Introduction
For ultrasonography (US), contrast agents were first introduced in 1996 and have since been used mainly for echocardiography, vascular US, Doppler US, and abdominal US in Europe and Asia. Although contrast-enhanced US (CEUS) has several advantages over the contrast-enhanced computed tomography (CECT) or contrast-enhanced magnetic resonance imaging (CEMR), such as no radiation, no harmful effects to the kidney or thyroid, easy accessibility, and comparable comfort during use with patients [1], it has not been widely used in abdominal applications. In the case of first-generation US contrast agents, early breakdown of microbubbles limits the contrast effect during continuous scanning. With the development of second-generation US contrast agents in 2001 and the advances of the contrast-specific mode with a low-mechanical index (MI) technique in US machines, a real-time evaluation has become possible, resulting in an increasing interest in US contrast agents and CEUS. In this review, we discuss the principles and types of US contrast agents and the clinical application of CEUS with a focus on abdominal imaging.
US Contrast Agents
General Principles
Conventional grayscale US is used to obtain anatomical information, and Doppler US can be performed if information about blood flow is needed. However, Doppler US can depict blood flow information only in relatively large vessels due to a low signal-to-noise ratio, and cannot evaluate the blood flow of microvessels and tissue perfusion [2]. US contrast agents overcome this limitation by their physical properties. US contrast agents consist of microbubbles containing air or various gases within a shell. When a US contrast agent is administered into the vasculature, it enhances the backscatter of the ultrasound waves by resonance within sonic windows [3]. This results in a marked amplification of the signals from the blood flow and provides additional information about the microvasculature [2]. By using a US contrast agent and the contrast-specific mode of the US machine, dynamic CEUS images can be obtained in the same manner as CECT or CEMR images, but with different enhancement patterns that are not always comparable with those of CECT or CEMR. This is attributed to the fact that a US contrast agent is retained only within the blood vessels (known as blood-pool contrast agent), whereas a contrast agent of computed tomography (CT) and magnetic resonance imaging (MRI) moves into the extracellular space after administration until the concentration of the contrast agent is balanced between the intravascular space and the extracellular space [4].
The MI is defined as the peak rarefactional (or negative) pressure divided by the square root of the ultrasound frequency. Most commercially available US machines display the MI values on their screens, and an MI value of 0.1-2.0 (mostly more than 1.0) is usually used for conventional grayscale US [2,5]. The MI value is related to the insonation power of the microbubble within the ultrasound field. At a very low MI, microbubbles stay static and only play a role in the scattering of the US beam. As the MI increases, microtubules oscillate at their resonance frequency linearly (MI < approximately 0.2) or nonlinearly (approximately 0.2 < MI < 0.5). In cases where the MI is more than 0.5, microbubbles oscillate strongly and expand beyond their limit, resulting in a disruption of the bubbles [2,5]. CEUS images can be created from either the signals of the nonlinear oscillation of microbubbles or the signals from the microbubble destruction [2].
To detect specific signals from a small amount of US contrast agent, the use of the contrast-specific US mode is essential [6]. Linear signals at a very low MI are generated from both the US contrast agent and the tissue; these signals are too similar to discriminate even with the most-recently developed US technology. Nonlinear signals generated by the US contrast agent can be separately detected by the CEUS-specific mode, and then, the corresponding CEUS images can be generated. Unfortunately, nonlinear signals are also generated by the tissue, and US equipment and technology that can discriminate between these signals and the US contrast agent signals are still under investigation. Because nonlinear signals from the tissue and the US equipment are proportional to the MI, the second-generation US contrast agents, which are used in the case of low MI, offer an advantage by decreasing non-US contrast agent signals [6,7].
Generations and Types
The generation of US contrast agents is categorized according to the type of gas within the microbubble shells. The first-generation US contrast agent, Levovist (Bayer Schering Pharma, Berlin, Germany), was introduced in 1996 and consisted of air within a shell of galactose microparticles (99.9%)/palmic acid (0.1%). After intravenous administration, it distributed itself within the blood pool within a few minutes, and about 2 minutes after its injection, the late phase or the liver/spleen-specific phase could be obtained [4,8,9]. The mechanism for the specific uptake of Levovist in the liver and spleen is not yet completely understood, but it is thought to be similar to the 99mTc-colloid uptake in scintigraphy or the super paramagnetic iron-oxide uptake in the reticuloendothelial system in liver MRI [10]. The mean size of Levovist microbubbles is 2-5 μm, and 97% of them are less than 7 μm in size. This size is sufficient for them to pass through the microcapillaries of the lung, which have a mean diameter of 7 μm without embolisms, and to travel to the left ventricle of the heart or liver for the generation of CEUS images [3,8].
The air within the Levovist contrast is made up of small molecules and can easily diffuse through the microbubble shell into the blood. Furthermore, because of the high solubility of air in blood, the diffused air outside the microbubbles can easily dissolve into the blood more quickly than preferred [3]. To improve the stability of the microbubbles, there was an attempt to make a shell with a polymer-based material, but it was not successfully commercialized due to poor image quality [3]. However, another effort to stabilize microbubbles by replacing air with a more inert and slowly diffusing gas such as sulfur hexafluoride or perfluorobutane was successful, and second-generation US contrast agents such as SonoVue (Bracco, Milano, Italy), Definity (marketed in North America as Luminity by Lantheus Medical Imaging, North Billerica, MA, USA), Optison (GE Healthcare, Princeton, NJ, USA), and Sonazoid (GE Healthcare, Oslo, Norway) were introduced. Definity consists of octafluoropropane gas within a lipid shell, and Optison consists of octafluoropropane within an albumin shell. Both have been approved only for cardiac applications [3,6]. SonoVue consists of sulfur hexafluoride (SF6) within a phospholipid shell. SF6 is an inert molecule that does not interact with any other molecule in the body [2]. After destruction of the microbubble, SF6 gas is excreted only through the lungs without any excretion through the kidney or the liver. The shell consists of a monolayer of an amphiphilic phospholipid. As the outer side of the shell, which is in contact with blood, has hydrophilic properties and the inner side has hydrophobic properties, the shell can stably contain SF6 gas [2]. SonoVue was initially approved for the evaluation of the heart and macrovascular (cerebral and peripheral arteries, and the portal vein) and microvascular structures (characterization of focal lesions in the liver and the breast) [6]. However, its cardiac application has been temporarily suspended by the European Medicines Agency (EMEA) [11]. Sonazoid consists of perfluorobutane within a hydrogenated egg phosphatidylserine (HEPS) shell. In contrast to SonoVue, Sonazoid can be used to obtain both vascular-phase and Kupffer-phase images, which are usually obtained 10-15 minutes after the administration of the US contrast agent. In the Kupffer phase, microbubbles are trapped by the Kupffer cells and lead to a homogeneous enhancement in normalfunctioning liver parenchyma [3]. Sonazoid has only been approved in Japan and Korea for the evaluation of focal liver lesions.
A high MI (MI > 0.7) was used for the first-generation US contrast agents. With the high-MI technique, CEUS images are created using signals from microbubble destruction; hence, the continuous acquisition of CEUS images is impossible. As a result, only intermittent scanning can be performed for a few seconds, and during the CEUS examination, images are recorded frame by frame. With some intervals, CEUS images can be obtained again if the undestroyed US contrast agent is replenished. Recorded images can be reviewed frame by frame after the examination without any time constraint [8,12]. In comparison, the low-MI technique (MI < 0.3) can be applied to second-generation US contrast agents, and continuous, real-time scanning is possible in this case [8].
Safety Considerations
A US contrast agent is excreted via the lung after the destruction of the microbubbles; hence, it is not nephrotoxic. Further, it does not contain iodine, thereby not having any effect on the thyroid functions. Although the US contrast agent is very safe, it can be regarded as a foreign material by the immune system; therefore, a hypersensitivity reaction is possible [1]. The incidence of a severe hypersensitivity reaction was reported in about 0.002% in largescale abdominal application studies [13,14]. The overall reported incidence of the hypersensitivity reaction was less than that occurring with the use of an iodine contrast agent in CT and was similar to that of the use of a gadolinium chelate contrast agent in MRI [1].
Contraindications of SonoVue are a recent acute coronary syndrome or clinically unstable ischemic cardiac disease, right-toleft shunts, severe pulmonary hypertension, uncontrolled systemic hypertension, and adult respiratory distress syndrome [11,15]. Recently, the use of SonoVue in echocardiography has been temporarily suspended because of possible severe hypertension, bradycardia, cardiac arrest, and acute myocardial infarction, which were mostly reported during echocardiography as an idiosyncratic hypersensitivity reaction [11]. Sonazoid is contraindicated in patients with right-to-left shunts, severe pulmonary hypertension, and adult respiratory distress syndrome. It should not be used or, if it must be used, it should be used with extreme caution in patients with egg allergies, because the Sonazoid shell is made of HEPS sodium [15]. Because the safety of SonoVue and Sonazoid has not been evaluated in pregnant women, in women who are breast-feeding, or in patients who are younger than 18 years of age, both US contrast agents should be avoided in these patients.
Although not yet proven in vivo with human subjects, there is a possibility that microbubbles with insonation can cause harmful effects to cells or tissue, such as microvascular rupture, hemolysis of red blood cells, increased heating around the US contrast agent, and killing of phagocytic cells that have engulfed the US contrast agent [14]. The European Federation of Societies of Ultrasound in Medicine and Biology (EFSUMB) guidelines have recommended caution as damage to the microvessel can be clinically harmful to the eye or the brain [16]. According to previous in vivo animal studies [14], an MI value of more than 0.4 rapidly accelerates this harmful biological effect; hence, MI values should be maintained as low as possible during the entire examination.
Clinical Application of CEUS
Guidelines
The need for CEUS guidelines increased in the early 2000s and discussion on this matter began at the EUROSON Congress in 2003. The first version of a CEUS guideline was published in January 2004 [4]. This guide dealt with the general considerations for CEUS, including an overview of US contrast agents, imaging techniques, and safety considerations. In terms of clinical applications, the first EFSUMB guideline focused on the evaluation of focal liver lesions [4]. The updated version, which was published in 2008, included the monitoring of focal liver lesions after ablative treatment and applications to other organs, such as applications to the kidney and pancreas, transcranial US, and US for blunt abdominal trauma [6]. A recent guideline update dealt with nonhepatic applications of CEUS, such as applications to the gastrointestinal tract, spleen, vesicoureteral reflux, scrotum, lung and pleural lesions, breasts and inflammatory joints [16]. In contrast to its use in Europe, CEUS has not been widely used in North America. The main reason for this is that the second-generation US contrast agents, which can be used for applications other than cardiac indications, have not yet been approved by regulatory agencies such as the United States Food and Drug Administration (USFDA). Recently, the revised American Association for the Study of Liver Disease (AASLD) practice guideline for the management of hepatocellular carcinoma (HCC) removed CEUS from its diagnostic flow algorithm because the second-generation US contrast agents used for liver imaging are not yet approved in the United States and because intrahepatic cholangiocarcinoma (ICC) might be falsely diagnosed as HCC on CEUS [17]. However, because there is criticism about the updated AASLD guideline, and phase III trials of liver CEUS are ongoing [18,19], CEUS might be included in future diagnostic guidelines with broader applications.
Evaluation of Focal Liver Lesions
Dynamic contrast enhanced images of the liver can be obtained with US contrast agents similar to CECT and CEMR. The arterial phase starts about 10-20 seconds and lasts for 30-45 seconds after the administration of a US contrast agent. Vascularity of the focal lesion can be evaluated during the arterial phase. The portal venous phase (PVP) starts at 30-45 seconds and continues for 2-3 minutes [16]. On the portal-venous-phase images, the degree of washout between the focal liver lesion and the adjacent liver parenchyma can be compared [4]. “Washout” is defined as the transition of hyperenhancement or isoenhancement to hypoenhancement as compared to the adjacent normal liver parenchyma [16]. In comparison to SonoVue of which only 7.3% is phagocytized by Kupffer cells, 99% of injected Sonazoid is phagocytized by Kupffer cells, and thus, a Kupffer-phase image can be additionally obtained about 10-15 minutes after the administration of Sonazoid [20]. The equilibrium phase of CECT or CEMR does not exist on CEUS. This is because the US contrast agent is a pure intravascular contrast agent, and the concentration equilibrium of the US contrast agent between the extracellular space and the intravascular space cannot be achieved [6].
Detection of Focal Liver Lesions
The sensitivity for detecting liver metastasis has been reported to be about 87%-91% even with Levovist [21]. However, there were limitations for the evaluation of the whole liver because of insufficient examination time due to the instability of the US contrast agents, a result of microbubble destruction occurring during examinations. The sensitivity was reported to be about 79%-100% with second-generation US contrast agents, which is significantly higher than that of conventional grayscale US and comparable with that of CT [21-24]. For a metastasis smaller than 1 cm, the sensitivity improved markedly from 29.1%-35% to 63.3%-76.6% upon the use of US contrast agents as compared to that in the case of conventional grayscale US; this sensitivity improvement was comparable with that of CT [24]. Recently, preoperative or postoperative chemotherapy has been frequently performed in patients with colorectal cancer in order to decrease the tumor burden or to kill the possible remnant cancer cells. Chemotherapy can insult the liver parenchyma and lead to fat depositions, resulting in a texture change of the liver and decrease in the contrast resolution between the metastasis and the adjacent liver parenchyma. As the metastasis presents with hypoenhancement as compared to the adjacent liver parenchyma during the PVP or the late phase, CEUS can improve the diagnostic sensitivity for detecting metastasis in these patients from 63.2% to 79.5%, and thereby enable more accurate surgical planning [25]. Further, intraoperative CEUS can help to accurately diagnose the retracted liver metastasis after chemotherapy, and its diagnostic performance is comparable with that of MRI [26,27]. However, there have also been conflicting results that show multiple detector computed tomography (MDCT) having better sensitivity than CEUS, and surgical plans have also been changed in some patients because of a metastasis that was not detected on CEUS but was detected on CT [26,28]. Hence, CEUS cannot yet solely replace MDCT in the evaluation of liver metastasis, and further studies on the diagnostic performance of CEUS with second-generation US contrast agents and a state-of-the-art CEUSspecific mode are warranted.
Differential Diagnosis of Focal Liver Lesions
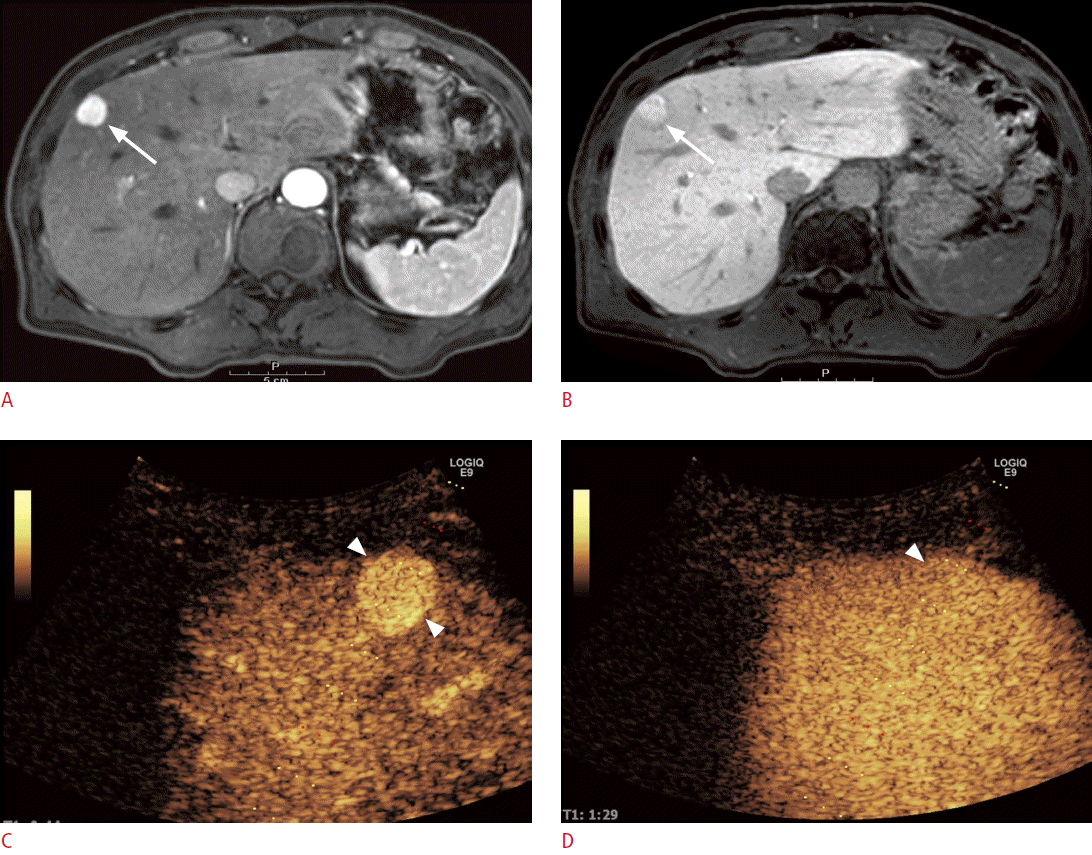
On CEUS, most benign lesions show hyperenhancement or isoenhancement as compared to adjacent normal liver parenchyma in the PVP (Fig. 1), whereas malignant lesions usually present as hypoechoic in the PVP or the delayed phase (Fig. 2) [6]. According to the meta-analysis studies, CEUS can accurately differentiate between benign and malignant focal liver lesions with a sensitivity of 93% and a specificity of 90%, and the diagnostic performance of CEUS is similar to that of CECT and CEMR [29,30]. In some reports, CEUS has been reported to provide a conclusive diagnosis when focal liver lesions were incidentally found on conventional grayscale US [31,32]. Furthermore, CEUS could improve the diagnostic accuracy from 42%-44% to 89%-92% for lesions that have been found to be inconclusive on CECT and improve the diagnostic confidence level [32]. Hence, CEUS can be used as a second-line diagnostic tool for the evaluation of incidentally found indeterminate focal liver lesions on conventional grayscale US or contrast-enhanced CT.
Most lesions that are less than 1 cm in size in patients with an extrahepatic malignancy are too small to characterize on CECT. The differential diagnosis of these lesions between metastases and benign lesions such as hepatic cysts is important for treatment planning. In general, conventional grayscale US is used as a first-line tool for differential diagnosis because it can accurately differentiate cysts from solid lesions. However, some lesions are also inconclusive on conventional grayscale US, and additional assessment with MRI or intraoperative US may be needed for the differential diagnosis. In a previous study, CEUS was used to successfully diagnose 98.5% of the inconclusive cases on both CECT and conventional grayscale US and led to changes in the treatment plans in 11.6% of these patients [33].
Conventional grayscale US has been used as a screening tool in patients with chronic liver disease due to its noninvasiveness, relatively low cost, lack of radiation exposure, and easy accessibility. However, the reported diagnostic accuracy for HCC was not considered sufficient, particularly for small lesions, even though early detection is a major prognostic factor for improving patient survival [34]. Furthermore, nodules other than HCC, such as regenerative nodules or dysplastic nodules, commonly accompany in a cirrhotic liver, and as HCC can present with nodules with various echogenicities, conventional grayscale US is limited in the detection and differentiation of HCC in patients with chronic liver disease [35]. For the differential diagnosis of the detected nodules, understanding the vascularity within a lesion is crucial, and usually CECT or CEMR is used as a secondary diagnostic tool after the detection of nodules in a cirrhotic liver. CEUS has also been used to evaluate lesion vascularity with a diagnostic accuracy similar to that of CECT [34]. Small HCCs, those less than 2 cm in size, were accurately diagnosed using CEUS with a sensitivity of 81% and a specificity of 86% [36]. HCC usually appears as a hypervascular lesion in the arterial phase and as a hypovascular lesion in the PVP or the delayed phase [6,35]. However, another problem is that enhancement patterns vary depending on the degree of differentiation. In previous studies, about 13% of HCC did not appear as hypervascular lesions as compared to the adjacent liver parenchyma in the arterial phase, and well differentiated or poorly differentiated HCC tended to appear as lesions other than the hypervascular lesions in the arterial phase. Furthermore, 9% of HCC did not show hypovascular lesions in the delayed phase and 78% of them were well differentiated HCC [37,38]. In short, moderately differentiated HCC tends to show a typical enhancement pattern, which is arterial enhancement and washout in the delayed phase, whereas well or poorly differentiated HCC frequently presents with an atypical enhancement pattern. Hence, careful assessment is needed to evaluate nodular lesions in patients with chronic liver disease [34].
In the comparison of imaging modalities, gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA [86%])-enhanced MRI showed better sensitivity than both contrast-enhanced CT (74%) and CEUS (72%), and when Gd-EOB-DTPA and CEUS were used together in the evaluation, the sensitivity improved up to 90% [39]. In addition, when CEUS and MDCT were used together to assess tumor vascularity, the combination of the evaluation techniques improved the sensitivity for differentiating HCC from benign nodules in patients with liver cirrhosis, by decreasing the false-negative rate [34,40].
Intraoperative CEUS also improved the diagnostic accuracy of HCC. According to a previous report, intraoperative CEUS with Sonazoid could newly detect about 9.4% of HCC, and the surgical extent was changed in 42.9% of patients in whom HCC was newly detected during CEUS [41].
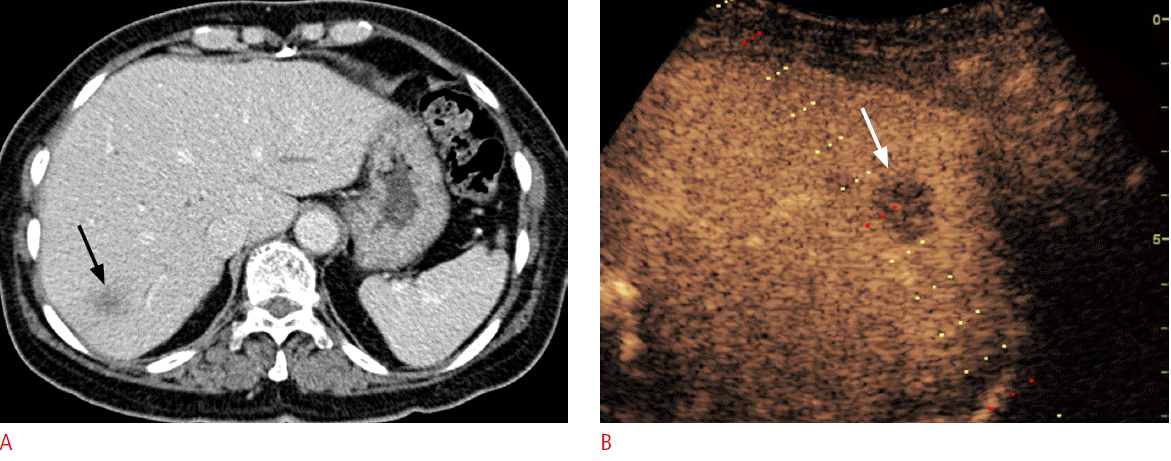
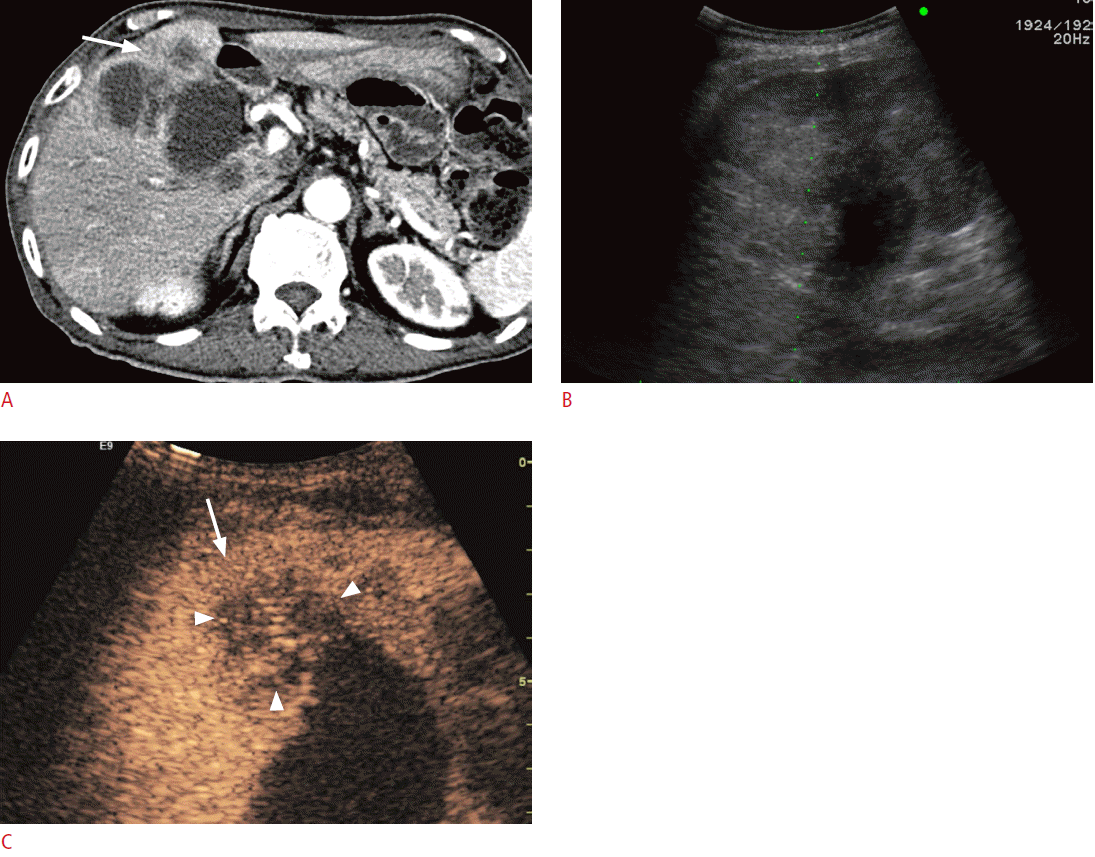
Portal vein thrombosis is frequently observed in patients with liver cirrhosis or HCC. In these patients, differentiation between tumor thrombosis and bland thrombosis is critical to determine treatment plans or to predict prognosis because tumor thrombosis is one of the common excluding reasons for not performing surgery, transplantation, or radiofrequency ablation (RFA) [42]. Furthermore, even if there is portal vein thrombosis without definite evidence of a mass-forming lesion in the liver on conventional US, a careful further examination is needed to check whether there is an undetected infiltrative HCC. Although Doppler US was used to diagnose tumor thrombosis in previous research, the sensitivity and the diagnostic accuracy were only about 20% and 50%, respectively [42]. On CEUS, tumor thrombosis can be diagnosed when there is an enhancement in the arterial phase, in which the sensitivity and the diagnostic accuracy can be improved to 88%-100% and 92.5%, respectively (Fig. 3) [35,42,43].
The diagnostic performance of CEUS was found to be significantly lower in patients with chronic hepatitis than in patients without chronic liver disease [31,44]. This might be due to technical difficulties such as limited time for the whole liver assessment; limited sonic window due to anatomical changes such as hypotrophy of the right hepatic lobe; heterogeneous enhancement of the liver parenchyma due to the arterioportal shunt, which is often found in cirrhotic livers; and the relatively less extensive enhancement of the liver parenchyma as compared to the normal liver [6,35].
In the cost-effectiveness analysis of CEUS as a surveillance tool for HCC in patients with compensated hepatitis C virus-related liver cirrhosis, it was shown that CEUS surveillance using Sonazoid is a cost-effective strategy when the annual incidence of HCC is more than 2% and the sensitivity of CEUS for detecting HCC is more than 80% [45]. However, there is still not enough evidence for using CEUS as a screening tool, and CEUS may rather be used as a problem-solving method, particularly for nodules sized between 1 and 2 cm [38,46].
Sonazoid introduced an additional advantage to CEUS by providing the Kupffer phase, which usually starts from 10-15 minutes to 120 minutes after Sonazoid administration [47]. In the Kupffer phase, areas of normal functional Kupffer cells showed enhancement due to microbubbles being phagocytized by Kupffer cells, with specificity being improved as compared to conventional B-mode US from 89.2%-94.9% to 97.8%-98.2% in HCC detection [48]. The histologically more advanced HCC might appear as more hypoechoic than the adjacent liver parenchyma, which was comparable to the signal intensity difference in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI [49,50]. Another advantage of Sonazoid-enhanced US is the defect reperfusion image in which both Kupffer-phase and arterial phase images can be evaluated simultaneously and with the same slice by reinjection of Sonazoid during the Kupffer phase [51]. According to a previous study, Sonazoid-enhanced US showed better diagnostic accuracy (95%) than CECT (82%) in the depiction of malignant hepatic lesions by using the defect reperfusion technique [52].
Differential diagnosis between ICC and HCC in patients with chronic liver disease or liver cirrhosis is a controversial issue, and the recently updated AASLD guideline removes CEUS from the diagnostic procedure for HCC due to the possibility of the falsepositive diagnosis of HCC in patients with ICC [17]. This decision by AASLD is based on an article that reported that 47.6% of ICC showed homogeneous intense enhancement in the arterial phase and washout in the delayed phase on CEUS, findings that were not distinguishable from HCC [53,54]. However, the following studies showed that the enhancement pattern was somewhat different between the two tumors because HCC is more likely to appear as homogeneous or heterogeneous hyperenhancement, whereas ICC often presents with peripheral rim-like enhancement or heterogeneous hypoenhancement in the arterial phase [55]. In the quantitative analysis with the time-intensity curve, ICC showed a more rapid and marked washout than HCC, although there was significant overlap between the two [56,57]. Because ICC develops in patients with liver cirrhosis or chronic hepatitis, and ICC smaller than 3 cm is more likely to appear as homogeneous hyperenhancement in the arterial phase with delayed washout, a finding also typical of HCC [55,58], careful interpretation of CEUS is needed in smaller nodules that develop in patients with liver cirrhosis or chronic hepatitis.
CEUS-Guided Procedures
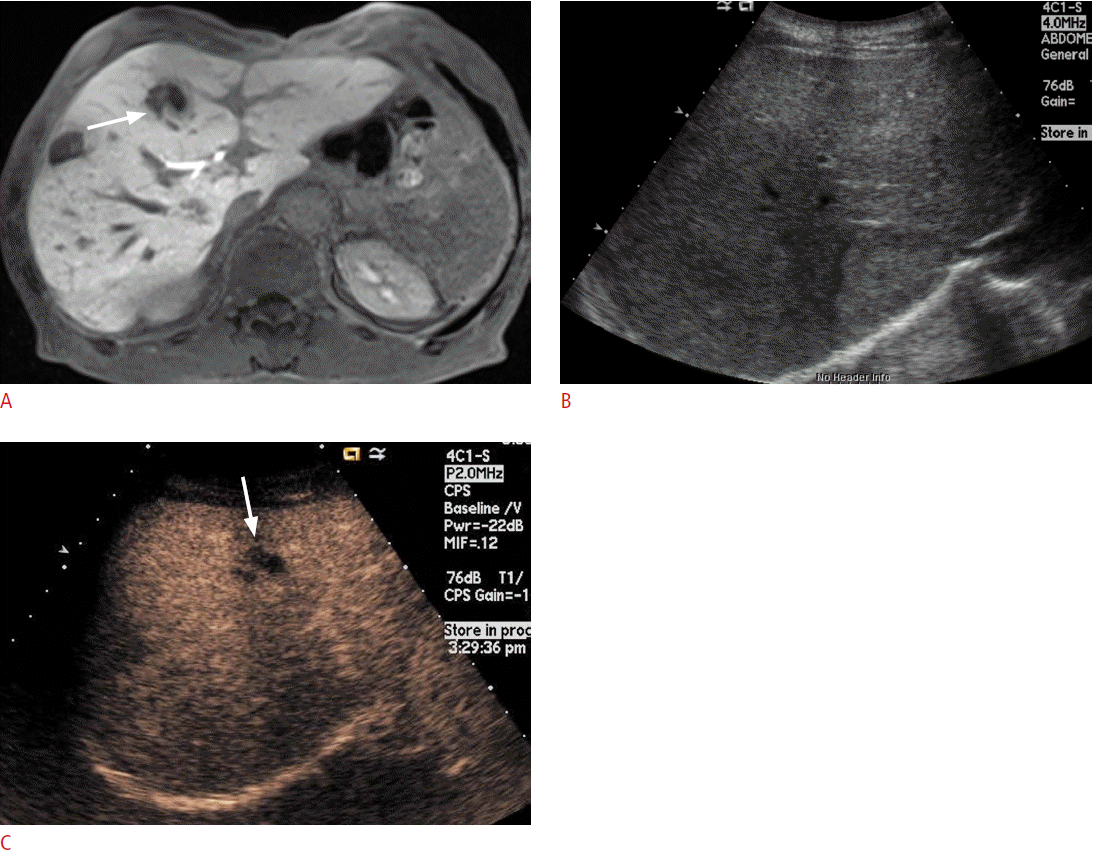
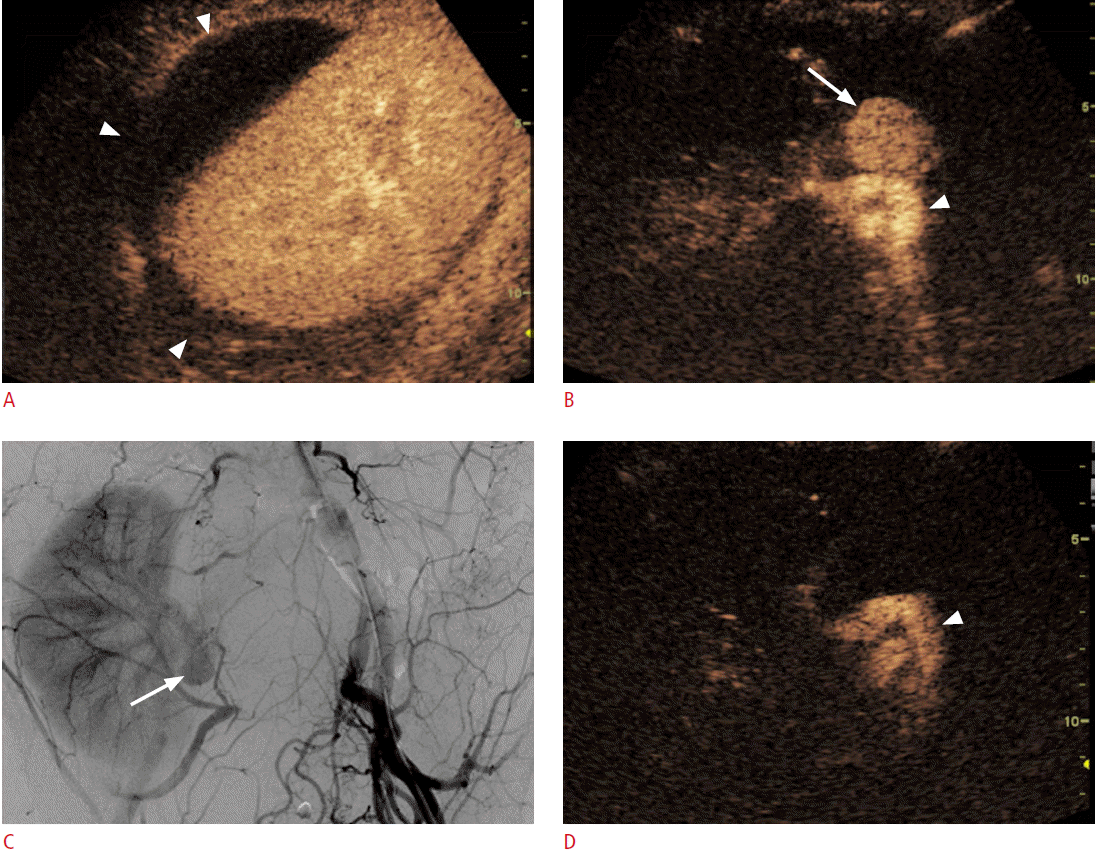
If the lesion was not clearly delineated on conventional US, but was well visualized on CT or MRI, a biopsy can be performed after the administration of a US contrast agent (Fig. 4). In lesions that have a large proportion of central necrosis, it may turn out that tissue is obtained only from the necrotic portion of the liver on a conventional grayscale US-guided biopsy, making a pathologic diagnosis impossible and requiring a re-biopsy. On CEUS, the viable portion of the tumor can be differentiated from the necrotic portion by the presence or absence of enhancement, and a targeted biopsy can be performed at the viable portion of the tumor (Fig. 5).
During the RFA of inconspicuous lesions, fusion imaging with conventional grayscale US and CECT or CEMR can be used for RFA guiding. If the lesion is inconspicuous even with fusion imaging with conventional grayscale US, CEUS can be additionally used. According to a previous study, about 83% of HCC, which was inconspicuous on fusion imaging with conventional grayscale US, was well visualized after the contrast enhancement of US [59]. After RFA of the hepatic tumor, it is essential to evaluate the treatment response. Although CECT has been used for this purpose, there are several limitations, including possible harmful effects of radiation and the iodinated contrast agent, and an inaccurate assessment of the ablated margin. Furthermore, even though a residual viable tumor is detected on CECT, it is sometimes difficult to visualize and target the residual viable tumor on conventional grayscale US, particularly immediately after RFA [60]. Because CEUS can overcome these disadvantages, it can be used in the evaluation of the post-RFA response. For hypervascular lesions such as HCC, if the intratumoral vascularity, which was noted before treatment, disappeared on postprocedural CECT or CEUS, complete ablation of the tumor can be confirmed. For hypovascular lesions such as metastasis, successful ablation can be confirmed if the ablated zone does not show enhancement on CECT or CEUS, including the target lesion. It is important to secure a sufficient safety margin (>5 mm) to ablate satellite nodules around the main lesion and to prevent marginal recurrence. According to previous studies, the diagnostic accuracy of CEUS (93.8%- 100%) for the evaluation of the RFA treatment response with either SonoVue or Sonazoid was comparable with CECT or CEMR [47,60- 63]. The optimal time to assess the treatment response to RFA is 3 hours after the procedure because the treated tumor margin is the most clearly perceived at this time [60]. Uniform rim enhancement can be seen up to 30 days after RFA, and this rim enhancement should not be misdiagnosed as marginal tumor recurrence [6]. Efforts have also been made to evaluate the treatment response after trans-arterial chemoembolization (TACE) with CEUS. Although CEUS could detect residual intratumoral vascularity after TACE in previous studies, it is not feasible to use it as an evaluation tool after TACE because of the difficulty in evaluating multiple lesions, deeply located lesions, and poorly enhancing or diffusely growing lesions [63,64].
Quantitative Analysis
The most widely used assessment tool for treatment response is the response evaluation criteria in solid tumors (RECIST); these criteria are based on the serial change in the lesion size. Recently, antiangiogenic agents have been developed for several diseases, and concerns have been raised that an evaluation of only size is not sufficient for an accurate assessment. Hence, the recently suggested modified RECIST (mRECIST) and Choi criteria aim to include not only the size changes but also the changes in enhancing patterns within the tumor on contrast-enhanced images [65,66]. Besides studies on conventional CECT or CEMR, perfusion studies have been performed to predict the treatment response as early as possible, but radiation exposure during perfusion CT and a lack of standard protocols or the technical complexity of perfusion MRI limit the generalized use of perfusion imaging for tumor assessment. CEUS can potentially assess the response by a perfusion study because it is technically easy and sufficiently feasible for daily practice and there is no harm from radiation or from the contrast agent. Furthermore, several quantitative parameters such as the degree of peak enhancement, mean transit time, and time to peak (TTP) enhancement can be obtained by analyzing the time-intensity curve [16]. In previous studies, quantitative parameters calculated from CEUS showed a moderate correlation with that of CECT [67,68]. Further, peak enhancement, regional blood volume, regional blood flow, and rise time have been reported to be significantly higher in the case of benign focal liver lesions than in the case of malignant lesions [69,70]. Perfusion CEUS can also predict the microvessel status within the tumor [71-73], and the treatment response of the anti-angiogenic regime shows that the TTP enhancement increases significantly in the responder group as compared to the nonresponder group [74].
There are some drawbacks of a CEUS quantitative perfusion study. Quantitative parameters from CEUS perfusion can affect various conditions such as acoustic attenuation, field differences, US system settings, and cardiac output. Therefore, normalization of such quantitative parameters has been recommended to solve this problem [67]. Furthermore, motion correction is still not possible in CEUS perfusion studies; hence, the technical success rate was found to be lower than that of CECT (100% vs. 70%) [68]. Lastly, just as CEUS is used for diagnostic purposes, a quantitative analysis with CEUS cannot be routinely performed for deep-seated lesions or in patients with severe fatty liver because most of the ultrasound beam is scattered or reflected before reaching the targeted lesion.
Evaluation of Other Organs
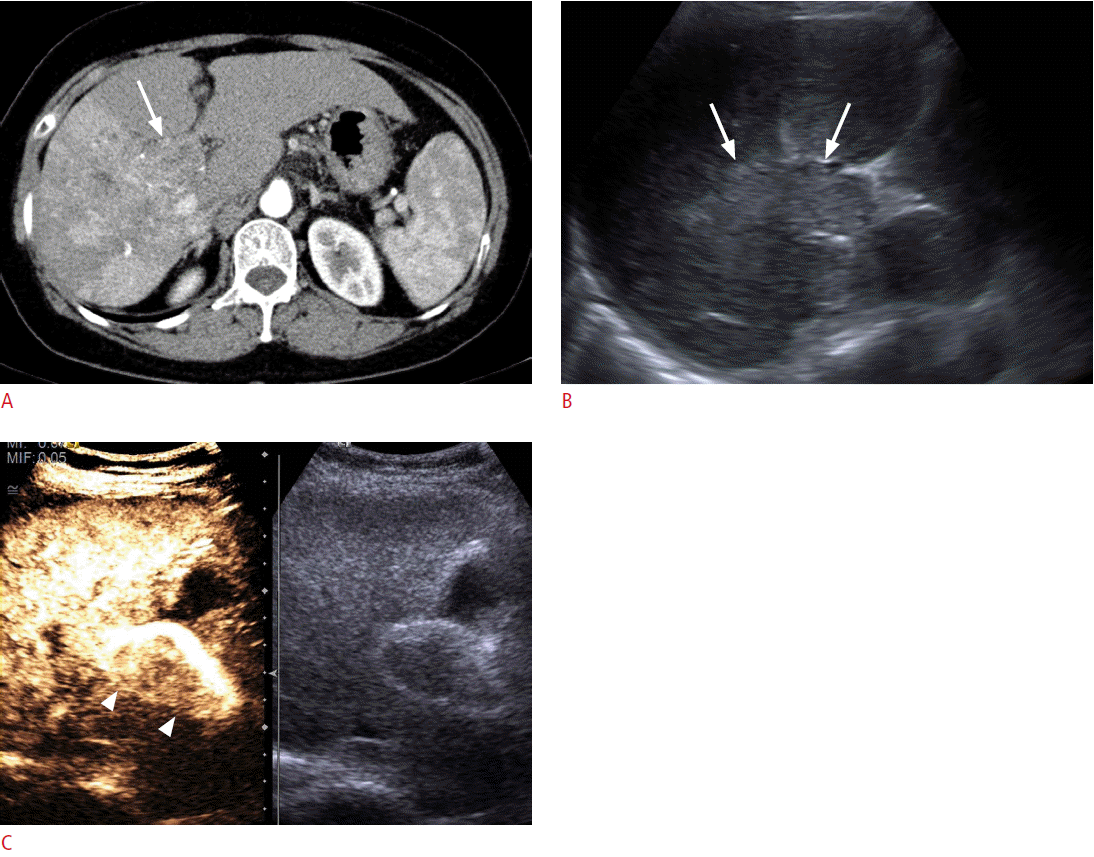
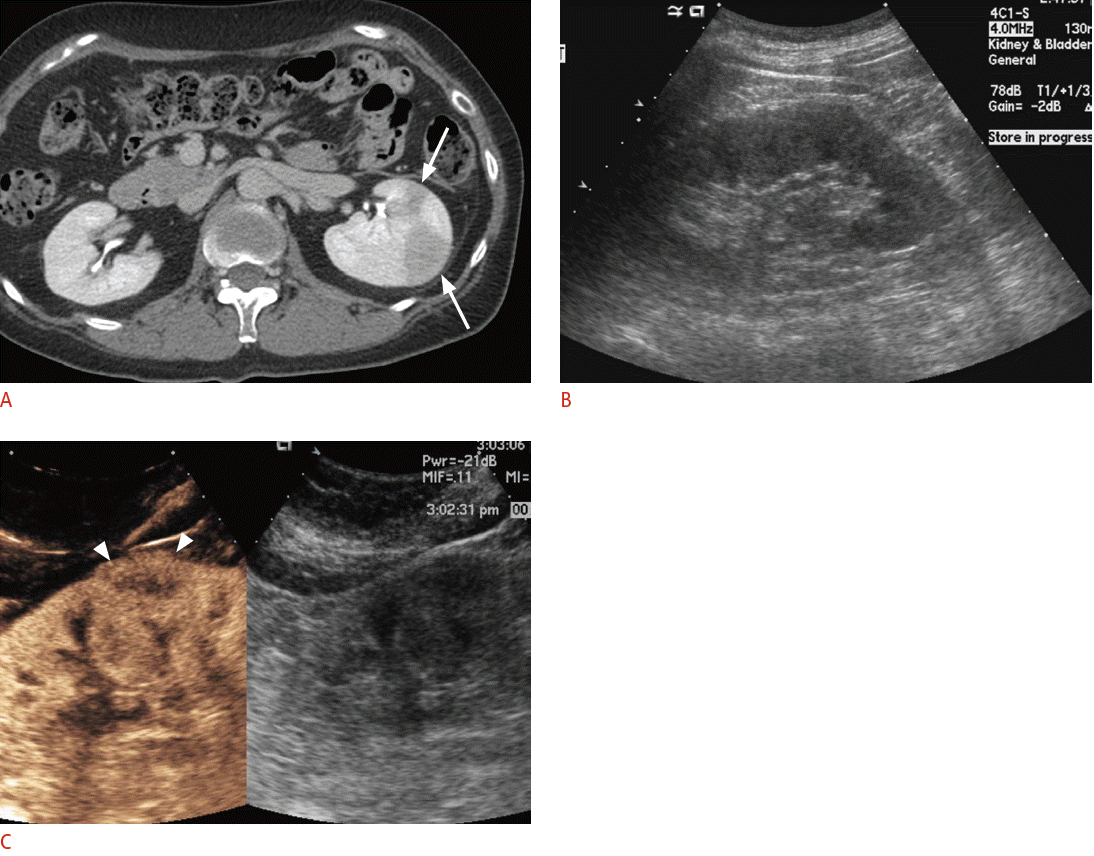
For the differentiation between neoplasms and pseudotumors, such as the column of Bertin or the dromedary hump in the kidney, CEUS can be used. If the enhancement pattern is similar to the adjacent renal parenchyma and there is no mass effect on the renal vessel, the mass of interest is more likely to be a pseudotumor than an actual neoplasm. CEUS can also delineate lesions that are well visualized on CECT but are not noted on conventional grayscale US (Fig. 6). For complex cystic lesions, the presence or absence of enhancing mural nodules and thick irregular septa can be evaluated with CEUS in addition to Doppler US [6]. After kidney transplantation, CEUS can be helpful in the evaluation of vessels and their anastomosis sites if CECT is not feasible due to a renal function that is not yet restored or stabilized (Fig. 7). In the case of pancreatic applications, CEUS is still used to acquire additional information after cross-sectional imaging, rather than for the detection or differentiation of focal pancreatic lesions [16]. Most pancreatic adenocarcinomas appear as hypovascular on CEUS, and CEUS can visualize the tumor margin and the relationship between a tumor and its adjacent vessels more clearly [75,76]. Enhanced mural nodules are very suggestive findings for malignant cystic pancreatic neoplasms, and CEUS can differentiate a mural nodule from a hematoma or a sludge ball [16]. CEUS can improve the visualization of the aortic lumen and an endoleak in patients with abdominal aortic aneurysm or dissection. CT angiography can also accurately detect an endoleak, but CEUS can provide additional information for identifying the endoleak type by a real-time examination (Fig. 8) [16]. Although CECT is still the gold standard for evaluating blunt abdominal trauma, CEUS can be an alternate option in patients with low-energy blunt abdominal trauma in which CECT does not seem mandatory but in which an abdominal evaluation is still needed [6]. In terms of a diffuse liver injury, CEUS can depict injured areas without requiring that patients be moved to the CT room even if conventional B mode US cannot detect any abnormalities in the liver [77].
Conclusion
CEUS has unique advantages over CECT and CEMR, as US contrast agents are safer than iodinated or gadolinium chelate contrast agents given that they have no effect on the kidney and thyroid function while having no radiation and being easily accessible. The development of second-generation US contrast agents that enable an actual real-time study with low-MI techniques boosts the use of CEUS in many applications. The detection rate and the diagnostic accuracy of focal liver lesions can also be improved by CEUS. Although there is debate on whether CEUS plays a crucial role in the management of HCC, CEUS may improve diagnostic performance in patients with liver cirrhosis or chronic hepatitis. CEUS can also be applied to organs other than the liver; some examples of such applications are the detection and differentiation of kidney lesions, clearer delineation of pancreatic cancer and the adjacent vessels, evaluation of aortic or other vascular abnormalities, and the evaluation of blunt abdominal trauma. For patients who receive antiangiogenic drugs, it has the potential to be a tool complementary to cross-sectional imaging in the assessment of the treatment response, while providing additional enhancement or perfusion information along with size changes. In summary, CEUS exhibits a better diagnostic performance than conventional grayscale US and an accuracy similar to that of CECT or CEMR in the evaluation of liver and vascular diseases, although there are some limitations due to the physical properties of US. Having the advantages of no radiation exposure or no nephrotoxicity, CEUS may be more widely used as a first-line or problem-solving tool in the future.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




