Urethral configuration and mobility during urine leaking described using real-time transperineal ultrasonography
Article information
Abstract
Purpose
The aim of this study was to explore differences in the bladder neck configuration and segmental urethral mobility during the cough stress test (CST) in the supine and standing positions between women with and without stress urinary incontinence (SUI).
Methods
This prospective study included 100 control women and 100 incontinent women who had a CST with transperineal ultrasonography. The bladder neck configuration and urethral mobility were described in terms of urethral funneling, bladder neck descent (BND), retrovesical angle (RVA), urethral rotation angle, and urethral mobility at six points along the urethra (vectors 1 to 6). The two groups’ ultrasound findings in the two positions were compared.
Results
Valid data were collected from 78 control women and 90 women with SUI. Significant differences were found in age and body mass index between the two groups (P<0.01). Urethral funneling was found in 33 women (36.7%) with SUI and five continent women (6.4%) and altered little in the standing position. In the standing position, the mean RVA significantly increased (160° to 179°, P<0.001) in the SUI group; The mean vector of points 1 to 6 significantly increased in the control group (all P<0.001). The RVA, BND, and vectors 1 to 4 were significantly greater (all P≤0.01) in women with SUI than without, in both positions.
Conclusion
Urethral funneling was an intrinsic anatomical characteristic relative to SUI. Weak upper- and mid-urethral support and an unstable connection between the trigone and proximal urethra were the anatomical signs of SUI.
Introduction
Female stress urinary incontinence (SUI) is involuntary urinary leakage that results from increased abdominal pressure during sneezing, coughing, and exercising, with a high prevalence of 20% and a severe impact on patients’ physical and mental health as well as their social relationships [1]. The cough stress test (CST) is a generally used clinical diagnostic test recommended by several clinical practice guidelines for SUI diagnosis [2,3]. Evaluating the real-time anatomic changes during urine leaking is vital for understanding the underlying anatomic mechanisms of SUI.
Pelvic floor ultrasonography has been applied to SUI assessment for more than 30 years. When abdominal pressure is increased, the urethra can usually be seen to rotate down around the symphysis pubis. Anatomical changes such as bladder neck mobility, funneling, and segmental urethral mobility can be measured with high reliability [4-7]. A previous study by the authors showed that transperineal ultrasonography (TPUS) could be used to document anatomic changes during the CST in both the supine and standing positions [7].
This study aimed to describe the bladder neck configuration and urethral mobility in the CST in supine and standing positions and to explore the anatomical changes of the urethra in patients with SUI.
Materials and Methods
Compliance with Ethical Standards
Data collection was approved by the Human Research Ethics Committee of the Second Xiangya Hospital (No. 2020-038). Informed consent was sought from each subject.
Study Design
This was a prospective study of 100 incontinent women and 100 control women recruited from a gynecological clinic. All women underwent a standard interview with CST and four-dimensional TPUS between November 2018 and May 2020 at the Second Xiangya Hospital.
Exclusion criteria for the incontinent and control groups were a medical history of (1) previous pelvic or pelvic floor surgery or physiotherapeutic interventions; (2) recurrent infections; (3) voiding symptoms; (4) pelvic irradiation; (5) suspected fistulas; (6) other lower urinary tract symptoms such as urinary frequency, urgency, or dysuria; and (7) pelvic organ prolapse beyond the hymen.
The inclusion criteria for the control group were (1) older than 18 years, (2) no history of neurological and urinary diseases, and (3) not taking any medication.
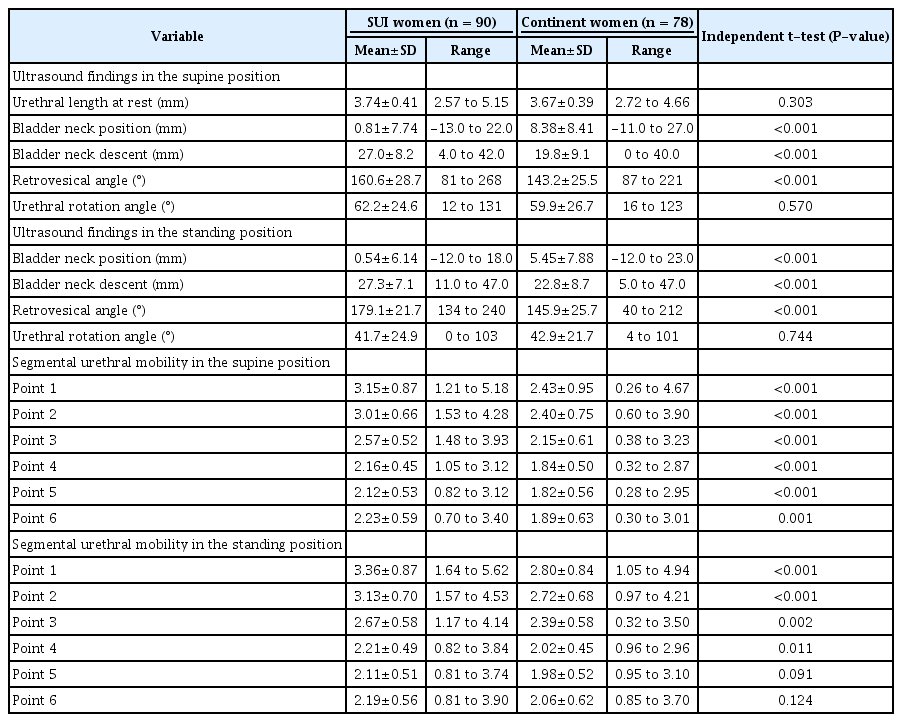
The inclusion criteria for the incontinent group were (1) a symptom of involuntary urine leakage from physical activity such as coughing, sneezing, or laughing and (2) a positive CST [3]. Based on the interview, the severity of urinary incontinence was classified using the Ingelman-Sundberg scale, as follows: grade I, urinary incontinence when coughing or sneezing; grade II, urinary incontinence when running or picking up items from the floor; and grade III, urinary incontinence when walking or climbing stairs. TPUS was performed using a Voluson E10 system (GE Healthcare, Milwaukee, WI, USA) equipped with a 4-8 MHz curved array transducer placed in the midsagittal direction with the bladder volume less than 50 mL. Ultrasound volumes of the pelvic floor were acquired during CST. Leakage was defined as urine being detected in the urethra or between the external urethral orifice and the probe surface (Fig. 1). Images of at least three Valsalva maneuvers per patient were acquired in both the supine and standing positions, by the authors Zhao and Wen.

Transperineal ultrasound images in the mid-sagittal plane during real-time urine loss.
A, B. Images at rest (A) and the maximal Valsalva maneuver (B) in the supine position are shown. Urine leakage was defined as the presence of urine in the urinary tract or between the external urethral orifice and the probe surface. C, D. Images in the standing position and demonstration of the urethral motion profile by tracing the urethra at rest (C) and during the Valsalva maneuver (D) are shown. Points 1 to 6 were automatically placed, and the x and y coordinates were determined by the UMP software. The vector of point 1 was calculated using the formula: SQRT [(x2-x1)2+(y2-y1)2]. BN, bladder neck; P, symphysis pubis; U, urethra; V, vagina; R, rectum; BL, bladder.
Post-processing analysis was performed at least 3 months later in the pelvic floor midsagittal plane by the author Wen, who was blinded to the clinical findings. Urethral funneling was defined as the internal urethral orifice being open during the Valsalva maneuver (Fig. 1). Bladder neck descent (BND), bladder neck position at the maximal Valsalva maneuver (BNP), urethral rotation angle, and the retro-vesical angle were measured according to the American Institute of Ultrasound in Medicine (AIUM)/International Urogynecological Association (IUGA) practice parameter [8].
Urethral segmental mobility was assessed using the semiautomated UMP software [9]. In brief, a semiautomated Excel macro automatically determined the x and y coordinates of six equidistant points placed along the length of the urethra, after manual tracing of the urethra in the midsagittal plane, from the bladder neck (point 1) to the external urethral meatus (point 6), relative to the dorsocaudal margin of the symphysis pubis on a bitmap image (Fig. 1). Segmental urethral mobility (vectors 1 to 6) was calculated using the formula SQRT [(Xvalsalva-Xrest)2+(Yvalsalva-Yrest)2].
A test-retest series with a total of 20 patients was performed. The intraclass correlation coefficients (ICCs) of inter-rater agreement were 0.86 for BNP and BND (95% confidence interval [CI], 0.78 to 0.96), 0.92 for the urethral rotation angle (95% CI, 0.85 to 0.97), and 0.91 for the retrovesical angle (95% CI, 0.83 to 0.97). The ICCs for vectors 1 to 6 ranged from 0.76 (95% CI, 0.61 to 0.84) to 0.93 (95% CI, 0.90 to 0.94), suggesting good to excellent interobserver reproducibility.
Statistical analyses were undertaken using SPSS version 21 (IBM Corp., Armonk, NY, USA). The normality of the continuous data was assessed using the Kolmogorov-Smirnov test. The Wilcoxon test was used for non-normally distributed data. Ultrasound findings in CST in the two positions were compared using the paired t-test, while ultrasound findings in women with and without SUI were compared using the independent t-test. A P-value of <0.05 was considered to indicate statistical significance.
Results
Of the 200 women, 22 control women and 10 incontinent women were excluded because the four-dimensional volume could not be used for UMP analysis. A total of 168 complete datasets were collected from 78 continent women and 90 women with SUI, including 28 women (31.1%) with grade I SUI, 46 (51.1%) with grade II, and 17 (17.8%) with grade III, according to the Ingelman-Sundberg scale.
Significant differences in age (P=0.005) and body mass index (BMI) (P<0.001) between the two groups were identified using the independent t-test. Eighty-nine (98.9%) women in the SUI group and 67 (85.6%) women in the control group had at least one vaginal delivery. No significant difference in parity and age at the first delivery was identified between women with and without SUI (Table 1).
In the SUI group, the retrovesical angle (P<0.001) and vector 1 (P=0.018) significantly increased, while the urethral rotation angle significantly decreased (P<0.001) in the standing position relative to the supine position. No significant differences in BNP, BND, and vectors 2 to 6 between the two positions were identified (Table 2). These findings did not change according to the degree of SUI.
For the control group, BNP, BND, and vectors 1 to 6 significantly increased (all P<0.01). In contrast, the urethral rotation angle significantly decreased (P<0.001) in the standing position relative to the supine position (Table 2).
Between the two groups, no significant difference was confirmed in urethral length. In the supine position, urethral funneling was found in five continent women (6%) and in 33 women with SUI (36.7%); in the standing position, urethral funneling was present in three continent women (3.8%) and 34 women with SUI (37.8%). A significant difference (P<0.001) was identified between the two groups in both positions.
The retrovesical angle, BNP, and BND were significantly greater (all P<0.001) in the SUI group than in the control group in both the supine and standing positions (Table 2).
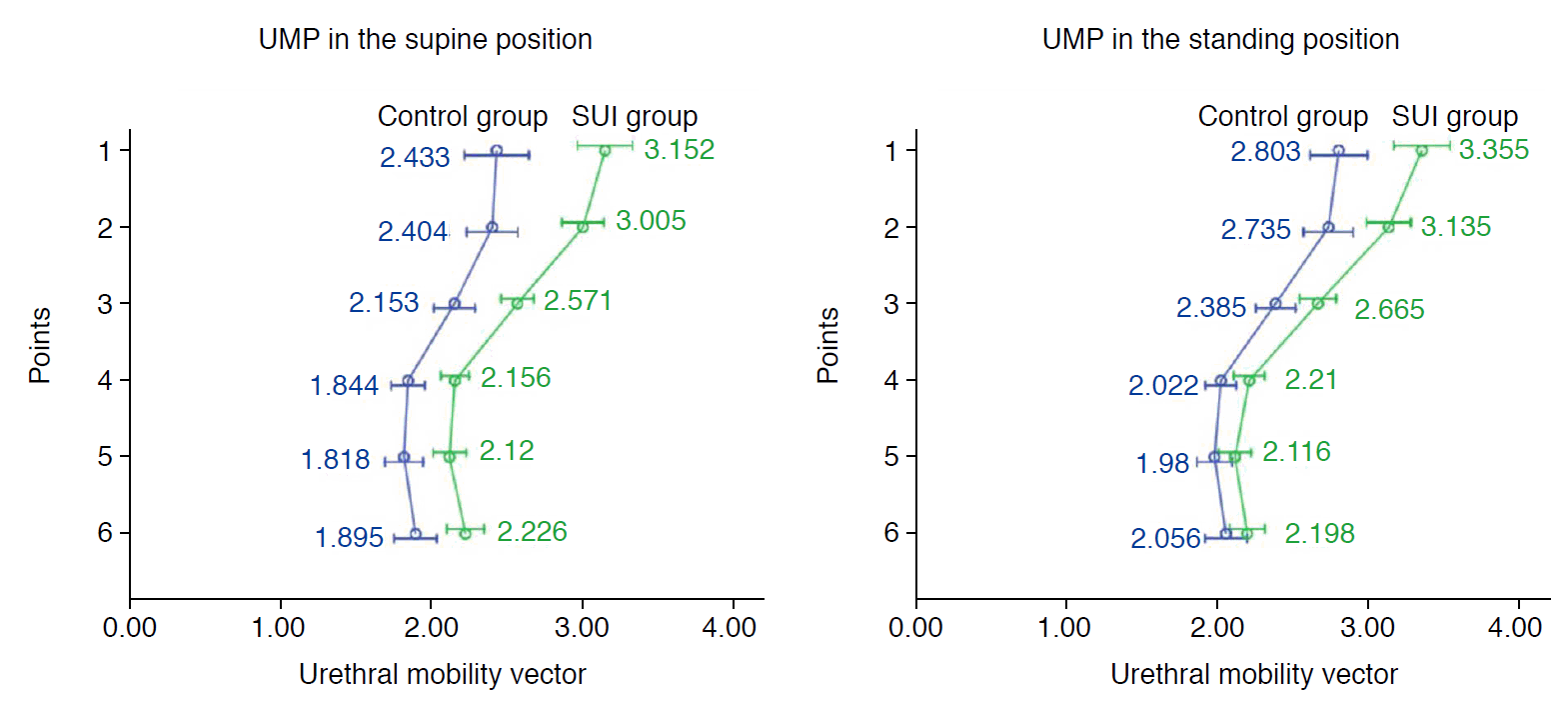
Vectors 1 to 4 were significantly greater in the SUI group than in the control group (Table 2) in both positions (all P≤0.01). The urethral mobility profile of the two groups in both positions is shown in Fig. 2.
Discussion
Pelvic floor ultrasonography is commonly employed to investigate pelvic floor disorders due to its low cost, accessibility, and practicability. TPUS can describe the lower genitourinary structures and the real-time anatomic changes during the Valsalva maneuver. Bladder neck and urethral mobility are usually only documented during the Valsalva maneuver in the supine position [4,5,9]. In this study, anatomic changes in the bladder neck and urethra during a CST were observed in the supine and standing positions. For continent women, the mobility of the entire urethra was more significant in the standing position, which indicated that the urethra was subject to considerable pressure. However, the retrovesical angle changed little. Although urethral mobility did not significantly increase in the standing position for incontinent women, the bladder neck and proximal urethral configuration changed. The retrovesical angle increased and was far more oblique. It seems that a stable connection between the trigone and proximal urethra is beneficial for continence, and a straightening connection may contribute to leakage, which agrees with the theory underlying the Green classification of cystocele that an open retrovesical angle is correlated with SUI [10,11].
Furthermore, upper- and mid-urethral mobility was significantly higher in women with SUI than in continent women in both positions, indicating weak stability of the upper-and mid-urethra in SUI. Magnetic resonance imaging demonstrated disruption of the paraurethral ligament in women with SUI [12]. Mid-urethral support damage is an important contributor to SUI [9,13,14].
A markedly higher prevalence of urethral funneling incidence was found in the SUI group. It did not change significantly between the two positions, which indicates that urethral funneling was an intrinsic anatomical characteristic of SUI, in agreement with previous studies [15-17].
SUI is a multifactorial disease influenced by anatomical structure changes and intrinsic anatomical characteristics, but it can be easily diagnosed by a CST. The suburethral sling/tape implant is an effective surgical procedure, although the incidence of postoperative voiding dysfunction or recurrent SUI is 10%-20% [18,19]. Therefore, investigating the anatomic changes of SUI may be beneficial for individualizing the surgical strategy [20]. Imaging showing real-time anatomical changes is meaningful.
There are many limitations to this study that need to be acknowledged. First, in this study, urethral sphincter function was not measured using urodynamic tests. Second, the higher BMI in the SUI group may have been a confounding factor in the urethral mobility measurements in the standing position. However, the effect was limited because the mean BMI (24 kg/m2) in the SUI group was within the non-obese range [21,22]. Third, ultrasonography in a standing position best simulates real conditions, but is difficult to perform in practice.
Ultrasound findings during the CST showed that urethral funneling was an intrinsic anatomical characteristic of SUI. Weak upper- and mid-urethral support and an unstable connection between the trigone and proximal urethra were the anatomical signs of SUI.
Notes
Author Contributions
Conceptualization: Wen L. Data acquisition: Zhao B, Wen L, Liu D, Huang S. Data analysis or interpretation: Zhao B, Wen L. Drafting of the manuscript: Zhao B, Liu D, Huang S. Critical revision of the manuscript: Wen L. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported
Acknowledgements
The authors thank Prof. Ka Lai Shek of Western Sydney University, Australia, for providing the semi-automated UMP software and the related training to Lieming Wen. This study was supported by the National Natural Science Foundation of China (NSFC) project (81901770), Natural Science Foundation of Hunan Province (2020JJ8047) and "The Project of New Clinic Techniques of Central South University".
References
Article information Continued
Notes
Key point
Transperineal ultrasound can be used to detect bladder neck and urethra's anatomic changes during a cough stress test in supine and standing positions. Stable connection between trigone and proximal urethra was beneficial for continence, and a straightening connection may contribute to leakage. Upper- and mid-urethral mobility was significantly higher in women with stress urethral incontinence (SUI) than continent women, which indicated the weak stability of upper-and mid-urethra in SUI.