Imaging features of complex sclerosing lesions of the breast
Article information
Abstract
Purpose:
The purpose of this study was to evaluate the imaging features of complex sclerosing lesions of the breast and to assess the rate of upgrade to breast cancer.
Methods:
From March 2008 to May 2012, seven lesions were confirmed as complex sclerosing lesions by ultrasonography-guided core needle biopsy. Final results by either surgical excision or follow-up imaging studies were reviewed to assess the rate of upgrade to breast cancer. Two radiologists retrospectively analyzed the imaging findings according to the Breast Imaging Reporting and Data System classification.
Results:
Five lesions underwent subsequent surgical excision and two of them revealed ductal carcinoma in situ (n=1) and invasive ductal carcinoma (n=1). Our study showed a breast cancer upgrade rate of 28.6% (2 of 7 lesions). Two lesions were stable on imaging follow-up beyond 1 year. The mammographic features included masses (n=4, 57.1%), architectural distortion (n=2, 28.6%), and focal asymmetry (n=1, 14.3%). Common B-mode ultrasonographic features were irregular shape (n=6, 85.7%), spiculated margin (n=5, 71.4 %), and hypoechogenicity (n=7, 100%). The final assessment categories were category 4 (n=6, 85.7%) and category 5 (n=1, 14.3%).
Conclusion:
The complex sclerosing lesions were commonly mass-like on mammography and showed the suspicious ultrasonographic features of category 4. Due to a high underestimation rate, all complex sclerosing lesions by core needle biopsy should be excised.
Introduction
Complex sclerosing lesions are rare benign breast lesions that include sclerosing lesions with a variety of epithelial proliferative lesions [1]. Radial scars exceeding 1 cm in diameter are known to be complex sclerosing lesions [2-4]. Such radial scars are characterized by a central fibroelastic zone from which tubular structures radiate [5]. Complex sclerosing lesions have all of the features of radial scars and may exhibit nodular masses with or without other changes, such as papillomas, apocrine changes, and sclerosing adenosis around the periphery [5].
Most lesions are benign, but malignant cancer may coexist or develop [6]. Several studies have published data regarding radial scars and the radial scar/complex sclerosing lesion combination [7-9], but to date, few studies have reported imaging results of complex sclerosing lesions alone [9]. In particular, previous studies have rarely reported the radiological features of complex sclerosing lesions according to the Breast Imaging Reporting and Data System (BI-RADS) [9]. There are conflicting data regarding the risk of breast cancer subsequent to a complex sclerosing lesion [10-12]. The present study was conducted to assess the rate of upgrade from a complex sclerosing lesion to breast cancer and to evaluate the imaging features of complex sclerosing lesions according to BI-RADS [13].
Materials and Methods
This study was approved by our Institutional Review Board, which waived the requirement for patient consent for this retrospective review.
From March 2008 to May 2012, seven lesions were confirmed to be complex sclerosing lesions by ultrasonography-guided core needle biopsy. In all of the patients, subsequent surgical excision or a follow-up imaging study was performed and reviewed to assess the breast cancer upgrade rate. Medical records and pathology results were reviewed for the evaluation of clinical features and histology.
Mammography and ultrasonography were performed in all of the patients. Mammographic images were acquired using a Mammomat Inspiration (Siemens, Erlangen, Germany) and Selenia (Hologic, Bedford, MA, USA). The ultrasonographic images were acquired using a 7-15 MHz linear probe (HDI 5000, Advanced Technology Laboratories, Bothell, WA, USA; iU22 Ultrasound System, Philips Ultrasound, Bothell, WA, USA) and a 6-14 MHz linear probe (EUB-8500 scanner, Hitachi Medical, Tokyo, Japan).
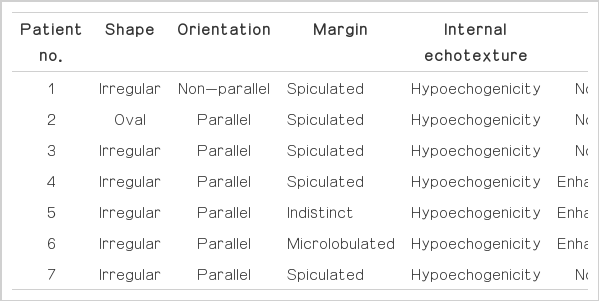
Two radiologists retrospectively analyzed the mammographic findings for shape, margin, and density, and the ultrasonographic findings for shape, margin, internal echogenicity, and posterior acoustic features including category according to the American College of Radiology BI-RADS [13].
Results
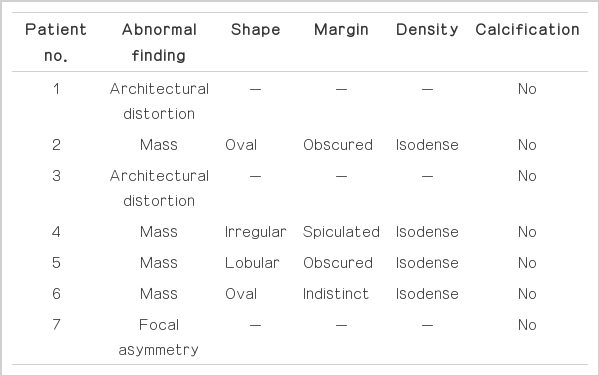
The clinical findings of the patients are summarized in Table 1. The median age of the patient was 34 years old (age range, 25 to 51 years). The lesions varied in size from 1.1 to 2.6 cm (median, 1.7 cm). On physical examination, two patients complained of a palpable mass, and one complained of mastalgia. The remaining four patients were asymptomatic. Five lesions underwent subsequent surgical excision: complex sclerosing lesion (n=2) (Fig. 1), complex sclerosing lesion with atypical ductal hyperplasia (n=1) (Fig. 2), ductal carcinoma in situ (n=1) (Fig. 3), and invasive ductal carcinoma (n=1). Two lesions showed stable imaging findings on follow-up imaging studies (12 and 24 months).

Complex sclerosing lesion in a 34-year-old woman with no symptoms (patient 1).
A. A mammogram shows focal architectural distortion (arrows) with a central radiolucent area (arrowhead). B. An ultrasonogram reveals an irregular, non-parallel, spiculated hypoechoic mass (arrows). Upon BI-RADS assessment, it was classified as category 4C, with moderate suspicion of malignancy. C. A photomicrograph of a histologic specimen (H&E, ×40) reveals a central fibroelastic core surrounded by a radiating compressed ductal structure, compatible with complex sclerosing lesion (arrows and arrowheads).

A complex sclerosing lesion in a 33-year-old woman with no symptoms (patient 4).
A. A mammogram shows an irregular spiculated isodense mass (arrows) without calcification or a central radiolucent area. B. An ultrasonogram reveals an irregular, spiculated hypoechoic mass (arrows) with posterior acoustic enhancement (arrowheads). Upon BI-RADS assessment, it was classified as category 5, highly suggestive of malignancy. C. A photomicrograph of a histologic specimen (H&E, ×40) from excision reveals a complex sclerosing lesion (arrows).
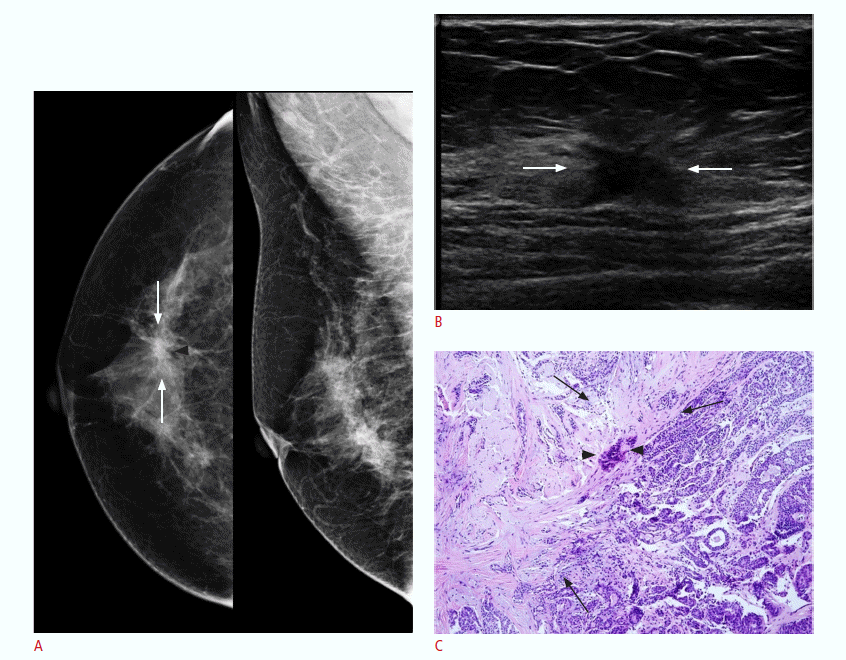
A complex sclerosing lesion in a 51-year-old woman with no symptoms (patient 7).
A. A mammogram shows focal asymmetry (arrows) with a central radiolucent area (arrowhead). B. An ultrasonogram reveals an irregular, parallel, spiculated hypoechoic mass (arrows) with no posterior acoustic features. Upon BI-RADS assessment, it was classified into category 4C, with moderate suspicion of malignancy. C. A photomicrograph of a histologic specimen (H&E, ×100) from excision reveals ductal carcinoma in situ (arrowheads) in the background of a complex sclerosing lesion (arrows).
The mammographic and ultrasonographic findings of the complex sclerosing lesions are summarized in Table 2 and 3. The lesions appeared as isodense masses in 57.1% of the cases (4 of 7), with an oval (50%, 2 of 4), lobular (25%, 1 of 4), or irregular shape (25%, 1 of 4). The margin was obscured (50%, 2 of 4), spiculated (25%, 1 of 4), or indistinct (25%, 1 of 4). The lesions that appeared as isodense masses did not exhibit a central radiolucent area or calcifications (Fig. 2A). The others exhibited architectural distortion (28.6%, 2 of 7) (Fig. 1A) or focal asymmetry (14.3%, 1 of 7) (Fig. 3A) with a central radiolucent area.
All of the lesions appeared as hypoechoic masses on ultrasonography. The shapes were irregular (85.7%, 6 of 7) or oval (14.3%, 1 of 7). The margins were spiculated (71.4%, 5 of 7), microlobulated (14.3 %, 1 of 7), or indistinct (14.3%, 1 of 7). In the final assessment, the lesions were classified as BI-RADS category 4C with moderate concern regarding malignancy (42.9 %, 3 of 7) (Figs. 1B, 3B), category 4B with intermediate suspicion of malignancy (28.6%, 2 of 7), and category 4A with low suspicion of malignancy (14.3%, 1 of 7). Moreover, one lesion was classified as BI-RADS category 5, with characteristics highly suggestive of malignancy (14.3%, 1 of 7) (Fig. 2B).
Upgraded lesions are those with an initial histopathologic diagnosis of complex sclerosing lesion based on ultrasonographyguided core needle biopsy that subsequently yield a diagnosis of ductal carcinoma in situ (DCIS) or invasive carcinoma after a surgical excision. After surgical excision, the rate of upgrade from complex sclerosing lesion to breast cancer (DCIS and invasive ductal carcinoma) was 28.6% (2 of 7).
Discussion
The association of the radial scar/complex sclerosing lesion with cancer is controversial [10-12,14]. Jacobs et al. [10] stated that radial scars are an independent risk factor for the development of breast cancer. In contrast, Berg et al. [11] stated that radial scars were not independent risk factors and that any increased risk was attributable to associated proliferative disease, such as atypical hyperplasia. Manfrin et al. [12] found that patients with coincident cancer generally had larger radial scar/complex sclerosing lesions. It has also been suggested that the size of the radial scar/complex sclerosing lesion may be important, with larger lesions being more likely to have malignant potential [12]. Therefore, many studies have suggested that a complex sclerosing lesion signifies the possibility of coincident cancer or precancerous lesions and that complete excision should be performed in older patients with larger radial scar/complex sclerosing lesions [2,9-11]. In our study, after surgical excision, two cases revealed malignancy, and one case revealed atypical ductal hyperplasia. Thus, the upgrade rate was 28.6%. The current study showed the diagnosis of complex sclerosing lesion after imaging-guided biopsy is an indication for surgical excision to exclude the diagnosis of breast cancer.
On mammograms, complex sclerosing lesions exhibited a mass density in four patients that most commonly manifested as an obscured, oval, isodense mass. The lesions that appeared as isodense masses did not show a central radiolucent area or calcifications. The other patients exhibited focal asymmetry (n=1) and architectural distortion (n=2) with a central radiolucent area. Radial scars commonly present as asymmetric densities or architectural distortions with central translucent areas [8,15]. Long, radiating spicules against a background of radiolucent fat create a black, star-like appearance [8,16]. This appearance is in contrast to most of the complex sclerosing lesions, which appeared as masses that resembled breast cancer.
On ultrasonography, complex sclerosing lesions commonly manifest as irregular hypoechoic masses with spiculated margins (Table 3). Three cases showed posterior acoustic enhancement. Six cases were classified as category 4, which is characterized by suspicious findings. Radial scars commonly appear as hypoechoic lesions with irregular or spiculated margins and posterior acoustic shadowing [9]. However, in our study, the complex sclerosing lesions commonly appeared as irregular, hypoechoic masses with spiculated margins and posterior acoustic enhancement (Fig. 2B).
The limitation of this study is the small sample size. Because of the retrospective nature of the analysis and the selection bias for patients likely to be at risk of malignancy, large patient numbers will be required to improve the reliability in future studies. Furthermore, subsequent surgical excision was not performed for two lesions and one case was reviewed by a short-term follow-up imaging study (12 months) that can raise the possibility of breast cancer.
In conclusion, the present study showed a rate of upgrade to cancer of 28.6%. A complex sclerosing lesion exhibited the etiology of a radial scar, but the imaging findings of a complex sclerosing lesion showed features distinct from those of a radial scar, such as their commonly appearing as a mass density on mammography and as an irregular, hypoechoic mass with a spiculated margin and posterior acoustic enhancement on ultrasonography. Therefore, all lesions that are pathologically proven to be complex sclerosing lesion by core needle biopsy should be excised.
Notes
No potential conflict of interest relevant to this article was reported
Acknowledgements
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.