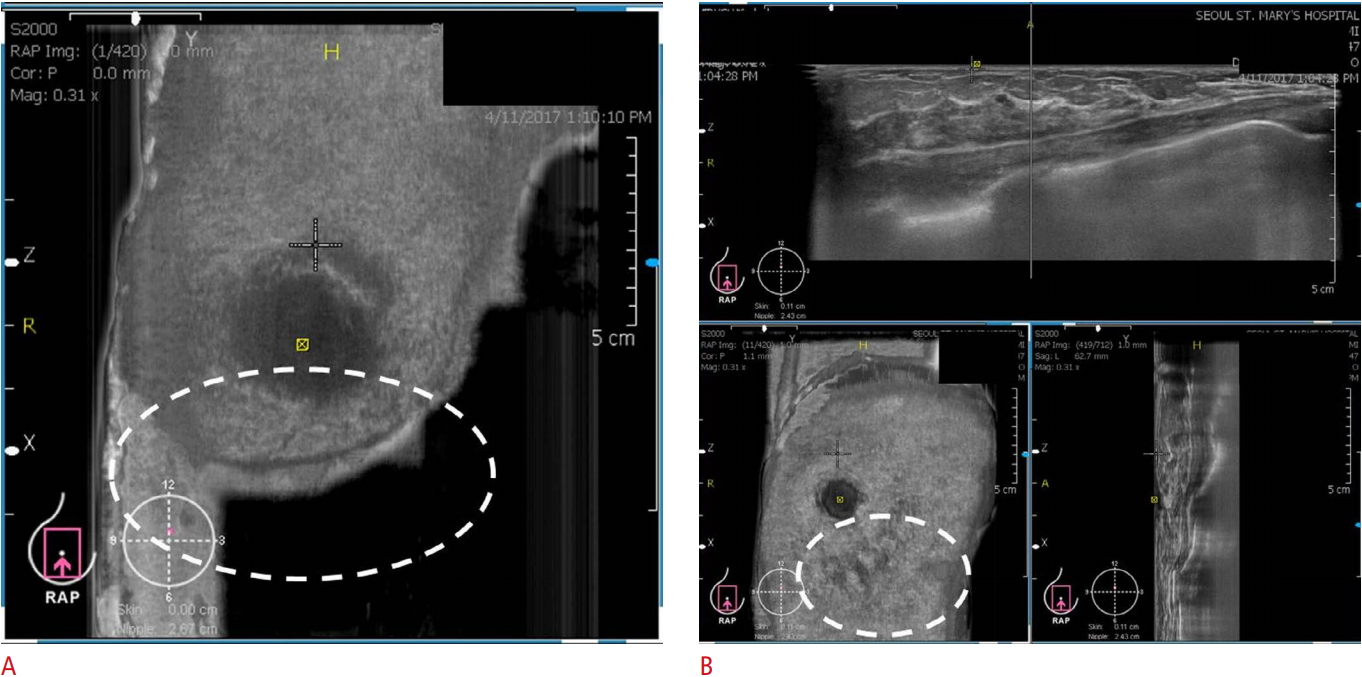
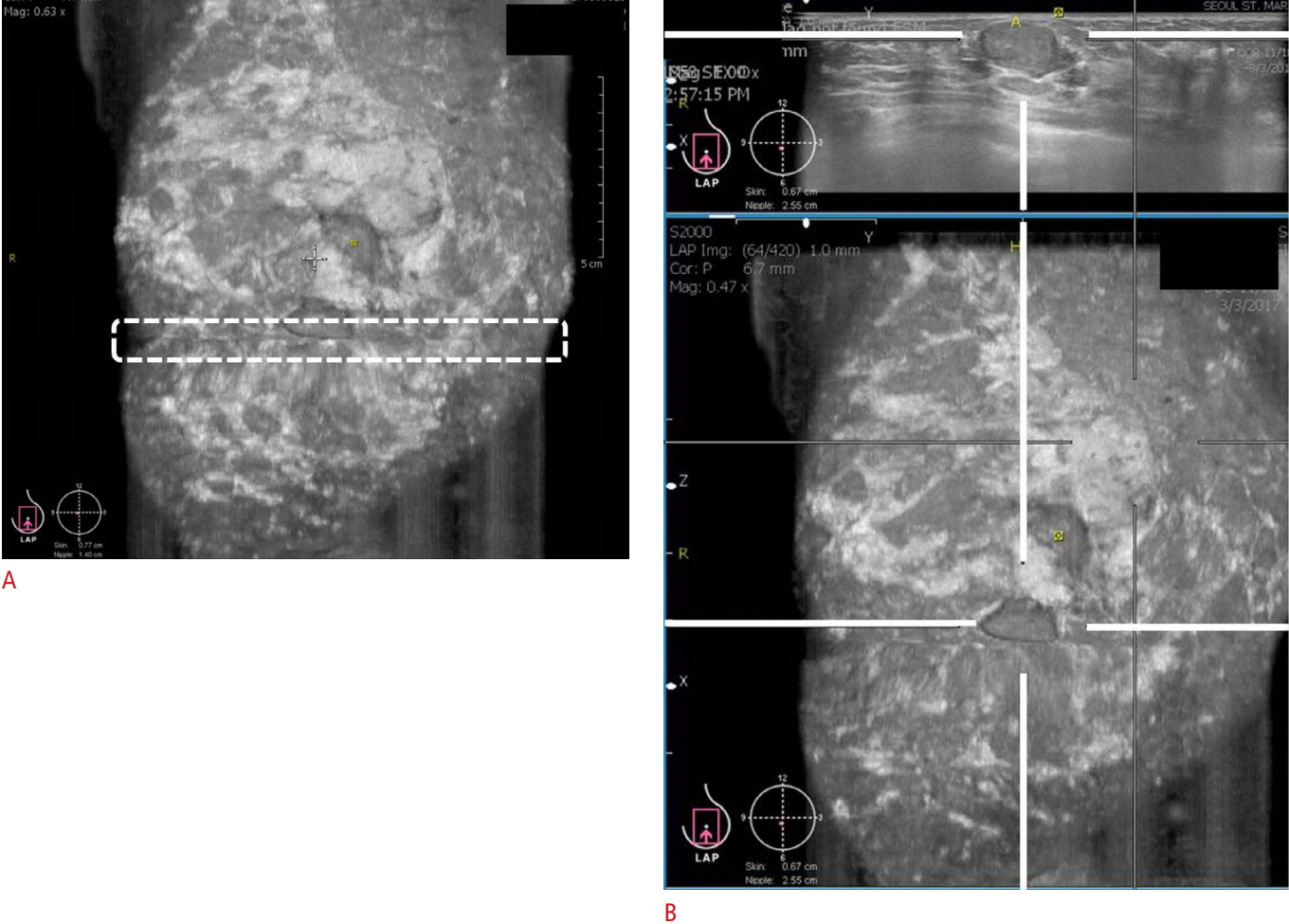
The breast tissues should be fully covered. Poor contact between the transducer and skin could prevent the complete coverage of the breast and adversely affect image acquisition (Fig. 2A). Therefore, gentle compression and skillful manipulation of the probe to cover the whole breast is important.
The depth of the scan is specified from A to D (depending on the breast size, as denoted by bra cup size) or from 3.5 to 5 cm (small, medium, and large), depending on the vendor, and then the scanning area is selected. The probe is placed on the selected area. The breast is scanned and the volume information is acquired.
Anteroposterior, medial, and lateral views are routinely acquired. If there are additional indications, such as for large breasts, superior and inferior views are also acquired.
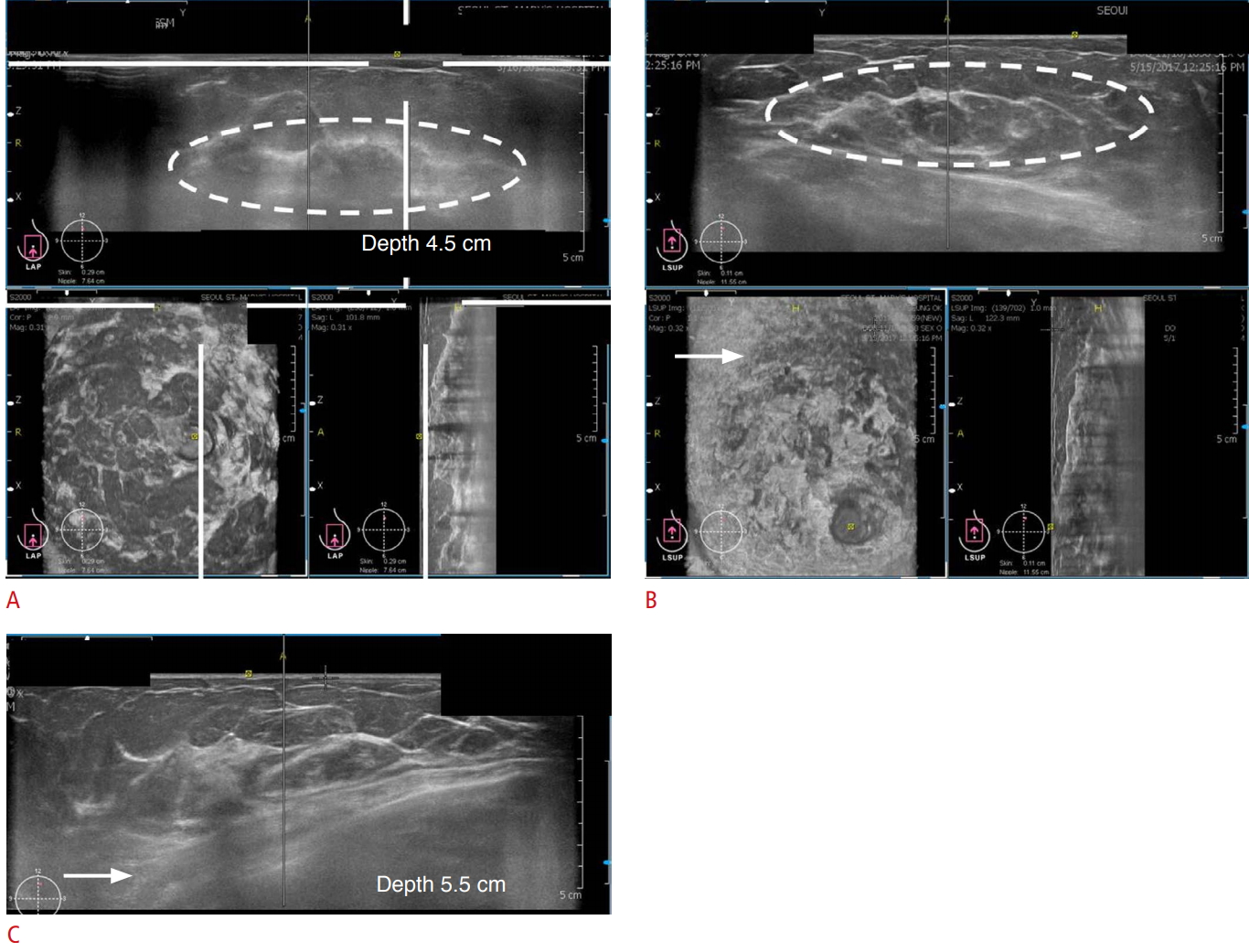
In the anteroposterior view, the nipple should be positioned at the center of the image. In the medial and lateral views, the nipple should be positioned at the periphery of the image (Fig. 2B). It is easier to acquire images in women with large breasts than in those with small breasts. The depth of the scan should be evaluated to ensure that the deep and peripheral breast tissues are included in the image fields. In women with large breasts (a bra cup size larger than an E), the upper and deep central breast tissues are frequently not covered. Therefore, additional views should be acquired after correcting for the error in depth (Fig. 3A-C).
Artifacts of ABUS
Because artifacts are not part of breast tissue anatomy or pathology, and generally interfere with the ability to make an accurate diagnosis, it is important that technologists and radiologists learn to identity and characterize artifacts on ABUS images. Common artifacts encountered in ABUS are described below.
Nipple Shadow and Reverberation Artifacts
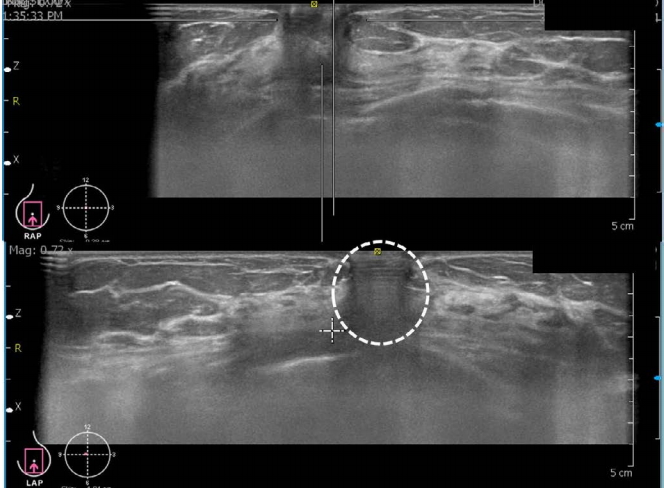
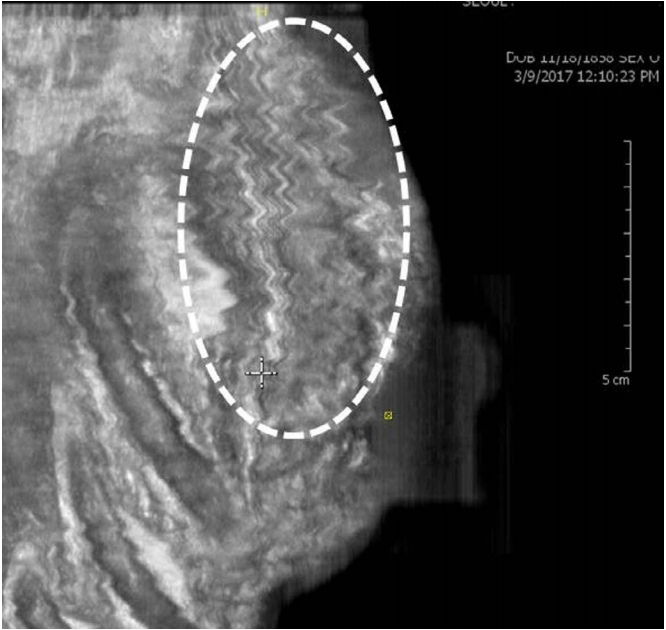
Nipple shadow and reverberation artifacts are problematic in ABUS (Fig. 5).
A reverberation artifact has the typical feature of multiple parallel lines that decrease in intensity and are equidistant from each other [12]. In addition, in the area around the nipple, the contact between the transducer and the skin is often imperfect, resulting in an acoustic shadow behind the nipple.
Skip Artifacts
A skip artifact is seen as a transverse anechoic line (Fig. 6A). It is common where there is a change in tissue stiffness, either due to a mass or simply due to moving from fat to dense tissue (Fig. 6B). Although artifacts generally interfere with image quality, this type of artifact can, however, help to characterize a lesion or other findings [13]. When a transverse anechoic line is shown, the radiologist must try to identify the cause of the change in tissue stiffness. Skip artifacts can be useful for detecting isoechoic masses, which are difficult to distinguish from the surrounding fat.
Motion Artifacts
Motion artifacts are seen as multiple zigzag lines. The probe is designed to move smoothly over the area of interest. However, when the probe is placed just above the rib cage and vigorous compression or patient motion occurs, the probe can move discontinuously and create these curly lines (Fig. 7) [13,14].
Contact Artifacts
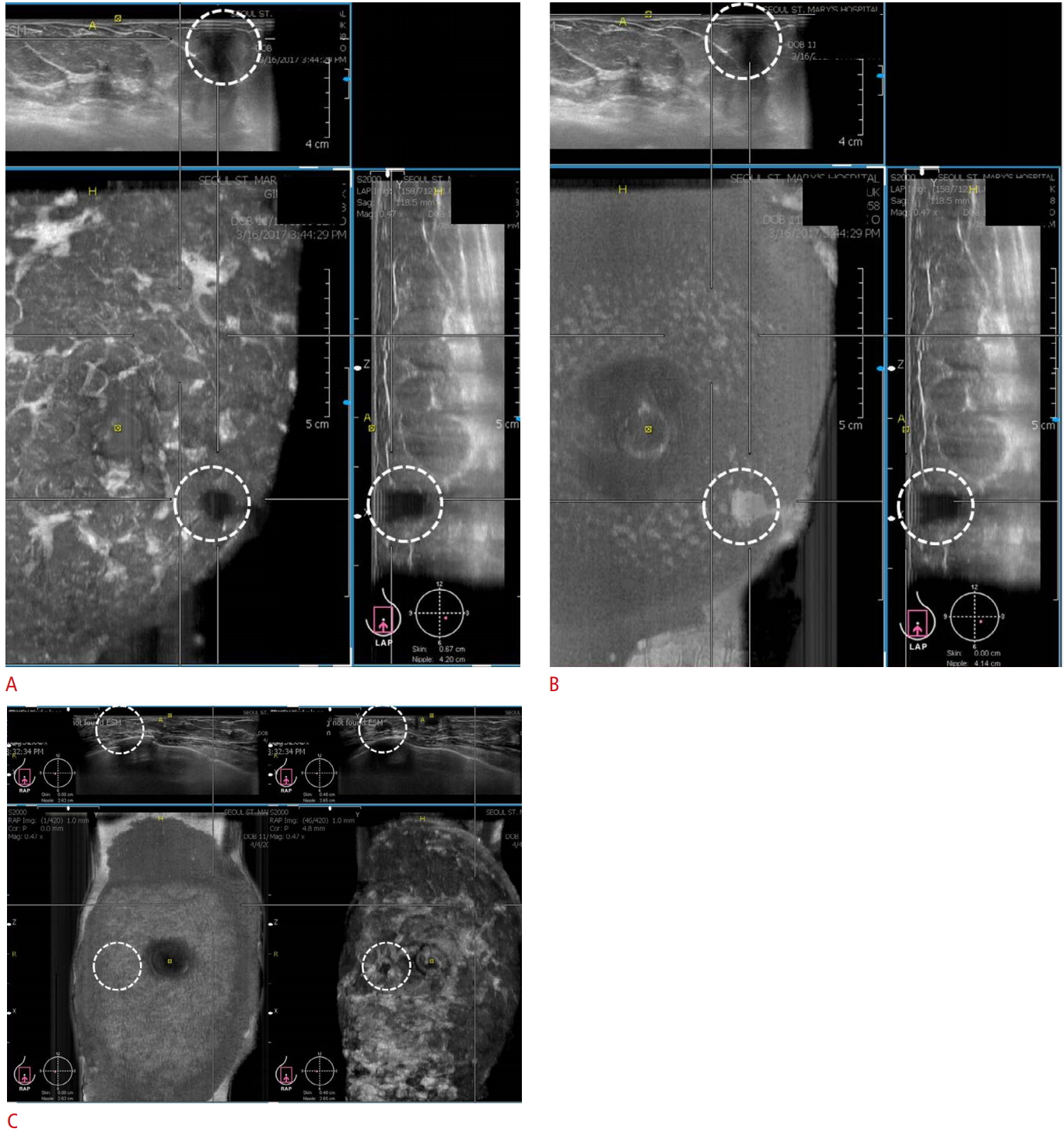
A contact artifact appears as an irregular hypoechoic to anechoic lesion depending on the area of poor contact between the transducer and skin (Fig. 8A). It can mimic a breast mass.
The difference between a true lesion and this artifact is that the hypoechoic area of this artifact changes to an echogenic area at the skin layer (Fig. 8B). Contact artifacts occur in the superficial portion of the breast, from the skin layer to the subcutaneous fat layer (Fig. 8A). However, true lesions are located at the layer of the breast parenchyma (Fig. 8C).
Section Thickness Artifacts
A section thickness artifact is associated with echo-signal averaging within the section thickness. It is shown as a false area of sludge or debris with an anechoic cystic structure, and is commonly seen in cysts and the bladder (Fig. 9) [12]. Since a wide fixed focal zone is applied in ABUS, these artifacts occur frequently.
How to Improve the Image Quality of ABUS
Poor image quality in ABUS will ultimately raise questions about its reliability in clinical practice.
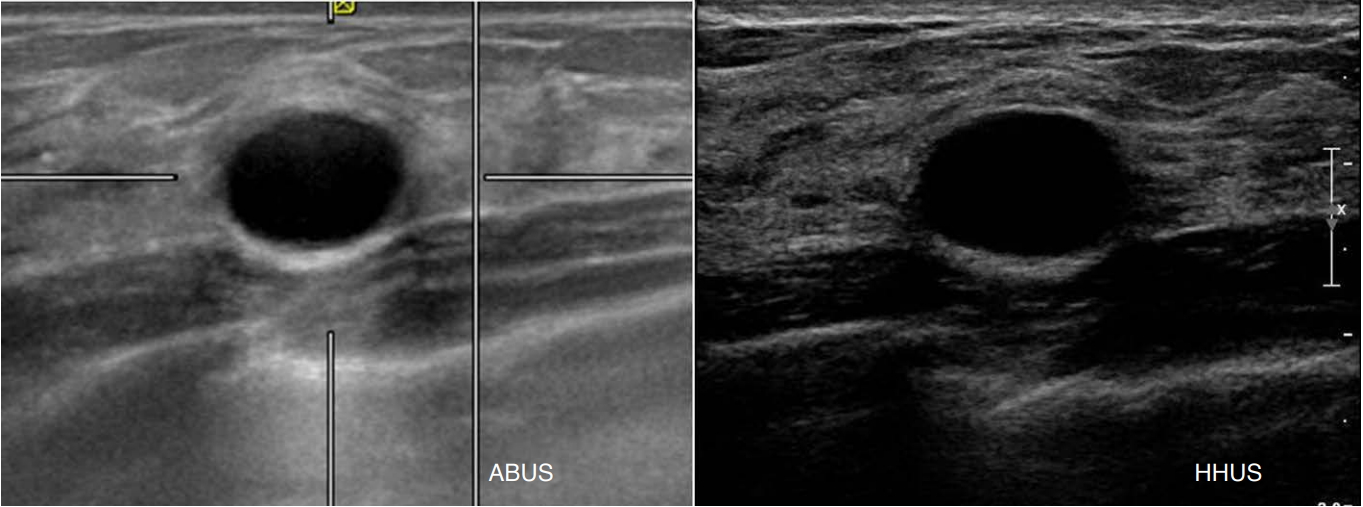
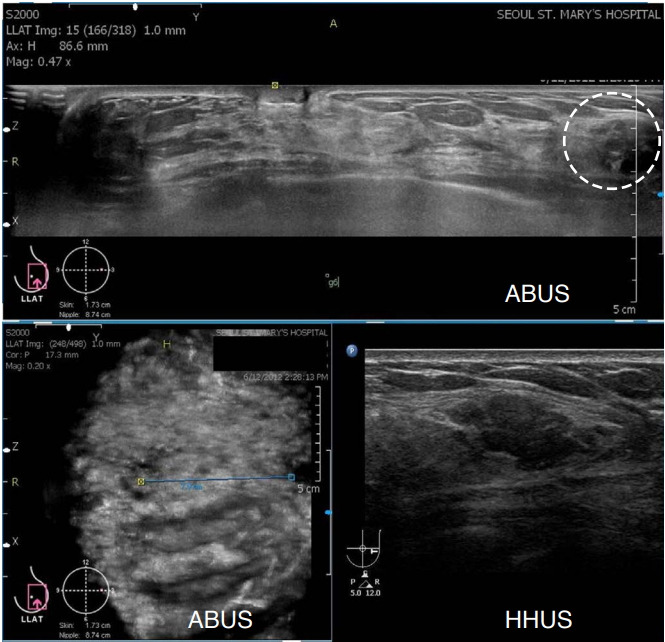
In a prospective study [15] comparing the image quality of HHUS and ABUS using identical image pairs of 411 lesions, the image quality of ABUS was identical or superior to HHUS in 97.1% of cases. However, in 2.9% of the lesions, ABUS was inferior, and in 0.5% of cases, the poor quality of ABUS images posed challenges for image interpretation due to incomplete lesion coverage and shadowing by a contact artifact (Fig. 10).
In order to obtain a good image, basic techniques should be followed, including an abundant use of lotion, full coverage and adjustment of depth, gentle compression, and proper transducer placement.
Nipple shadowing and reverberation artifacts are common. To reduce these artifacts, ABUS systems have adopted new processing algorithms [14]. The reverberation removal algorithm determines whether tissue contact is present and removes data corresponding to areas without tissue contact. The adaptive nipple shadow reduction tool analyzes data to enhance the retroareolar structure. Applying abundant lotion often resolves this problem [9,16].
To reduce motion artifacts, the patients should be instructed to breathe superficially and not to talk during the acquisition [16]. Technicians should check for the presence of a contact artifact before the completion of each acquisition and rescan the image if a contact artifact is present [16].
Innovative means of addressing the challenges posed by artifacts should be put forth. A prospective study showed that gel pad application had an effect on scan coverage and image quality [17]. As the probe moves on the dome-shaped breast horizontally, pressure imbalance and contact defects occur (Fig. 11A). Therefore, image quality can be degraded at the periphery.
A commercial gel pad was used as a specialized coupling agent to improve contact defects (Fig. 11B). As the gel pad was placed on the breast, the breast became more flattened, with fewer contact defects at the periphery. With regard to the coverage of the breast, the area of the breast properly scanned was increased by using a gel pad (Fig. 11C).
In terms of image quality, no significant differences were found between images acquired with or without gel pad application in terms of conspicuity, contrast, degree of retroareolar shadowing, and retroareolar lesion visibility (Fig. 11D, E). When a gel pad is placed on the breast, the deep portion may not be included in the scanning field because of the thickness of the gel pad. Therefore, it is necessary to consider the gel pad thickness when making tissue depth adjustments (Fig. 11F).
In another study, the authors proposed an automated image quality assessment system [9] that evaluated the three aspects of nipple position, nipple shadowing, and breast shape at the point of image acquisition. They applied image processing and machine learning algorithms on ABUS images. The algorithms detected approximately 55% of images that were rated as low-quality. They concluded that the algorithm was fast and reliable.
Conclusion
The image quality and diagnostic performance of ABUS and HHUS are comparable. However, in some cases, image artifacts such as nipple shadowing, reverberation artifacts, and improper acquisition might be obstacles to the optimization of image quality. Hence, radiologists and technologists need to be familiar with these image defects and how to reduce and utilize them, where possible, for proper diagnoses to be made in clinical settings.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




