Introduction
Acute-onset pelvic pain is an extremely common symptom in premenopausal women presenting to the emergency department (ED). The various potential etiologies for pelvic pain, both gynecologic and nongynecologic, can result in a diagnostic quandary for the ED clinician, as accompanying signs and symptoms are often nonspecific (i.e., nausea, vomiting, fever, and leukocytosis). After excluding pregnancy in reproductive-age women, either using urine or serum human chorionic gonadotropin levels, the differential diagnosis can be limited further using supporting evidence from history, physical examination, and laboratory data.
The role of ultrasonography as one of the main tools in the radiologists’ arsenal for evaluation of pelvic pain in premenopausal patients is well established. According to the American College of Radiology appropriateness criteria, in reproductive-age women with a negative pregnancy test in whom a gynecologic etiology for pelvic pain is suspected, ultrasonography is the recommended primary imaging modality. When the ultrasonography is inconclusive or nondiagnostic, computed tomography (CT) or magnetic resonance imaging (MRI) can then be considered for further imaging. Of course, both ultrasonography and MRI have the advantage of no ionizing radiation dose to the patient, a major consideration when imaging in this patient population. Ultrasonography also tends to be both readily available in most emergency settings, as well as lower cost than either CT or MRI. However, the drawbacks of ultrasonography include its dependence on the skill of the operator, along with technical limitations related to patient body habitus and bowel gas [1]. Ultimately, the primary goal of imaging in these patients is to distinguish between adnexal causes of acute pelvic pain that may be managed conservatively or medically, and those requiring emergency/urgent surgical or percutaneous intervention. In this paper, we review common adnexal causes of acute pelvic pain and their sonographic appearance.
Functional and Hemorrhagic Ovarian Cysts
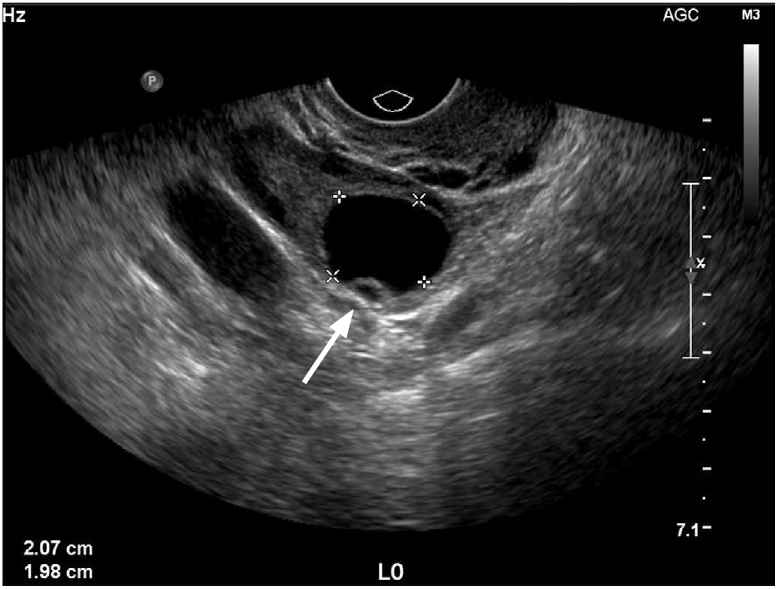
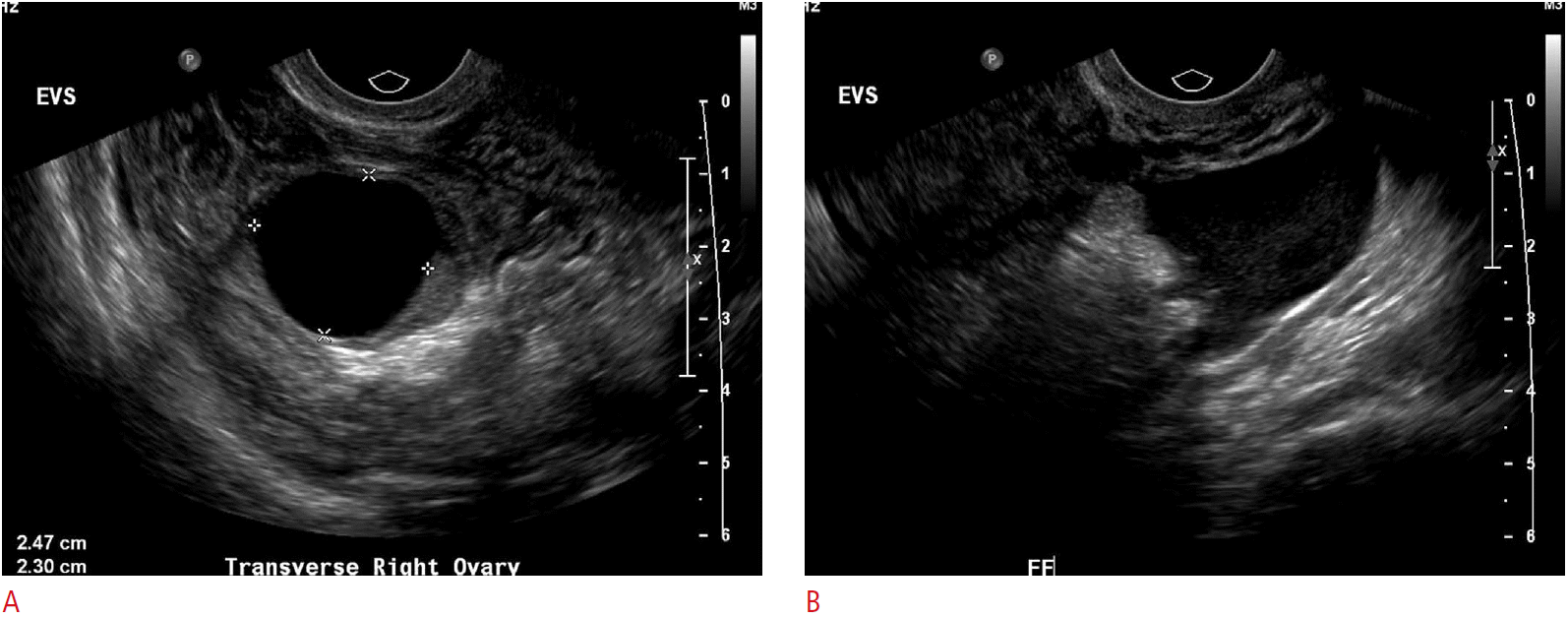
Normal premenopausal ovaries generally have a homogenous echotexture and several small follicles are often seen at the periphery of the ovarian parenchyma. During the early proliferative phase of the menstrual cycle, several follicles will increase in size under the influence of both follicle-stimulating hormone and luteinizing hormone. One of these follicles becomes the dominant follicle and continues to grow, reaching up to approximately 2.5-3.0 cm (Fig. 1). When the fluid in one of the nondominant follicles fails to be resorbed and continues to grow, it is termed a follicular cyst. On ultrasonography, the follicular cysts are thin-walled and avascular and most often unilocular, containing anechoic fluid resulting in posterior acoustic enhancement. They can sometimes grow to be quite large, ranging in size from 3-8 cm (Fig. 2). Pain from these cysts may develop secondary to rapid cyst growth, hemorrhage, or rupture.
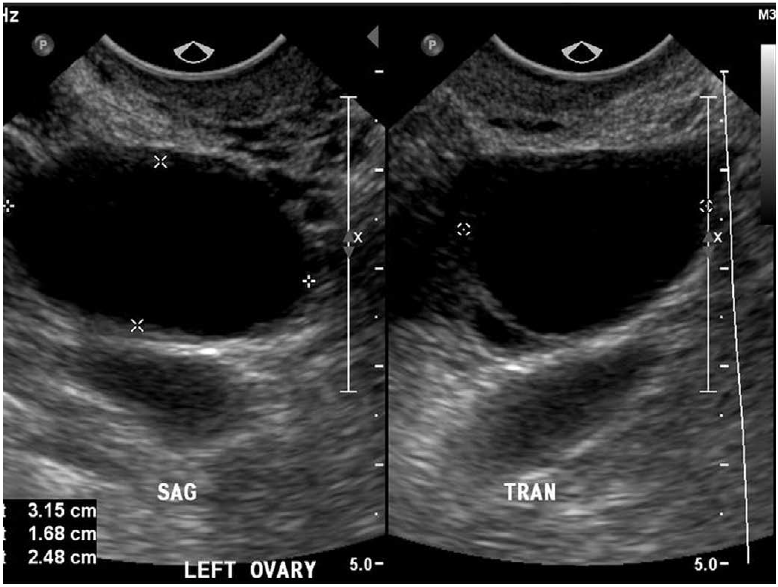
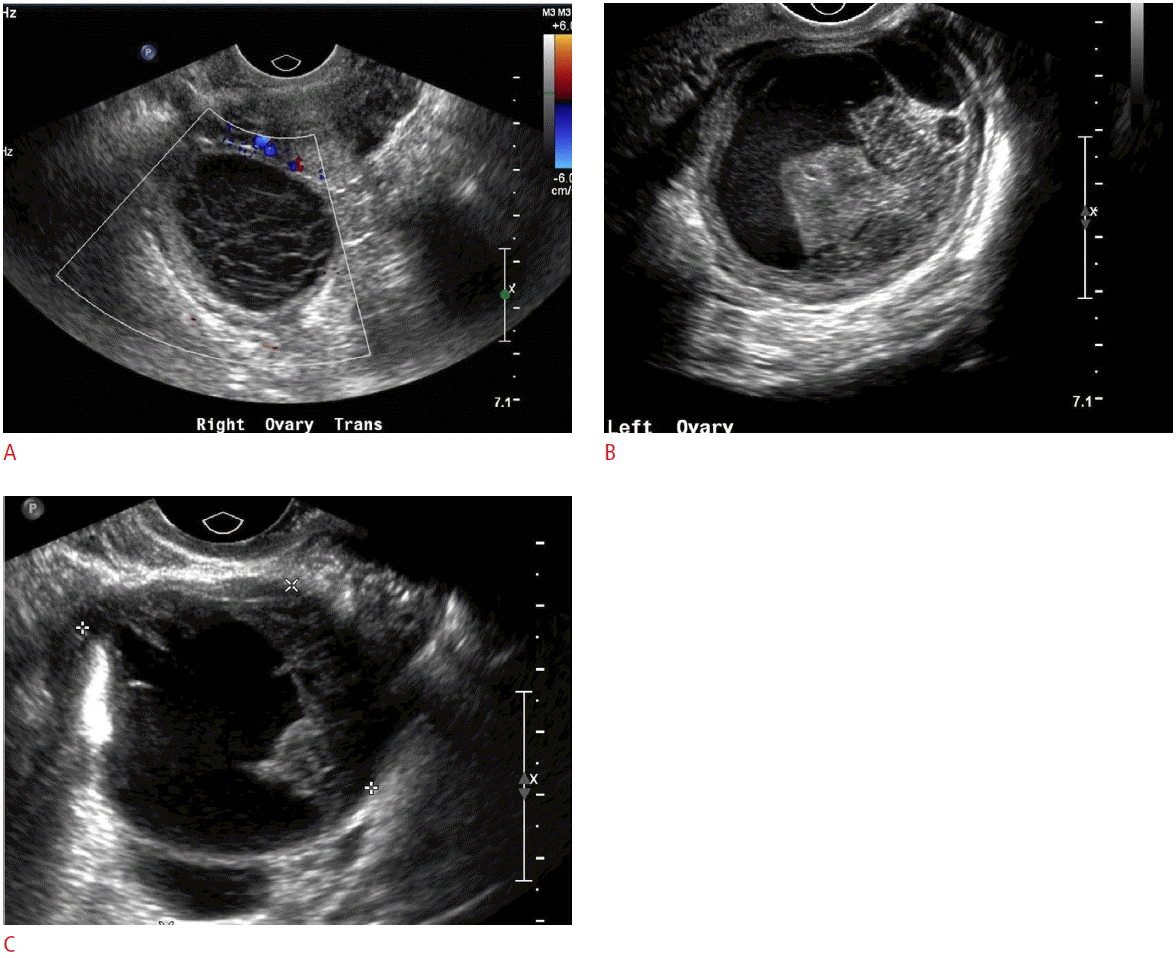
Following ovulation, the corpus luteum forms (Fig. 3) and usually will slowly involute throughout the remainder of the menstrual cycle up to the time of menstruation. Occasionally, fluid may persist within the corpus luteum and accumulate to a greater volume, resulting in a corpus luteal cyst. Corpus luteal cysts, unlike their follicular counterparts, demonstrate a thicker, and often irregular, wall. Doppler evaluation will depict the peripheral vascularity of the corpus luteum from hypervascularized luteinized granulosa and theca cells. While corpus luteal cysts are less common than follicular cysts, they tend to grow larger, are generally more symptomatic, and are more likely to become hemorrhagic due to their vascularity (Fig. 4) [2-4].
While both follicular cysts and corpus luteal cysts can result in acute pelvic pain by virtue of their size, it is more common for them to become symptomatic when there is acute intracystic hemorrhage or intraperitoneal leak/rupture. The sonographic appearance of hemorrhagic ovarian cysts is complex and variable, depending on the stage of blood products within the cyst. The appearance of hemorrhagic cysts will slowly evolve over time, reflecting the gradual change from acute blood to subacute blood, clot formation, and finally clot retraction. Blood is echogenic at the time of early hemorrhage. The blood products will gradually evolve, becoming progressively more hypoechoic over time.
One of the classic sonographic appearances of a hemorrhagic cyst is the “lace-like” or “spider-web” pattern of internal reticulation. These fine septations are avascular on color Doppler imaging, and are not true tissue septations. Rather, they represent fibrin strands that form as the blood clot hemolyzes. A retracting clot will produce a thicker-appearing peripheral septation or mural nodule, often with a vaguely triangular appearance. The blood products can also layer into fluid-fluid or fluid-debris levels (Fig. 5).
Endometriosis
Endometriosis is defined as the presence of functional endometrial mucosa outside the uterus. While this extrauterine endometrial tissue can be found anywhere in the body, it is most commonly found in the ovaries, broad and round ligaments, fallopian tubes, posterior cul-de-sac, and along the serosal surface of the uterus. The vast majority (80%) is found on the ovaries. Endometriosis is a disease of exclusively premenopausal women with a prevalence of approximately 10%. The ectopic endometrial tissue responds to the cyclical hormones of the menstrual cycle and, consequently, bleeds in a cyclical fashion. This results in hemorrhage-filled cysts (endometriomas) and bloody ascites in the abdomen and pelvis.
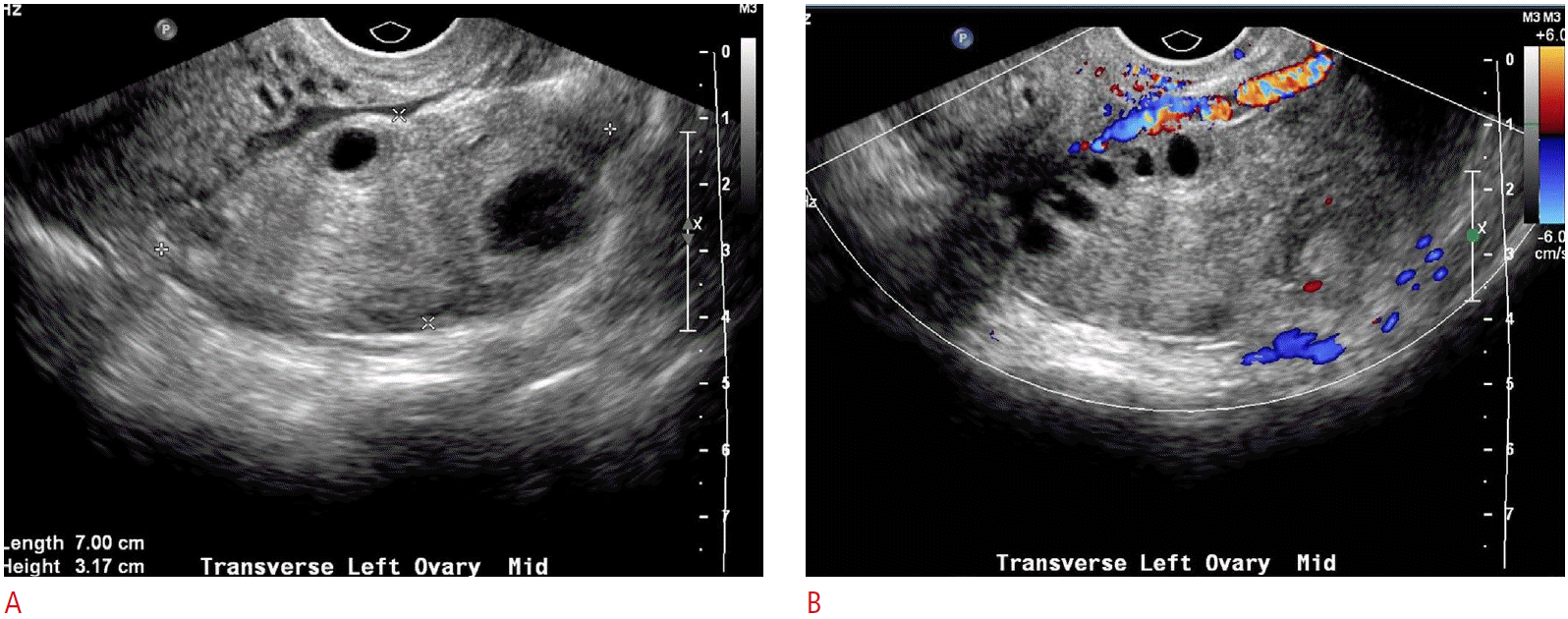
Not unlike hemorrhagic cysts, endometriomas can demonstrate a range of sonographic appearances-once again depending on the stage of blood product degradation. Initially, the appearance of an endometrioma can be indistinguishable from a hemorrhagic cyst. Over time, endometriomas will gradually develop their most characteristic appearance: that of a cystic mass with homogeneous low-level internal echoes, sometimes referred to as a “ground glass” appearance (Fig. 7). Fluid levels and peripheral nodules resulting from clot retraction can also be seen. Multiple, small, echogenic foci within the wall of the homogeneous cyst are also quite typical, representing cholesterol deposits accumulating in the wall [3,4]. Doppler analysis is not useful in distinguishing endometriomas from other types of cysts or masses, as low-resistance waveforms can be seen in endometriomas, although flow is usually more perilesional rather than internal vascularity [7].
Ovarian Torsion
Ovarian or adnexal torsion is an acute surgical emergency whereby the ovary is partially or completely rotated along the axis of its pedicle, compromising its blood supply. Torsion is uncommon, with a prevalence of only 2.7%, and often poses a diagnostic challenge, although it requires prompt and accurate diagnosis and treatment [8]. While an otherwise normal ovary can develop torsion, it is also commonly seen in the setting of a benign adnexal mass, which can act as the center of rotation about which the remainder of the ovary and broad ligament can twist. The risk is highest in children and in women of reproductive age, most notably during the early weeks of pregnancy and in the early postpartum period. Patients will present with acute pelvic pain, although this is frequently accompanied by nonspecific symptoms of nausea and vomiting.
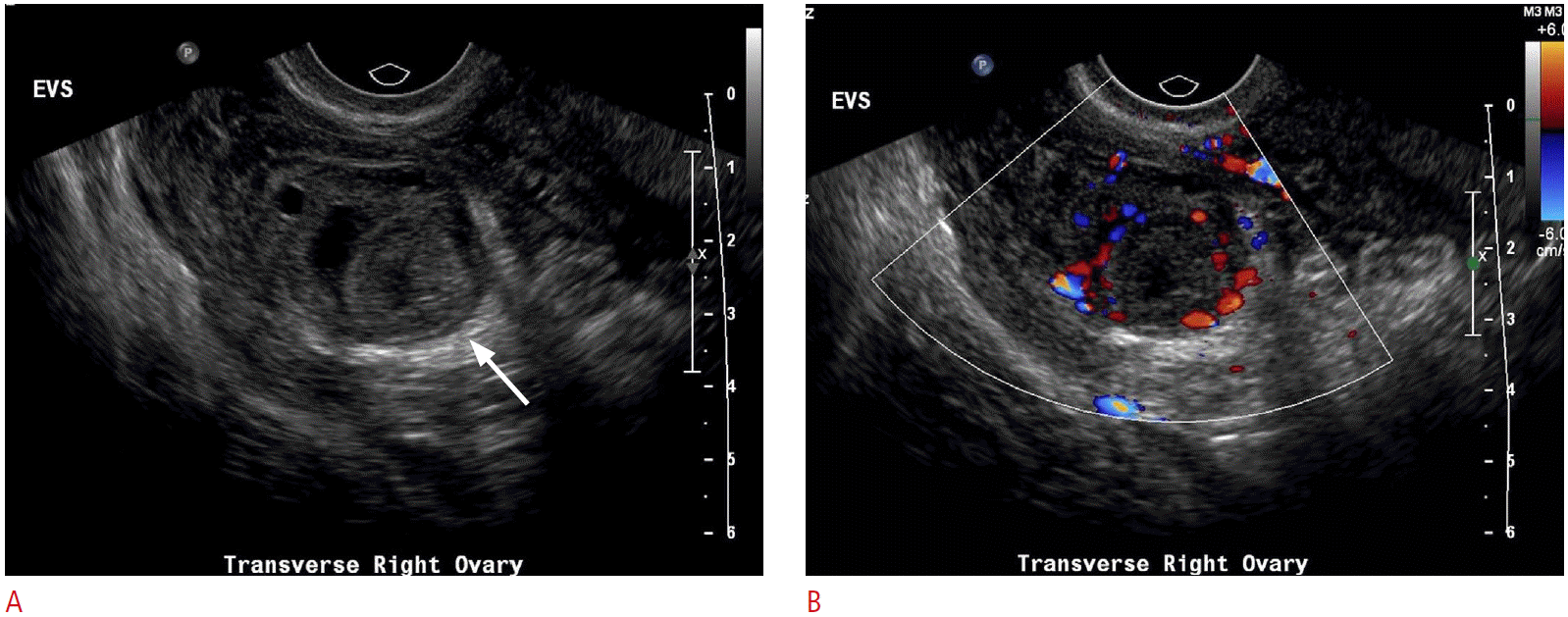
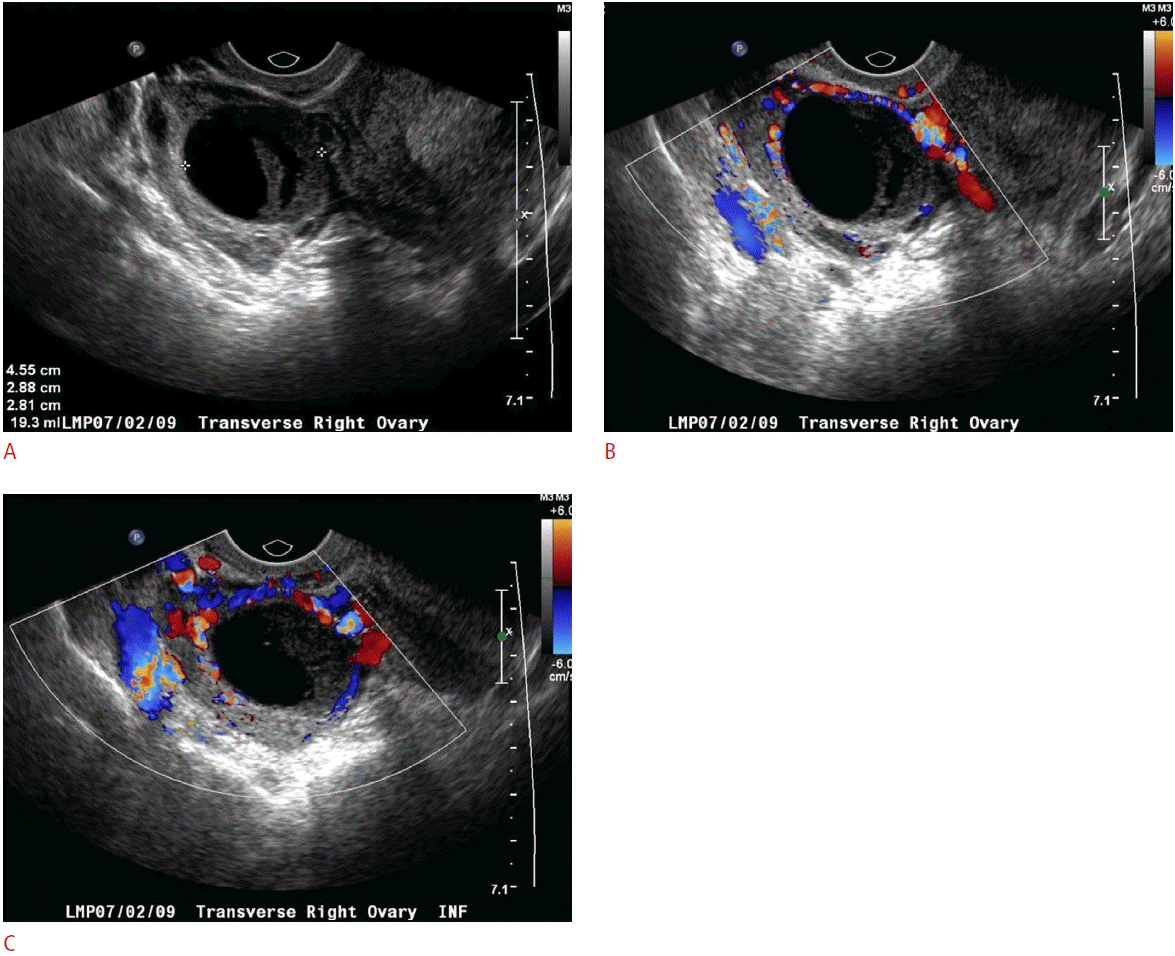
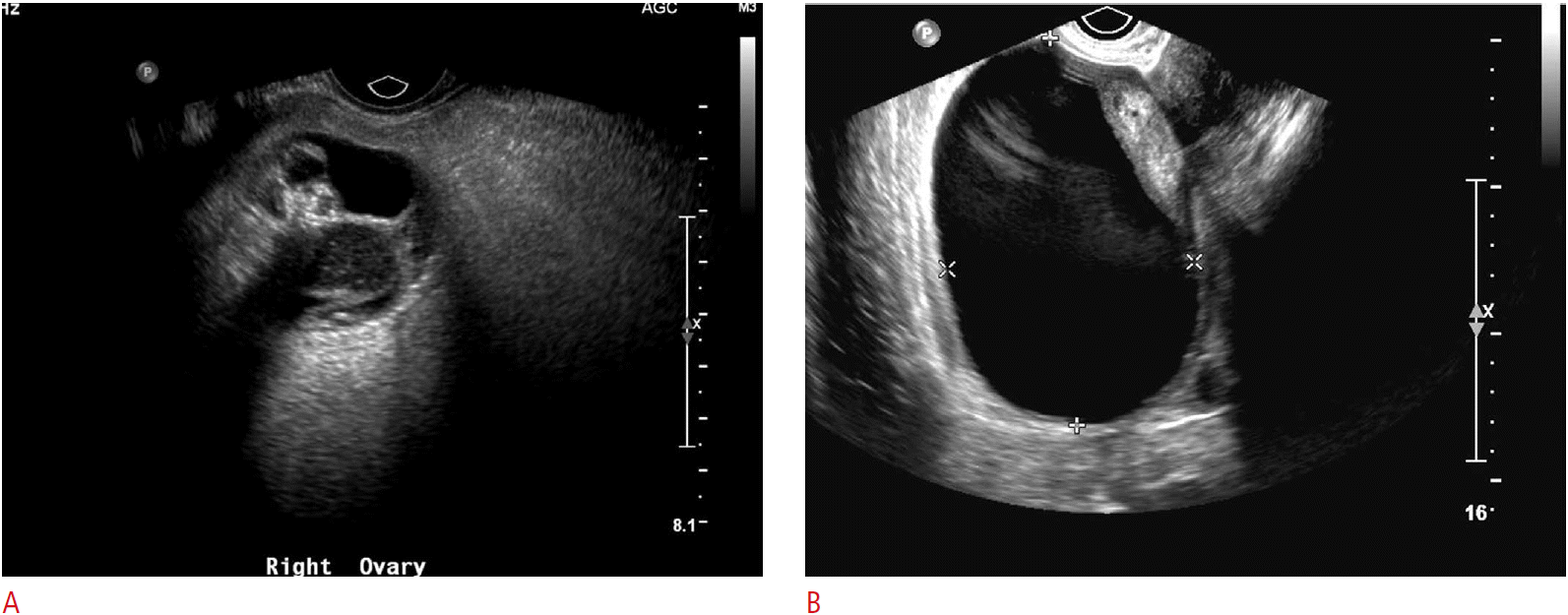
Not unlike other entities discussed here, the sonographic appearance of ovarian torsion can vary significantly depending on the degree of pedicle twisting, degree of vascular compromise, time delay from initial onset of symptoms to imaging, and presence or absence of an ovarian mass or cyst. As the lymphovascular pedicle of the ovary twists, the low-pressure and more flexible venous and lymphatic channels are the first to occlude. The obstruction of lymphatic and venous outflow results in engorgement of the ovarian parenchyma and edema, resulting in enlargement of the ovary, which may appear relatively hypoechoic with prominent and peripherally-located nonovulatory follicles (Fig. 8). The follicles are thought to become larger due to the build-up of internal fluid secondary to the venous and lymphatic outflow obstruction. A similar appearance can be seen with a very rare entity known as massive ovarian edema, a tumor-like enlargement of the ovary due to the accumulation of interstitial fluid and peripheral orientation of multiple follicles [9]. Free fluid can accumulate around the affected ovary or in the cul-de-sac. As vascular compromise of the ovary persists, the parenchyma will often become progressively more heterogeneous with patchy areas of increased echogenicity due to areas of hemorrhagic infarction interspersed with parenchymal edema. Alternatively, ultrasonography may depict only the cyst or mass that led to torsion initially. Depending on the size of the cyst or mass, the ovarian parenchyma may or may not be easily visible. This is especially true in cases of very large cysts or teratomas, where the only visible imaging finding is the mass or cyst itself (Figs. 9, 10) [3,4].
Another clue to the presence of ovarian torsion is the unexpected or abnormal location of the ovary in question; it may be twisted to the midline, cranial to the uterine fundus, down posteriorly into the cul-de-sac, or even into the contralateral pelvis. A much more direct sign of adnexal torsion is the so-called whirlpool sign, which is the visible twisting of the vascular ovarian pedicle, with or without demonstrable flow within the twisting vessels. The sign refers to the swirling appearance as seen while scanning in the short-axis of the pedicle, although vascular twisting can also be seen in the long-axis (Fig. 10) [10,11].
Color and spectral Doppler imaging may show an absence of both arterial and venous flow to the affected ovary. One may also see absence of venous flow with persistent high-resistance arterial flow. These are highly specific signs of adnexal torsion and may help clinch the diagnosis. However, the presence of normal color flow and spectral waveforms does not exclude the possibility of torsion. In one study, Doppler flow was found to be normal in 60% of surgically confirmed cases of ovarian torsion [12]. The reason for persistent vascular flow despite torsion is not altogether known and likely multifactorial. The dual blood supply to the ovary via both the ovarian artery and the ovarian branch of the uterine artery is thought to be one source of continued blood flow in a torsed ovary. Another hypothesis is that venous thrombosis and infarction produce symptoms before the arterial supply to the ovary is compromised. Torsion may also be incomplete or even intermittent, with transient restoration of blood flow in between episodes of torsion. Therefore, in the appropriate clinical setting, a unilaterally enlarged ovary should raise the possibility of ovarian torsion, even with the presence of Doppler flow. For these reasons, it is important to make a careful comparison to the contralateral ovary for both the gray-scale appearance and Doppler findings in order to make the diagnosis, especially in subtler cases.
Pelvic Inflammatory Disease/Tubo-ovarian Abscess
Pelvic inflammatory disease (PID) refers to a spectrum of infectious processes in the pelvis, typically ascending from cervicitis to involve the upper reproductive organs: the uterus (endometritis), fallopian tubes (salpingitis), and ultimately, the ovaries. Most cases are caused by Chlamydia trachomatis and Neisseria gonorrhoeae. However, there is a high incidence of co-infection with other organisms, such as Streptococcus species, Escherichia coli, and Bacteroides species. If left untreated, the infection can progress to form pyosalpinx and tubo-ovarian abscess. The initial diagnosis of PID is usually made on clinical grounds in patients with pelvic pain and cervical motion tenderness on physical exam. Patients may also have fever and leukocytosis, although symptoms are often vague, and the extent of disease is often not evident clinically. For this reason, imaging can play a crucial role in both the diagnosis and subsequent management of the patient’s medical condition.
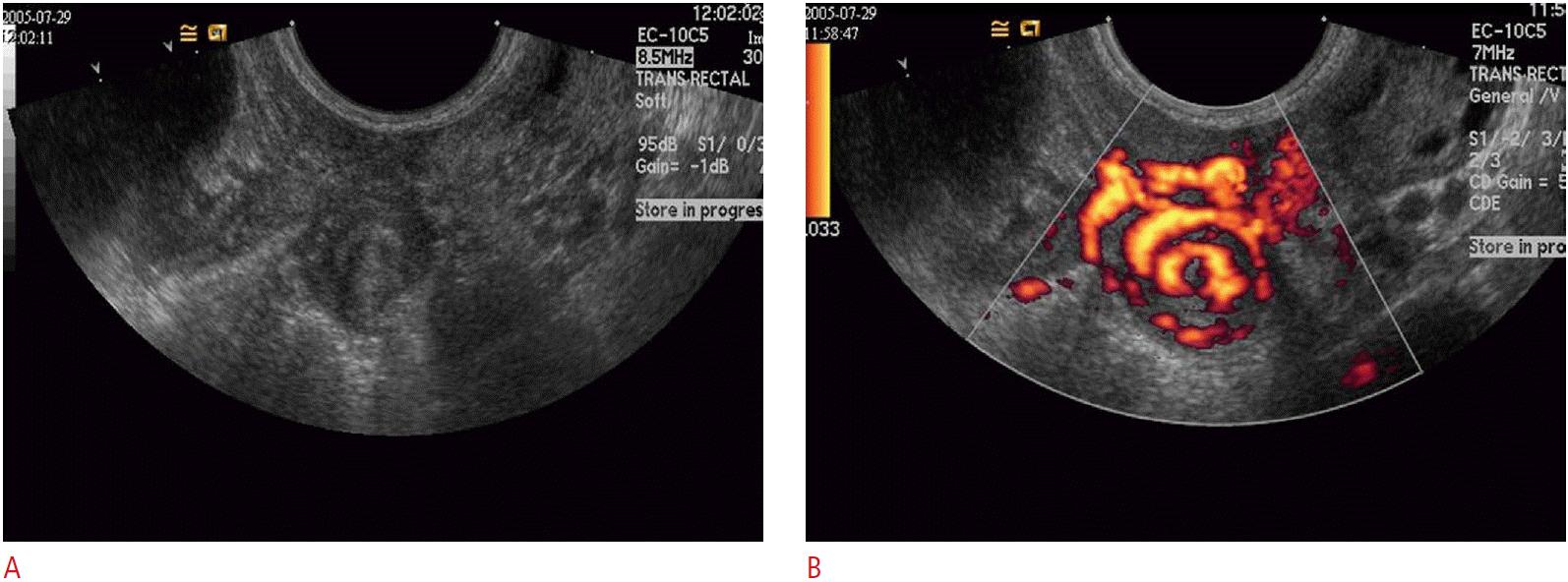
Sonographic findings of early stage PID in patients without pyosalpinx or tubo-ovarian abscess are usually very subtle and not easily detectable with ultrasonography; enlargement of the uterus and ovaries, indistinct soft tissue margins, thickening of the broad ligament and tubes, and fluid within the endometrial cavity or cul-de-sac. As the disease progresses and involvement of the fallopian tubes persists, tubal sonographic findings are some of the most specific hallmarks of PID. Dilated, fluid-filled, folded, and tubular structures in the adnexae are the key findings in hydro/pyosalpinx. The luminal fluid may be purely anechoic or complex with floating echoes. The walls of the tubes become thickened and hyperemic and in cross-section can adopt a “cog wheel” appearance due to the thickened endosalpingeal folds (Fig. 11). The inflamed ovary can acquire a reactive polycystic appearance, and eventually become adherent to the tube, often situated posteriorly and inferiorly in the region of the cul-de-sac. This is termed a tubo-ovarian complex. Persistent untreated disease leads to disruption of the normal adnexal and ovarian architecture with leakage of pus from the tube and the formation of a tubo-ovarian abscess (TOA), which appears as a complex, mixed solid and cystic mass in the pelvis (Fig. 12) [3,4,13]. Treatment for advanced PID involves the use of broad-spectrum antibiotics. Abscesses are drained using image guidance, either ultrasonography or CT, with catheters placed via a transgluteal, transvaginal, or transrectal route.
Summary
Acute-onset pelvic pain in premenopausal nonpregnant women is a very common symptom in ED patients. Adnexal causes of pain are numerous, and accurate diagnosis is important in order to distinguish between emergency surgical conditions and those that can be managed expectantly or with medical therapy. The prompt initiation of appropriate therapy is key for the successful management of patients with certain conditions, such as ovarian torsion or PID and TOA. As a radiation-free and relatively inexpensive imaging modality, ultrasonography plays a most important role in diagnosis and management of these patients.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+



 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




