Ultrasonography of pediatric urogenital emergencies: review of classic and new techniques
Article information
Abstract
Urogenital emergencies are fairly common in the pediatric population, and a timely and correct diagnosis is necessary to avoid possible future infertility. In this field, ultrasonography is essential, as it has the advantages of being radiation-free and readily accessible. In particular, a high-frequency transducer allows precise evaluation of the morphology and vascularity of the scrotum, which is on the surface of the body. Beyond conventional techniques, new advanced imaging techniques have been developed, including elastography and contrast-enhanced ultrasonography. However, several pitfalls remain in the diagnosis of urogenital diseases using ultrasonography. Thus, accurate knowledge and sufficient experience with the technique are essential for making a correct diagnosis. This review provides an overview of pediatric urogenital emergency pathologies and recent ultrasonography techniques.
Introduction
In the pediatric population, urogenital emergencies are quite common, and may have serious consequences. They include testicular torsion, epididymitis, testicular appendage torsion, testicular trauma, acute idiopathic scrotal edema (AISE), ovarian torsion, and emergencies involving associations of urogenital anomalies such as Wunderlich syndrome. These diseases are diverse, and there are pitfalls in their diagnosis. Along with conventional ultrasonography (US), new techniques have been developed, such as contrast-enhanced US (CEUS), tissue elastography, and 3-dimensional sonography. CEUS is used off-label in children, but a recent review suggested that the use of US contrast agents is safe in children [1]. These new techniques are also presented in this review.
Testicular Torsion
An etiological study of acute conditions of the scrotum showed that the distribution of diseases varied according to age [2]. In younger children (0-12 years), appendage torsion was most common (47%), followed by testicular torsion (34%) and epididymitis (4%). In those aged 13-21 years, testicular torsion dominated (86%), followed by appendage torsion (9%). Thus, testicular torsion should always be considered in the differential diagnosis, especially in adolescents.
Testicular torsion is a true emergency and is of the most concern among acute scrotum conditions. Testicular torsion is the rotation of the testis with torsion of the spermatic cord; this leads to vascular insult, which results in testicular ischemia and ultimately necrosis. The golden time for torsion reduction is 6-8 hours. The salvage rate is almost 100% if treated within 6 hours after onset but decreases quickly with time, declining to 70% within 6-12 hours and 20% within 12-24 hours [3]. Thus, the correct diagnosis and prompt reduction are essential. However, nearly 50% of clinically diagnosed cases of testicular torsion are false-positives [3], making a sonographic diagnosis especially important.
There are two representative types of testicular torsion: extravaginal and intravaginal. Intravaginal torsion is much more common than extravaginal torsion.
Intravaginal Torsion
Intravaginal twisting of the spermatic cord occurs due to excessive mobility of the testis and spermatic cord, owing to the abnormal attachment of the tunica vaginalis, which is known as the “bellclapper deformity” [4]. This type of torsion occurs commonly in adolescents, although it also occurs in neonates. The estimated frequency is 4 per 100,000 for boys aged <18 years [5].
The typical presentation is scrotal pain with sudden onset and subsequent scrotal swelling. However, in one study the initial symptoms were abdominal pain and vomiting without scrotal pain in 11% (9 of 84) of patients [6]. In a series published by Pogorelic et al. [6], the scrotum was not evaluated in six of nine patients, and five testicles were lost due to necrosis. Testicular torsion should always be kept in mind in the differential diagnosis of cases of lower abdominal pain [6].
On gray-scale sonography, the echotexture is initially normal. After 4-6 hours, the testicular echotexture shows a low echoic signal due to edema and swelling (Fig. 1A, B). After 24 hours, it becomes more heterogeneous, reflecting congestion, ischemia, and/or hemorrhage [3]. Heterogeneity of the testis suggests nonviability, whereas a homogenous echotexture bodes extremely well for testicular viability [7].
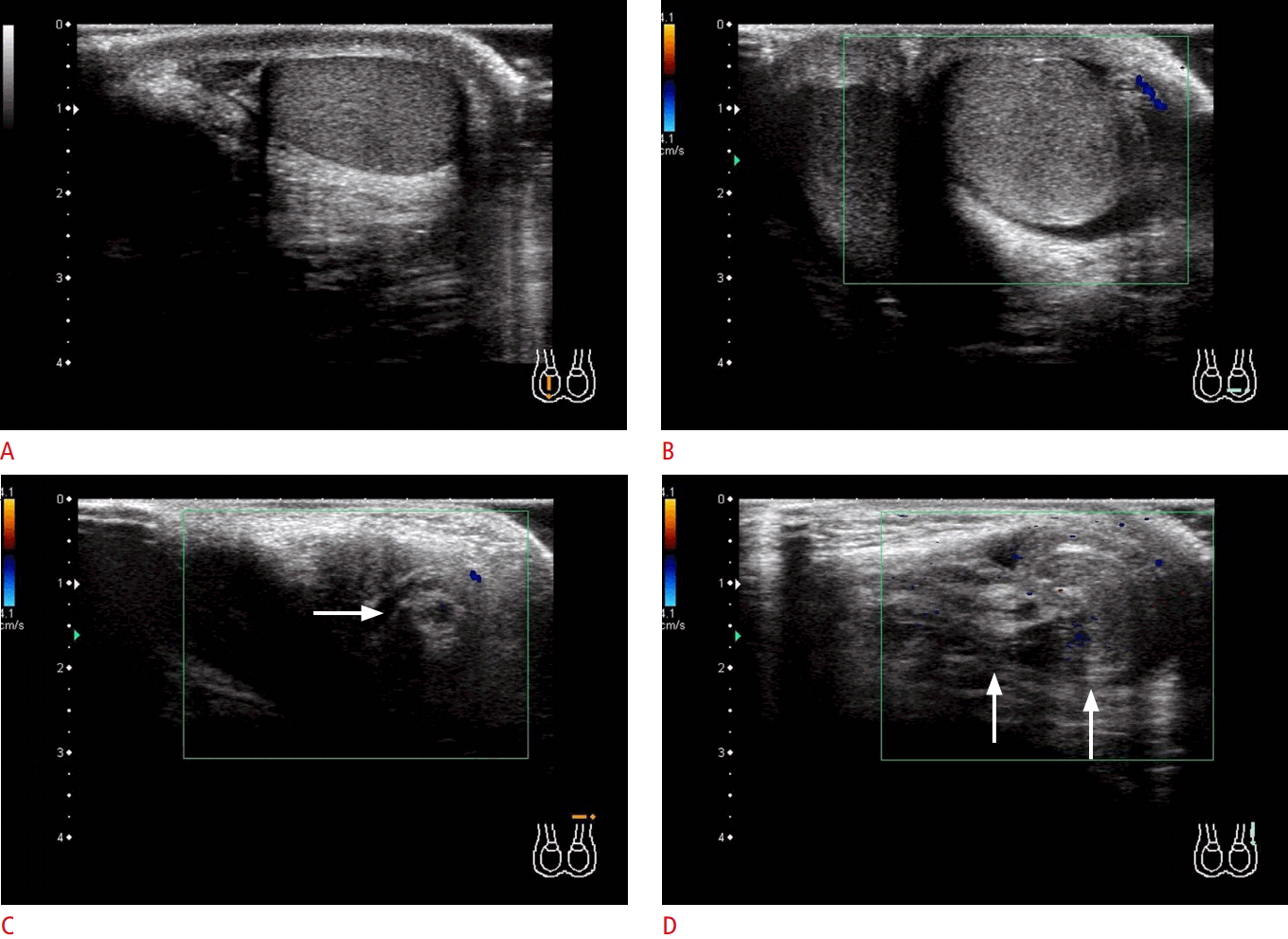
Testicular torsion (intravaginal torsion) in a 12-year-old boy with sudden-onset left inguinal pain.
A, B. Compared with the normal right testis (A), the left testis is enlarged and heterogeneous, and its vascularity is lost (B). These findings strongly suggest testicular torsion. C, D. The twisted spermatic cord (arrows in C and D) is seen in the axial (C) and sagittal (D) images. This is the direct finding of testicular torsion. The operative findings showed a testis twisted by 360° with discoloration. With surgical detorsion, the testis was preserved.
Using color Doppler or power Doppler, the vascularity of the testis can be evaluated. Decreased or absent affected testicular flow has a sensitivity of 86%-100%, and a specificity of 97.9%-100% [8-11]. Power Doppler is more sensitive in detecting testicular flow than is color Doppler (97% vs. 88%) [12]. This method is very valuable because of its simplicity, feasibility, high sensitivity, and high specificity. However, apparently preserved vascularity on color Doppler does not exclude testicular torsion. Testicular torsion may present as hyperemia in its early stages due to venous dilation and preserved arterial flow [13,14]. In such cases, the spectral wave form may be informative, showing an increased testicular resistance index with absent diastolic flow, indicating severe venous obstruction [13]. False-negative Doppler evaluations can also occur in the setting of spontaneous detorsion or intermittent torsion [15]. Intermittent torsion can exhibit hyperemia [16] and may be misdiagnosed as orchitis. A horizontal testicular position [17,18] and episodes of pain relief are useful for making a correct diagnosis. A quarter of cases of intermittent torsion have repeated episodes [19], and recurrent episodes are highly suggestive of intermittent testicular torsion when other causes have been excluded [19,20].
Although color Doppler and power Doppler images have high sensitivity and specificity, they are used to identify indirect findings suggestive of testicular torsion. The most direct finding with respect to testicular torsion is a twisted spermatic cord, referred to as the “whirlpool sign” (Fig. 1C, D) [16]. Indeed, the sensitivity and specificity of the whirlpool sign are up to 100% [16,21,22]. The whirlpool sign is also useful in cases of incomplete torsion, in which testicular vascularity may be preserved. Thus, the whirlpool sign should be carefully sought to avoid this pitfall, even if testicular vascularity is preserved. The location of the twisted spermatic cord varies: from just distal to the external ring to above or posterior to the testis [16]. In the case of an undescended testis, a twisted spermatic cord may be located in the inguinal canal. Thus, the entire route should be screened when seeking a twisted spermatic cord [16].
Observation of the epididymis is also important. Galina et al. [23] showed that the position and morphology of the epididymal head were abnormal in all nine of the boys in that study with acute testicular torsion but preserved testicular flow. This was also true in four cases with apparently normal-looking spermatic cords. That is, an abnormal configuration and displaced position of the head of the epididymis may be the only sonographic findings indicative of torsion. Thus, observation of the epididymis is especially important in cases of incomplete torsion with preserved testicular flow. Additionally, reactive hydrocele and scrotal wall thickening may be seen [7,23,24].
Similar to Doppler images, CEUS may show a lack of intratesticular enhancement [14,25]. Although no advantage over Doppler US has been found in the few series that have been published [25], CEUS has the potential to provide improved sensitivity in detecting flow in the pediatric testis during infancy because it is often difficult to demonstrate perfusion within a normal testis using color Doppler sonography [25]. Moreover, CEUS can add useful information for evaluating segmental infarction, which may be secondary to intermittent torsion. A focal lack of enhancement helps to differentiate segmental infarction from tumors [26].
On elastography, stiffness increases in testicular torsion [27,28], and in an animal model, stiffness changed according to time and severity [29]. Elastography may also offer information on spermatogenesis after testicular torsion. Higher stiffness values have been associated with qualitatively and quantitatively decreased spermatogenesis in an animal model [30].
Extravaginal Torsion
Extravaginal torsion occurs as a result of premature attachment of the tunica vaginalis to the scrotal wall. This leads to torsion of the tunica vaginalis, including its contents [31]. This mechanism is most common in the prenatal to neonatal period. Among cases of torsion in neonates, approximately 80% are prenatal, which are difficult to salvage, and approximately 20% are postnatal, which may be salvageable [32]. Although contralateral prophylactic orchiopexy is controversial [33], this may be indicated to avoid the risk of anorchia [32-34].
The sonographic findings of extravaginal torsion vary according to the stage [34]. In the acute stage, marked enlargement occurs, with heterogeneity and loss of vascularity (Fig. 2A, B). Other findings include linear hypoechoic striations (oriented radially from the mediastinum testis) (Fig. 2A), subtunica fluid, and hydrocele (Fig. 2A). In the later stages, shrinkage of the testis with increased echogenicity occurs (Fig. 2C, D). Knowledge of these changes is important because testicular flow is frequently not detectable even in a normal testis in neonates [35]. In this setting, elastography also may be useful to visualize the hardening of a necrotic testicle [27].
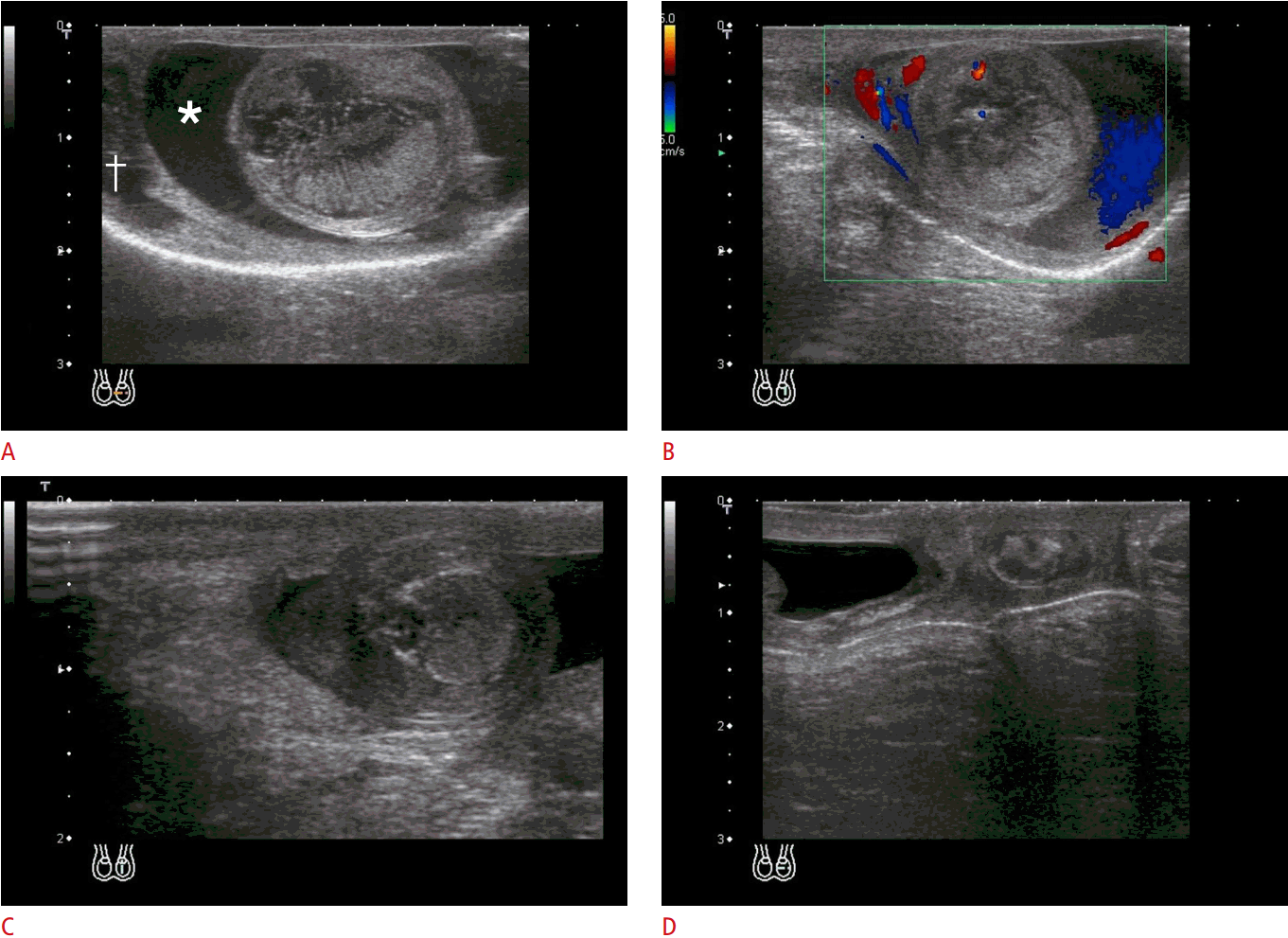
Testicular torsion (extravaginal torsion) in a neonate with left scrotal swelling.
A, B. Ultrasonography on day 3 after birth (A), the enlarged testis shows heterogeneous echogenicity with linear hypoechoic striations, hydrocele (asterisk), and subtunica fluid (dagger) without vascularity in the testis on color Doppler image (B). These findings are characteristic of neonatal testicular torsion. C, D. On day 25 (C) and 2 months (D), ultrasonography shows shrinkage with increased echogenicity and calcifications (courtesy of Dr. M. Mikiko, National Center for Child Health and Development, Japan).
Epididymitis
Epididymitis is also a common cause of acute scrotum. Epididymitis is more common in adolescents; cases commonly involve sexually transmitted organisms, such as Neisseria gonorrhoeae and Chlamydia [3,36]. In infants, representative pathogens are Escherichia coli, Pseudomonas, and Aerobacter [36]. The underlying abnormalities in infants include an ectopic ureteral opening to the seminal vesicle, posterior urethral valves, and vesicourinary reflux [37-40].
Although the need for imaging scrutiny regarding the underlying cause is controversial, some authors have suggested that patients with epididymitis, especially in infancy or after a second episode, should undergo an evaluation for underlying disease(s), via methods such as US and voiding cystourethrography [37,38]. This is because a correct diagnosis is needed, although epididymitis is typically treated conservatively.
Sonographic findings include swelling of the epididymis with decreased echogenicity (Fig. 3A, B) but may be heterogeneous, especially with hemorrhage. The head is commonly involved, and the entire epididymis may be involved in half of all cases (Fig. 3B). Severe swelling is frequently seen in patients with urogenital abnormalities [41]. Surrounding reactive changes, such as hydrocele and scrotal wall thickening, are also common. On color and power Doppler imaging, epididymal flow is increased (Fig. 3C) [3,14,36], and the resistance index decreases to less than 0.5-0.7 [3,42]. The spread of inflammation causes orchitis (epididymo-orchitis) in 20%- 40% of cases, showing enlargement, heterogeneous echogenicity, and increased vascularity [3,43]. CEUS is essentially unnecessary, but there is a place for CEUS in assessing complications. In the case of abscess formation or testicular infarction secondary to epididymoorchitis, CEUS can offer additional information. These conditions are visualized as a non-enhanced area, and abscesses show strong peripheral enhancement [14]. In cases of focal orchitis, a neoplasm can be included in the differential diagnosis. Elastography can differentiate orchitis, with soft elasticity, from neoplasms, with hard elasticity [44].

Epididymitis in a 5-year-old boy with right scrotal pain.
A-C. The epididymis shows marked enlargement in both the head (arrow in A) and body (arrows in B), and the epididymal vascularity is increased (C). Scrotal wall thickening is also seen (A-C). The testicular vascularity was also slightly increased (not shown), suggesting secondary testitis.
Testicular Appendage Torsion
In most cases (approximately 91%-95%) of testicular appendage torsion, the testicular appendage is involved, and epididymal appendage torsion is rare [3]. This occurs frequently in boys aged 7-14 years. Appendage torsion is associated with the cold season [45]. On physical examination, the twisted appendage can be seen as a bluish nodule through the overlying skin. This “blue dot sign” is specific for appendage torsion, but its sensitivity is as low as 21% [43]. Thus, a sonographic diagnosis is important.
On gray-scale sonography, the testicular appendage is typically seen on the medial side of the upper pole of the testis (Fig. 4, Video clip 1, 2). Depiction of the testicular appendage does not indicate torsion because the appendage is a normal structure. A twisted appendage was previously thought to be typically hyperechoic [46,47], but a recent study suggested that echogenicity changes with time [48]. A twisted appendage is initially hypoechoic but may be hyperechoic after >24 hours [48]. A hypoechoic appendage usually has a salt-and-pepper appearance, with multiple bright spots and septa [48,49]. Twisted appendages show swelling (ranging in size from 3 to 17 mm) (Fig. 5A) [46,47], compared with normal appendages (1-7 mm) (Fig. 4) [47,50]. Some have proposed a criterion of 5.6 mm as suggestive of testicular appendage torsion [47]. Additional findings include hydrocele testis and epididymal enlargement [46], which may potentially lead to the misdiagnosis of epididymitis. Appendage torsion is treated conservatively, which is also true for epididymitis. However, in the case of appendage torsion, manual reduction is also applied under sonographic monitoring [48,49]. In the case of epididymitis, scrutiny regarding the underlying cause may be necessary. Thus, an accurate diagnosis remains important. The success rate of manual reduction varies according to echogenicity: 91% for hypoechoic, 75% for isoechoic, and 50% for hyperechoic appendages [48].

Normal testicular appendage in a 3-year-old boy with right hydrocele testis.
A. Sagittal image shows the appendage (arrow) at the groove between the epididymis (asterisk) and testis. B. An axial image at the level of the lower edge of epididymal head (asterisk) shows the appendage (arrow) on the medial side of the epididymis (asterisk).

Testicular appendage torsion in a 12-year-old boy with left scrotal and back pain.
A, B. Color Doppler images were obtained in sagittal (A), and axial view (B). The testicular appendage (arrows in A and B), located at the upper pole of the testis, was swollen to >8 mm. Vascularity was lost in the appendage (arrows in A and B).
On color Doppler images, no vascular signal is seen in a twisted appendage (Fig. 5B) [43,47,48,50]. After reduction, appendages show increased vascularity and decreased size [48].
Testicular Trauma
Testicular trauma causes hemorrhage and infarction of the parenchyma, ultimately leading to necrosis. In serious cases, disruption of the tunica albuginea can occur, also known as testicular rupture, accompanied by parenchymal protrusion. This is an indication for urgent surgery, and early surgical intervention improves long-term testicular function [14]. Sonographic findings include changes in echogenicity due to hemorrhage and ischemia, and irregular testicular contours and/or discontinuity of the tunica albuginea suggest testicular rupture (Fig. 6A). Although tunica albuginea breach per se was visualized in only 50% of cases of testicular rupture in one study, US showed a sensitivity of 100% and a specificity of 65% for testicular rupture [51].

Testicular rupture in a 13-year-old boy hit in the right testis by a foul ball on the first bounce.
A. The continuity of the tunica albuginea (arrowheads) is obscure at the lower pole, and the heterogeneous testicular tissue had prolapsed (arrows). B. In a color Doppler image, the prolapsed tissue had lost its vascularity (arrows). Laceration of the tunica albuginea was confirmed; it was reconstructed surgically, and prolapsed necrotic testicular tissue was resected (courtesy of Dr. M. Mikiko, National Center for Child Health and Development, Japan).
CEUS has been found to show no significant defect in enhancement in cases of minor trauma, and weak, inhomogeneous, or patchy enhancement for severely fractured testicles. Hematomas have been found to show no enhancement [14]. Magnetic resonance imaging may be an alternative modality when the use of US results in an inconclusive diagnosis [52].
Acute Idiopathic Scrotal Edema
AISE is a self-limiting disease, commonly seen in children under 10 years of age (mean, 6 years) [53]. Its etiology is unknown, but infection, allergic reaction, and angioedema are presumed [53]. AISE shows spontaneous regression, but recurrence is also seen, in up to 21% of cases [53]. The typical presentation is scrotal erythema and edema without tenderness [53]. Erythema and swelling can extend into the perineal and inguinal region in approximately 50% of cases. The rate of unilateral versus bilateral presentation varies across reports; the rate of bilateral presentation is 10%-100% [53,54]. Patients are usually afebrile, but systemic fever can be seen in up to one-third of cases [53]. Laboratory examinations show normal ranges, except for the possible presence of eosinophilia, supporting the theory of an allergic etiology [53]. Sonographic findings include scrotal wall thickening with a heterogeneous striated and edematous appearance (Fig. 7A, B) and hypervascularity (Fig. 7C) (called the fountain sign in bilateral cases), and possible inguinal lymphadenopathy [53-55]. However, these findings are non-specific, and ruling out other diseases is important. In AISE, morphology and vascularity are normal in the testis, epididymis, and spermatid cord [43,53,55].
Two cases of acute idiopathic scrotal edema: a neonate with scrotal swelling (A) and a 6-year-old boy with scrotal pain (B, C).
A. The bilateral scrotal walls are markedly thickened (arrows). B, C. The scrotal wall is markedly thickened (arrow in B) with increased vascularity (arrowheads in C). Symptoms improved spontaneously in both patients.
Ovarian Torsion
Ovarian torsion refers to a twisted vascular pedicle, which leads to vascular insult, resulting in ovarian ischemia and ultimately necrosis. Autoamputation can occur, especially in neonatal torsion [56]. This disastrous condition is also important in the pediatric field. Salvage rates in pediatric series vary across reports, ranging from only 0%- 27% in one report [57] to a majority of cases in another [58]. There appears to be significant individual variation in the time to infarction, and there is no clear golden time for surgery, in contrast to testicular torsion [57]. The age distribution is bimodal, with the two peaks occurring in infants and adolescents [59]. Ovarian torsion is much more common on the right side because of the smaller free pelvic space on the left side due to the sigmoid colon [60]. Torsion usually occurs in association with an ovarian mass. The mass is usually benign, typically an ovarian cyst or teratoma; the reported malignancy rate was only 1.8% in pediatric cases of ovarian torsion [61].
The most common sonographic findings are enlargement and heterogeneity [58]. A mass of >5 cm is 83% sensitive for ovarian torsion [59], although this finding is not specific. Ovarian hemorrhage is rare in prepubertal children because endometriosis and tubo-ovarian abscess are exceedingly rare. Thus, a hemorrhagic ovarian cyst suggests ovarian torsion [58], although false-positives have been reported [62].
In addition to cystic ovarian masses causing ovarian torsion, intraovarian cysts should be evaluated carefully. Peripherally located cysts (Fig. 8A), which are not true cysts and may be attributable to the transudation of fluid due to congestion, have a relatively high specificity of 74% [62,63]. Ovarian enlargement with multiple peripheral cysts can show a high positive predictive value (87.5%) and specificity (93.3%) [63]. The follicular ring sign, or perifollicular hyperechoic rim, has also been reported to have a high specificity (80%), and is seen starting in the early stage of torsion [64]. This sign should be differentiated carefully from the acoustic shadow artifact of normal follicles in terms of the following features: it is seen concentrically around the follicles and exhibits a perceptible thickness (1-2 mm) [64]. A transvaginal scan is recommended, if available, but this sign can also be detected on a transabdominal scan [64]. The intrafollicular fluid-debris level is an additional follicular feature with a high specificity (85%) [65].

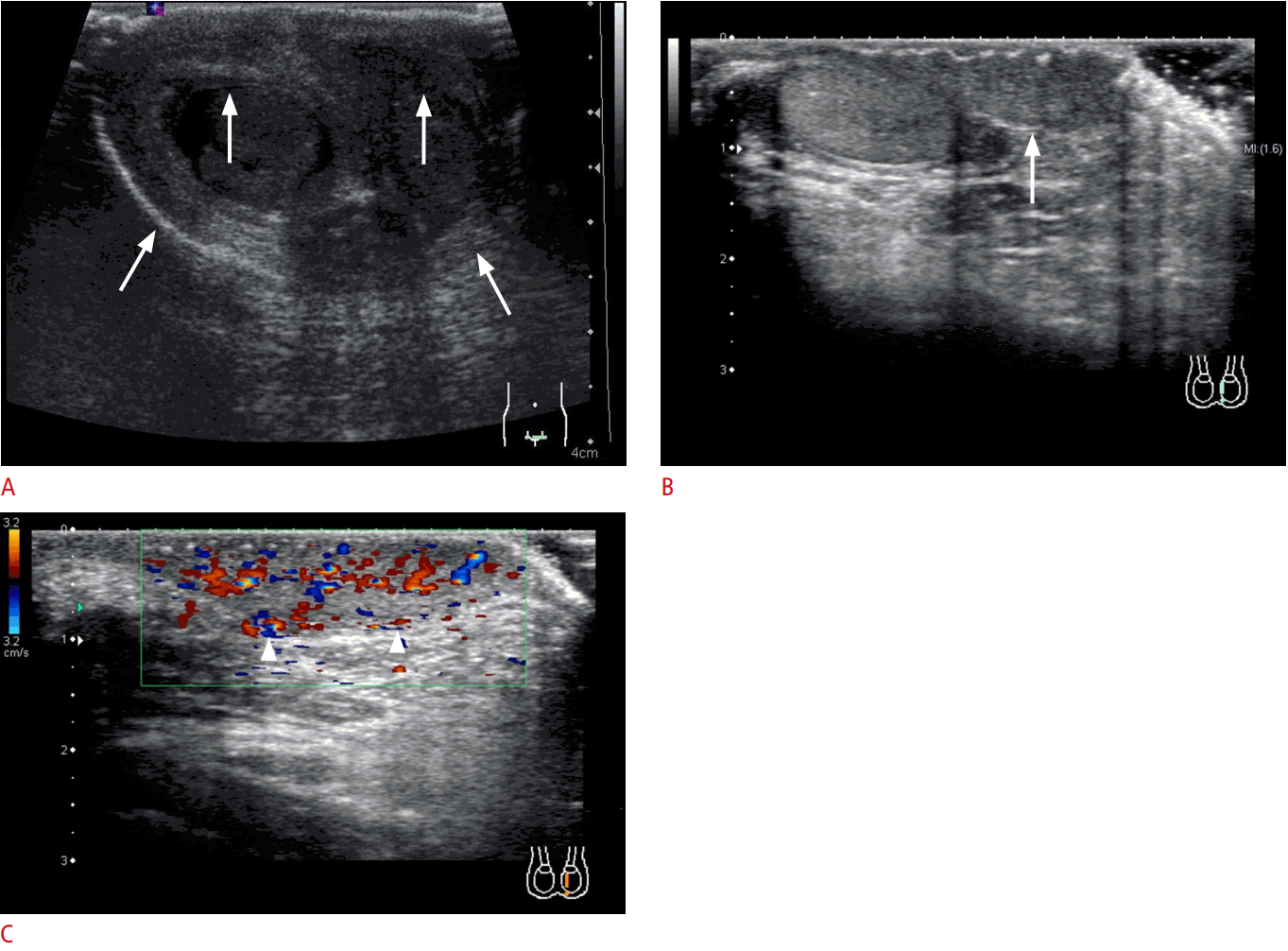
Ovarian torsion in a 16-year-old girl.
A. The affected ovary shows multiple peripherally located cysts (arrows). Note that the edge of the ovary is stretched (arrowheads) due to the torsion. B. The ovarian pedicle is twisted (in the center of spiral arrow) and can be seen as a whirlpool sign (Video clip 3). A large paraovarian cyst (the cause of torsion) is also seen (asterisk). C, D. The vascularity in the affected ovary is obscure (arrows in C) compared to the normal ovary (arrows in D) on superb monochrome microvascular imaging. E. An operative image shows the twisted pedicle (arrowheads) of the ovary (arrow). The asterisk indicates the transparent paraovarian cyst, and the dagger indicates the uterus.
Other classical signs include ovarian edema, abnormal ovarian position, free fluid in the pouch of Douglas, and the absence of ovarian vascularity (Fig. 8C) [58,63,66-70]. Venous flow is obstructed first, and arterial flow shows a “spiky” pattern. Both arterial and venous flow are then lost in the advanced stages [70]. The ability to make a diagnosis based on ovarian vascularity has considerable variation, with a positive predictive value of 19%-94% and a negative predictive value of 46%-100% [68,69,71]. There seem to be many factors affecting accuracy: dual supply from the uterine and ovarian arteries to the ovary, incomplete or intermittent torsion, the small size of vessels and low flow in children, and the deep location of the ovary. Moreover, the selected slice may not correctly reflect the vascularity of the entire ovary [72]. Recently, 3-dimensional assessment, which allows the evaluation of the whole ovary with quantitative vascular assessment, has been applied to ovarian torsion in adults [72-74], although this was performed with a transvaginal approach. Another solution could be CEUS. An animal study showed that the administration of contrast material enhanced the visualization of ovarian flow [75], and a case report showed decreased flow in a twisted ovary in a 6-year-old girl [76].
The whirlpool sign and a twisted pedicle have been reported to be useful and diagnostic (Fig. 8B, Video clip 3) [66,77,78]. This is a direct finding of torsion; however, the utility of the whirlpool sign has not been fully evaluated in pediatric series, where a transvaginal approach is not applicable [58]. For example, Lee et al. [78] showed 87% accuracy in an adult series, but they used a transvaginal probe in 47 patients, with the exception of four patients who were virgins or pregnant. They also stated that it was difficult to identify the whirlpool sign by a transabdominal approach [78]. This limitation seems to have resulted in the claim by some authors that US is not reliable in the diagnosis or exclusion of ovarian torsion in the pediatric population [59], although other authors insist on the utility of the whirlpool sign with a transabdominal approach [77]. The US diagnosis of ovarian torsion is not straightforward, and it is affected greatly by the operator (60%- 100%) [69]. Thus, other modalities should be used in equivocal cases.
Neonatal ovarian torsion should be considered separately because this condition usually occurs antenatally. Of patients with neonatal ovarian torsion, 91% had ovarian cysts on prenatal US [79]. US shows complex cystic masses, suggesting hemorrhage, with signs such as multi-septation, echogenic debris, and/or fluidfluid levels (Fig. 9) [67,79]. Calcification can also be seen in some cases [79]. There are several pitfalls in the diagnosis of neonatal torsion. Half of the ovaries with torsion may show an extrapelvic location and could be misdiagnosed as appendicitis or as an abscess [67]. Moreover, a thickened wall can show an echogenic inner rim, mimicking duplication cysts (Fig. 9A) [79].

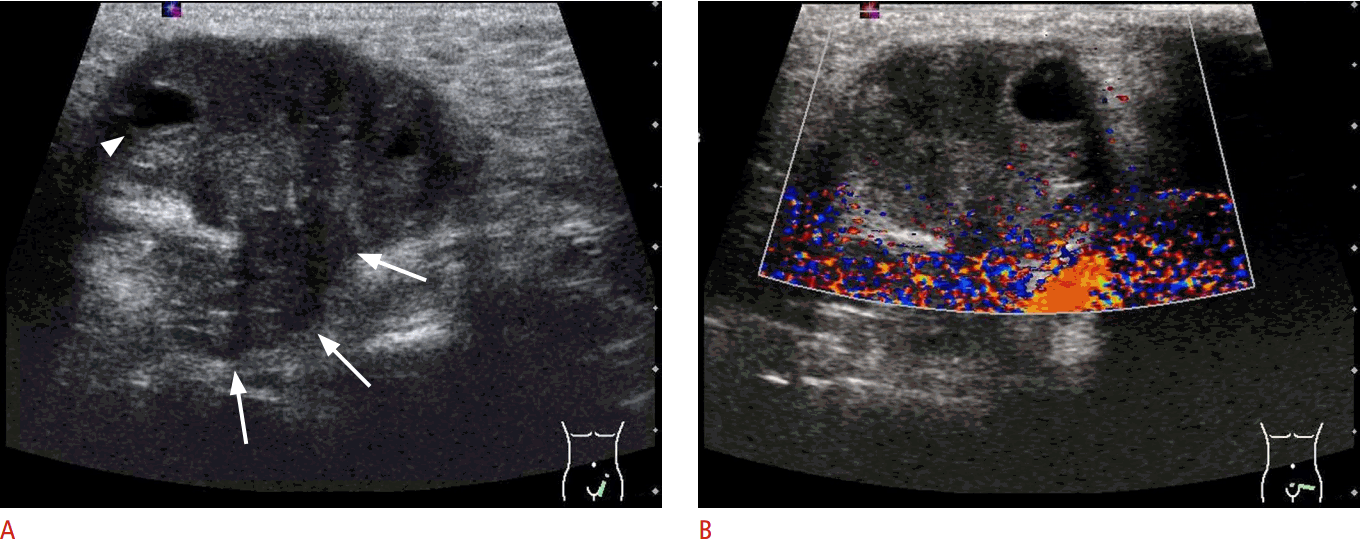
Two cases of ovarian torsion in neonates.
A. A cystic mass is seen in a day 0 baby. The cyst contains debris. The cyst wall shows thickening and an echogenic inner rim (arrows), which mimics a duplication cyst. B. A cystic mass is seen in a day 1 baby. The cyst wall is thickened, and the cyst shows multi-septation internally. This mimics a lymphatic malformation (lymphangioma).
Another special condition of ovarian torsion is seen in ovarian hernia [80-84]. Ovarian hernia is commonly seen in infants, and the incidence of irreducible ovaries is 6%-7% for inguinal hernias in girls [83]. As the ovarian vascular pedicle is narrowed in the internal ring, the size of the ovary is relatively greater than the pedicle. This causes a predisposition for the ovary to twist [84]. Ovarian hernia has a risk of torsion, and it is necessary to evaluate the possibility of torsion, including vascularity. If torsion exists, this should be treated as an emergency [84]. Unlike deep in the pelvis, US with a high-frequency linear probe is useful in evaluating inguinal hernia. Ovarian hernia is readily recognized as an oval mass with small cysts on US [80]. The sonographic findings of twisted ovarian hernia include multiple cysts, ovarian enlargement, and heterogeneous hyperechogenicity [82]. A twisted pedicle and decreased vascularity can also be seen (Fig. 10).
Ovarian hernia with torsion in an 8-month-old girl with left inguinal swelling.
A, B. The mass contains multiple cysts inside of it, suggesting that is an ovary. Twisting of the ovarian pedicle is seen (arrows in A), and vascularity is not detectable on a color Doppler image (B). The follicle contains debris (arrowhead in A).
Emergencies due to Associations of Urogenital Anomalies
If atresia of the uterine cervix, vagina, or hymen is present, menstrual blood discharge is obstructed at menarche, leading to hematometra, also called menstrual molimina. Usually, atresia goes unnoticed until abdominal pain occurs. Thus, radiologists sometimes encounter cases of hematometra presenting with acute abdominal pain. US shows uterine dilatation with echogenic fluid (Fig. 11), with or without cervical dilatation, according to the area of atresia [85]. Fluid-fluid levels can be seen in some cases (Fig. 11).
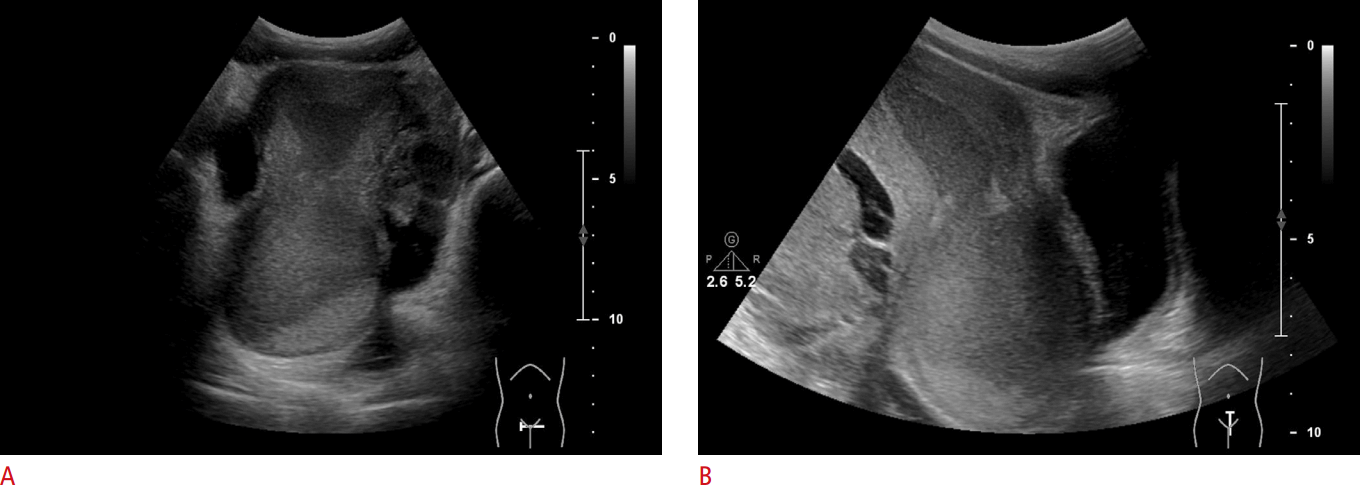
Hematometra in a 10-year-old girl before menarche, with recurrent abdominal pain over a few months (menstrual molimina).
Axial (A) and sagittal (B) images show a dilated uterine cavity with echogenic fluid, with fluid levels suggesting hydrometra with uterine cervical atresia. Note the intra-abdominal hematoma with complex echogenicity.
Uterine or vaginal atresia with Müllerian duct anomalies is frequently accompanied by renal anomalies. In particular, renal aplasia is highly predictive of an ipsilateral obstructive Müllerian anomaly, with this association found in more than 50% of cases [86]. The combination of unilateral renal aplasia, a duplicated uterus, and vaginal occlusion with Gartner pseudocyst was initially reported by Herlyn and Werner in 1971 (Herlyn-Werner syndrome) [87]. Wunderlich [88] reported the combination of renal agenesis and hemicervical atresia in 1976 (Wunderlich syndrome). In 1979, these cases were reported as Herlyn-Werner and Wunderlich syndromes [89]. These concepts are often combined as Herlyn-WernerWunderlich syndrome, although the claim has been made that such a syndrome does not exist [90]. Recently, the term obstructed hemivagina and ipsilateral renal anomaly has also been commonly used because this concept incorporates all kinds of uterine anomalies other than a duplicated uterus. Thus, the definition of syndromes is complex; however, the key fact is quite simple: there is an association of urogenital anomalies on the same side.
The typical presentation of these syndromes is abdominal pain at menarche. This diagnosis is relatively easy for pediatric radiologists familiar with these anomalies. However, these cases are sometimes initially misdiagnosed, probably because it is not possible to predict whether patients will initially visit emergency rooms, physicians, surgeons, or pediatricians. Moreover, radiologists may not always be involved. Thus, these patients should be noted before symptoms with routine US. In patients with renal dysplasia, the importance of investigating hemiuterine atresia has been stressed [91]. In the neonatal period, the uterus is relatively large because of maternal and placental hormones, and is suitable for the investigation of uterine anomalies (Fig. 12). However, the uterus becomes smaller after this period until adolescence and is difficult to see whether a uterine anomaly exists (Fig. 13). Thus, in cases of renal dysplasia, especially renal aplasia, an investigation to determine whether uterovaginal anomalies are present is recommended in the neonatal period [92].

Right renal agenesis with duplicated uterus and ipsilateral vaginal fluid (2-day-old neonate).
A. The right kidney shows agenesis. B-D. A pelvic scan clearly shows the duplicated uterus (arrows in B and C), with intravaginal fluid ipsilateral to renal agenesis (arrowheads C and D), suggesting obstructed hemivagina and ipsilateral renal anomaly. The uterus is mature in this period, in contrast to that of a 6-year-old girl, shown in Fig. 13. This period is suitable for screening for uterine anomalies.
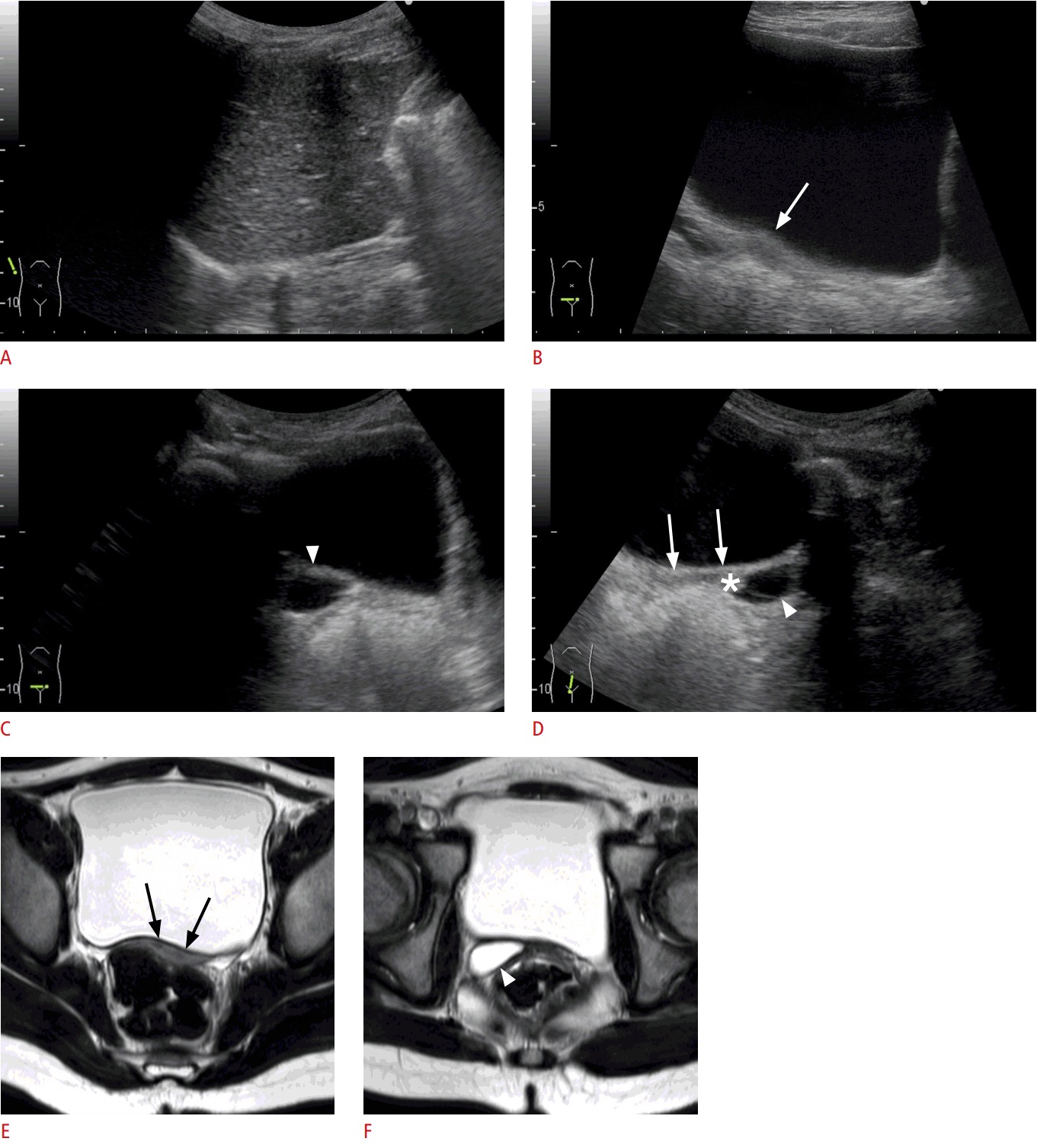
Right renal dysplasia with Müllerian duct anomalies with ipsilateral vaginal fluid (6-year-old girl).
A. The right kidney was not seen due to atrophy from the previously diagnosed multicystic dysplastic kidney. B -D. Intravaginal fluid is seen ipsilateral to renal dysplasia (arrowheads in C [axial image] and D [sagittal image]). The asterisk in D indicates the vaginal portion of the uterine cervix. The uterine anomaly could not be recognized due to its small size at her age (arrows in B [axial image] and D [sagittal image]). E. On magnetic resonance imaging, two lumina were barely recognized (arrows). F. A caudal slice shows that the vaginal fluid was ipsilateral to renal dysplasia (arrowhead). The size of the uterus is small at this age, in contrast to Fig, 12 in a neonate. Uterine anomalies should be screened during the neonatal period.
Conclusion
The US of pediatric urogenital emergencies is reviewed in this article. Although many pitfalls exist in making a correct diagnosis, knowledge of the correct approach can be of great help. New techniques, including elastography, 3-dimensional US, and CEUS, are also discussed here. These techniques should be used as additional tools for making correct diagnoses.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary Material
Video clip 1.
Normal testicular appendage in sagittal view scanned from lateral to medial side.
Video clip 2.
Testicular appendage torsion in sagittal view scanned from lateral to medial side.
Video clip 3.
Twisted pedicle (whirlpool sign) in ovarian torsion.
