Introduction
Lymphoma involving the abdomen usually demonstrates on ultrasonography as a homogeneous mass with marked hypoechogenicity. Non-homogeneous echogenicity is considered an unlikely finding for lymphoma, essentially because lymphoma lesions are mostly homogeneous and rarely demonstrate necrosis [1,2]. Post-transplantation lymphoproliferative disorder (PTLD) represents a range of lymphoid proliferation, from abnormal lymphoid hyperplasia to malignant lymphoma, that occurs after organ transplantation and administration of immunosuppressive agents. Immunosuppression and Epstein-Barr virus (EBV) infection are the two major risk factors associated with the development of PTLD. The imaging findings of lymphoma associated with PTLD can be nonspecific and atypical. Here, we describe a lymphoma case associated with PTLD that presented on ultrasonography as a mass with striking heterogeneous echogenicity. This study was approved by the Institutional Review Board of our institution, and a waiver of informed consent was obtained.
Case Report
A 30-year-old man with nephrotic syndrome underwent successful renal transplantation from a cadaveric donor. Prior to transplantation, he had been diagnosed as a hepatitis B virus carrier with liver cirrhosis and had been treated with a stent graft for aortic dissection. After transplantation, he received a triple immunosuppressive regimen that consisted of tacrolimus (4 mg/ day), mycophenolatemofetil (MMF; 2,000 mg/day), and prednisolone (5 mg/day). Seventeen months after transplantation, he denied any specific symptoms, but his laboratory tests showed elevated aspartate transaminase and alanine transaminase levels during regular follow-up.
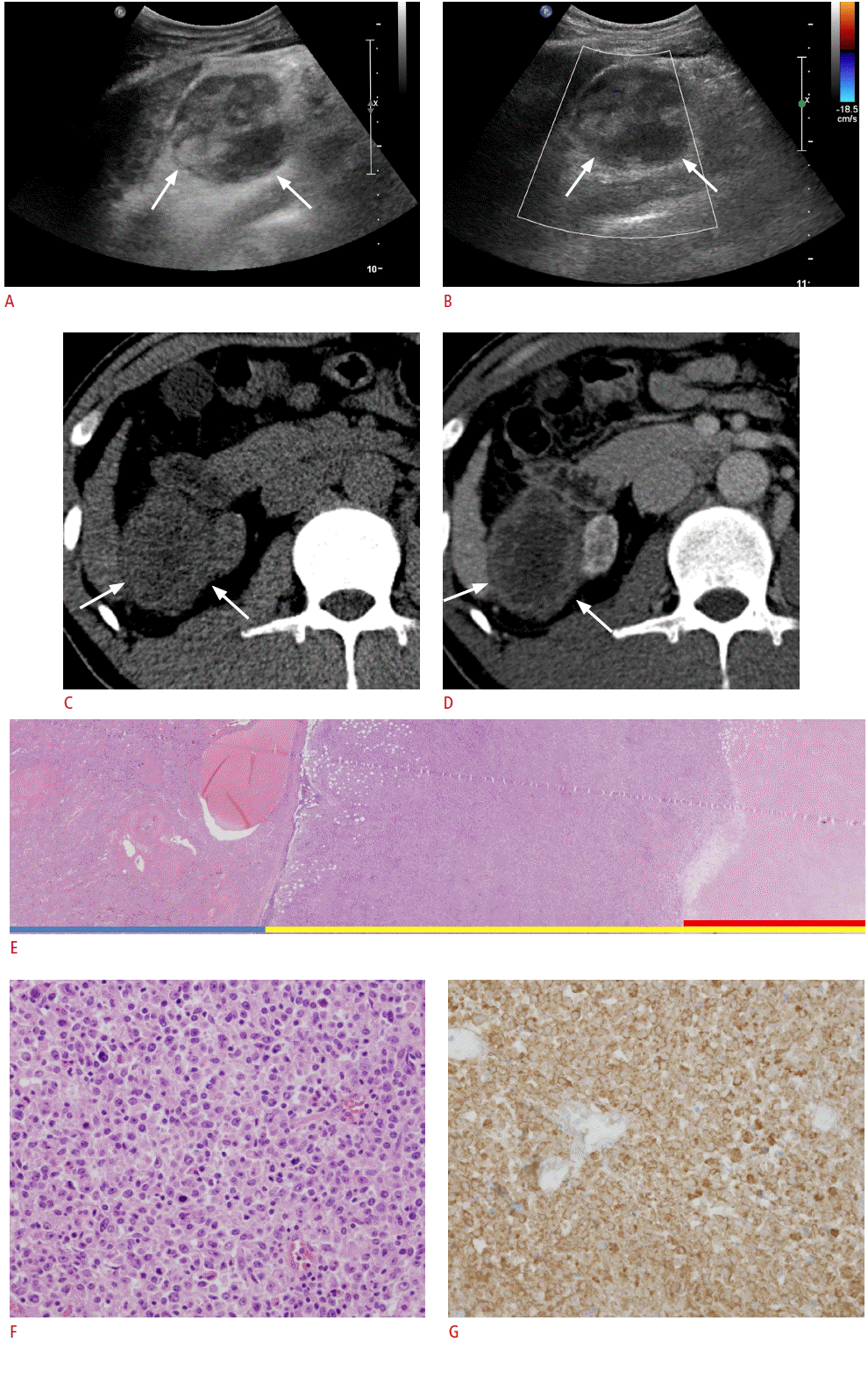
Abdominal ultrasonography for further evaluation of hepatitis was performed using Philips iU22 Ultrasound System (Philips Healthcare, Best, The Netherlands) with a 1.0-5.0-MHz (C5-1) wideband convex transducer. Underlying liver cirrhosis with multiple nodular lesions and atrophic native kidneys were observed. In addition, an incidental mass measuring 5.5 cm├Ś4.3 cm with marked heterogeneous internal echogenicity was found between the liver and the atrophic right kidney (Fig. 1A, B). The patient underwent subsequent triple-phase contrast-enhanced abdominal computed tomography (CT) for further evaluation of the mass. The CT revealed a 5.5-cm-sized right-sided retroperitoneal mass that showed mild peripheral enhancement, but the center of the mass seemed to lack perceivable intensification, suggesting that extensive necrosis had occurred. The mass was abutting the right kidney, liver segment 6, and the duodenum (Fig. 1C, D). The retroperitoneal location of the mass and the assumed extensive necrotic change led to the impression of retroperitoneal sarcoma. Desmoid tumor, inflammatory pseudo-tumor, and tuberculous abscess were other disease entities considered for the differential diagnosis. We excluded lymphoma because extensive necrosis is a very unusual finding.
The patient underwent surgery. During the operation, the mass was found to be adherent to the right native kidney; therefore, enbloc resection of the mass and right nephrectomy were performed. Upon gross examination of the resected mass, extensive internal necrosis was observed (Fig. 1E). Microscopic examination of the non-necrotic portion of the mass revealed abundant large tumor cells with atypia (Fig. 1F). On immunohistochemical staining, tumor cells showing atypia were strongly positive for CD20 (B-cell marker) (Fig. 1G). Relatively sparse cells with normal lymphocyte appearance were positive for CD3 (T-cell marker), suggesting reactive infiltration of T lymphocytes. Pancytokeratin was negative. Collectively, the final histopathological diagnosis was non-Hodgkin lymphoma (diffuse large B-cell lymphoma).
After surgery, the doses of tacrolimus and MMF were reduced to 1 mg/day and 500 mg/day, respectively. The patient managed well and did not show evidence of rejection of the transplanted renal allograft. The patient received three cycles of chemotherapy with a regimen of cyclophosphamide, doxorubicin, vincristine, prednisolone, and rituximab (R-CHOP). No evidence of lymphoma recurrence was observed during the postoperative surveillance period of 20 months.
Discussion
On ultrasonography, lymphomas presenting as a mass usually demonstrate homogenous, markedly-low echogenicity irrespective of the nodal or extra-nodal status [3-5]. This is because lymphoma is fundamentally a homogeneous tumor that generates very few internal reflections. Under rare circumstances, however, if the lymphoma mass becomes heterogeneous, atypical imaging findings may be encountered. For example, a reported lymphoma case involving the liver and a hepatoduodenal ligament that carried intermingled adipose cells within the mass presented as a hyperechoic mass [6].
PTLD consists of a wide spectrum of conditions associated with lymphoid proliferation that occurs after organ transplantation and the administration of immunosuppressive agents. This malady ranges from reactive, polyclonal hyperplasia to aggressive non- Hodgkin lymphoma. In the literature, PTLD is described as occurring most frequently during the first year after transplantation, with the highest incidence after small bowel transplantation (~20%), followed by lung, heart, liver, and kidney transplantation (1%-10%) [7]. Immunosuppression and EBV infection status are two major factors associated with the development of PTLD [8,9].
In the current article, we report a post-transplantation lymphoma case that presented as a retroperitoneal mass showing striking heterogeneous echogenicity on ultrasonography. The patient underwent surgical resection, and we performed a radiologicalpathological correlation to account for such atypical imaging findings. As such marked inhomogeneity revealed on ultrasonography is very unusual for lymphoma [1,2], we mistakenly excluded lymphoma from our differential diagnosis. Based on the gross and microscopic features of the surgical specimen, the heterogeneous echogenicity was attributed to extensive necrosis within the mass. Unlike untreated lymphomas, which rarely contain necrotic portions, lymphomas in post-transplantation conditions have been reported to occasionally show central areas of necrosis [10,11], which was probably the case in our patient as well. Knowledge of such atypical ultrasonography features of lymphoma associated with PTLD seems to be important because ultrasonography is frequently used as the initial surveillance modality after transplantation; if the radiologist is familiar with these atypical imaging features, confusion can be avoided.
In conclusion, lymphoma can occasionally present on ultrasonography as a mass with markedly heterogeneous echogenicity, which represents internal necrosis when associated with the conditions of PTLD. If the patient has a clinical history of organ transplantation, is receiving immunosuppressive therapies, and has atypical findings, the clinician is cautioned not to exclude lymphoma from the differential diagnosis, and early tissue confirmation is indicated.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




