Ethanol ablation as a treatment strategy for benign cystic thyroid nodules: a comparison of the ethanol retention and aspiration techniques
Article information
Abstract
Purpose
We compared the efficacy and safety of two ethanol ablation techniques-ethanol aspiration and ethanol retention-for benign cystic thyroid nodules.
Methods
From October 2008 to September 2013, 113 patients who were treated using the ethanol aspiration technique (February 2008 to December 2010) and 108 patients who were treated using the ethanol retention technique (January 2011 to September 2013) were enrolled (male:female ratio, 53:168; mean age, 48.1 years; range, 18 to 80 years). The patient sample had 94 cystic and 127 predominantly cystic thyroid nodules. The volume reduction ratio (VRR) at the last follow-up, improvements in symptoms and cosmetic scores, vascularity, pain, and major complications were evaluated and compared between the ethanol aspiration group and ethanol retention group. We also performed a subgroup analysis according to the proportion of the solid component, in which VRR, symptom and cosmetic scores, and therapeutic success were compared.
Results
No statistically significant difference in VRR was found between the ethanol retention group and the ethanol aspiration group (83.2%±32.8% vs. 86.1%±18.4%, P=0.416) while patients who underwent the retention technique were more likely to experience pain after treatment (P=0.001). VRR, symptom and cosmetic scores, and therapeutic success did not significantly differ between techniques in either group in the subgroup analysis.
Conclusion
The ethanol aspiration technique may be preferable to the ethanol retention technique for treating benign cystic and predominantly cystic thyroid nodules, because a comparable VRR can be expected with less pain.
Introduction
As the incidence of thyroid nodules has increased, ultrasonography (US)-guided ablation techniques have become widely used to treat benign thyroid nodules [1-4] and recurrent thyroid cancers [5,6]. Even though simple aspiration has been suggested as the first-line management tool for both diagnostic and therapeutic purposes in patients with benign cystic thyroid nodules, a high recurrence rate has been reported, depending on the number and volume of the aspirated cysts. US-guided ethanol ablation (EA) has been recommended as powerful treatment strategy for symptomatic benign cystic (no solid portion) or predominantly cystic (cystic portion >50%) thyroid nodules [7-9], and it has been shown to be effective and safe in previous studies [10,11].
Two different types of EA techniques have been used; one is the ethanol retention technique, which refers to the retention of injected ethanol inside the cystic cavity of the nodule [12], and the other is the ethanol aspiration technique, in which the injected ethanol is completely aspirated after several minutes [11]. Generally, EA involves removing the cystic fluid from the thyroid nodule and then injecting a volume of 99% ethanol corresponding to roughly 50% of the aspirated fluid volume. Some authors have argued that the injected ethanol should be aspirated, as doing so can prevent complications such as pain and/or perithyroidal fibrosis due to ethanol leakage [13]. In contrast, the retention technique might be preferable since it is technically simple and can save time [12]. Some authors have suggested that the sustained presence of ethanol can result in continuous coagulative necrosis, small vessel thrombosis, hemorrhagic infarction, and reactive fibrosis, which might provide a delayed ablation effect, especially for predominantly cystic nodules [14,15].
To our knowledge, very few studies have compared the efficacy and safety of the retention and aspiration techniques. For example, Kim et al. [12] suggested that percutaneous ethanol injection without the aspiration of ethanol-mixed fluid seemed to be the preferable method for treating benign cystic thyroid nodules because of the simplicity of the procedure. Therefore, we compared the efficacy of these two techniques in treating benign cystic and predominantly cystic thyroid nodules.
Materials and Methods
Study Design
This retrospective observational study was approved by the Institutional Review Board of our institution. Informed consent for US-guided procedures was obtained from all patients prior to each procedure.
In this study, we enrolled 113 patients who were treated using the ethanol aspiration technique (from February 2008 to December 2010) and 118 patients who were treated using the ethanol retention technique (from January 2011 to September 2013). The inclusion criteria were as follows: (1) a thyroid nodule with a cystic proportion greater than 50%; (2) the presence of pressure symptoms or cosmetic problems; (3) serum levels of thyroid hormones, thyrotropin, and calcitonin within normal limits; (4) cytological confirmation that the lesion was benign after two separate US-guided fine needle aspiration (FNA) biopsies [16,17]; and (5) no malignant features on US examinations (i.e., a taller-than-wide shape, a spiculated margin, marked hypoechoicity, or the presence of microcalcifications) [18].
Pre-enrollment Procedures and Assessment
The US, US-guided FNA, and laboratory and clinical results were evaluated before ablation. Two radiologists (J.H.B. and J.H.L., with 20 and 15 years of US thyroid experience, respectively) performed US and US-guided FNA using a 5-14 MHz linear probe fitted to a real-time US system (iU22 unit, Philips Healthcare, Bothell, WA, USA; Hitachi Logos E, EUB-7500, Hitachi Medical System, Tokyo, Japan). The nodule size, the proportion of the solid component, and vascularity were assessed. The three orthogonal diameters (the largest diameter and 2 perpendicular diameters) and volume of each nodule were evaluated [19]. Nodules without an obvious solid component were classified as cystic, whereas those with a cystic component of more than 50% were classified as predominantly cystic [9,10]. Vascularity was graded using four categories (grade 0, no intranodular vascularity; grade 1, perinodular vascularity only; grade 2, intranodular vascularity <50%; and grade 3, intranodular vascularity >50%) [11]. At enrollment, patients were asked to rate their symptom score on a 10-cm visual analogue scale, and their cosmetic grade was recorded by a physician (1, no palpable mass; 2, a palpable mass but no cosmetic problem; 3, a cosmetic problem only apparent upon swallowing; and 4, a readily detected cosmetic problem) [20].
Procedure
All procedures were performed in an outpatient setting. Patients were placed in a supine position with mild neck extension. After skin sterilization and anesthesia with 2% lidocaine at the puncture site, a 16- to 18-gauge needle was inserted into the nodule under US guidance using the transisthmic approach [11]. The internal fluid was aspirated to the maximum extent possible, and saline irrigation was used to remove residual debris and/or colloid. Ethanol (99%) was then injected slowly into the cystic cavity. The volume of injected ethanol usually corresponded to roughly 50% of the volume of the aspirated fluid. In the retention group, the injected ethanol was not removed after the procedure, whereas in the aspiration group, it was completely removed after 2-5 minutes based on the nodule size and operator’s preference [11,21,22]. In this study, we used a single-puncture technique for ethanol aspiration (i.e., retention of ethanol with the needle in place, followed by aspiration of ethanol after a few minutes) which can reduce discomfort due to additional needling during aspiration, as discussed in a previous study [12]. During and immediately after the procedure, the presence of any discomfort or complications was evaluated. Procedure-related pain was graded using four categories (grade 0, no pain or mild pain similar to the pain experienced during the lidocaine injection; grade 1, pain greater than the lidocaine injection, but not needing medication; grade 2, pain needing medication; and grade 3, EA procedure terminated before completion due to severe pain) [11]. Patients stayed in the hospital for 30 minutes after the procedure for observation.
The volume reduction ratio (VRR) was defined as
Follow-up
US examinations were performed in all patients at 1-, 6-, and 12-month follow-up examinations. After 1 year of treatment, an US examination was performed annually. Treatment efficacy was evaluated by measuring the VRR, therapeutic success (VRR >50%) [23,24], and vascularity of the treated thyroid nodules on US, and by assessing the improvement of symptoms and cosmetic problems [11]. Any adverse events, including pain, were recorded. Repeat EA or radiofrequency ablation (RFA) was performed on patients with nodules showing a VRR <50% or a residual solid portion with internal vascularity on a follow-up US examination, and/or incompletely resolved symptoms (symptom score reduction <50%) or cosmetic problems [25,26].
Statistical Analyses
We compared the VRR, symptom and cosmetic score, vascularity, and complications between the ethanol retention and aspiration groups. A subgroup analysis was conducted according to the proportion of the solid component, and the VRR, symptom and cosmetic scores, and therapeutic success were compared in this analysis as well. The Fisher exact test and the Wilcoxon signed-rank test were used for the analyses. P-values <0.05 were considered to indicate significant differences. All statistical analyses were performed using SPSS for Windows ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
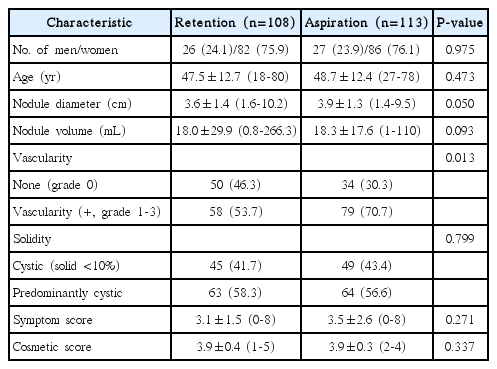
From January 2011 to September 2013, 118 patients treated using the ethanol retention technique were identified as being eligible for enrollment in this study. Ten patients were lost to follow-up. As a result, the retention group included 108 patients. By contrast, 113 patients treated using the ethanol aspiration technique between October 2008 and December 2010 were enrolled (27 males and 86 females; mean age±standard deviation, 48.7±12.4 years; range, 27 to 78 years). Therefore, a total of 221 patients (108 treated using the retention technique and 113 treated using the ethanol aspiration technique) were ultimately included in this study. The baseline characteristics of the patients in the two groups are summarized in Table 1.
The outcomes of the retention and aspiration groups at the last follow-up are summarized in Table 2. The mean follow-up period was 13.9±9.2 months (range, 1 to 31 months) in the retention group and 14.2±12.1 months (range, 1 to 36 months) in the aspiration group (P=0.836).
The VRR did not show a statistically significant difference between the two groups, as it was 83.2%±32.8% (range, -147.2% to 100%) in the retention group and 86.1%±18.4% (range, 8% to 100%) in the aspiration group (P=0.416). The mean symptom scores, cosmetic scores, and vascularity were not significantly different between the retention and aspiration groups (P=0.677, P=0.277, and P=0.062, respectively). There were no major complications in either group (P>0.99), although pain during the procedure was significantly greater in the retention group (P=0.001).
The subgroup analysis according to the proportion of the solid component is summarized in Table 3. No significant differences were found between the retention and aspiration groups in the cystic and predominantly cystic subgroups in the mean VRR at the last follow-up, symptom score, cosmetic score, or therapeutic success (P=0.364, P=0.129, P=0.671, and P=0.206 in cystic nodules, respectively; P=0.761, P=0.710, P=0.118, and P=0.070 in predominantly cystic nodules, respectively).
Discussion
This study showed that both the ethanol retention and aspiration techniques were safe and effective for the treatment of cystic and predominantly cystic thyroid nodules. The VRR was not significantly different between the ethanol aspiration and retention groups. Neither technique was associated with any major complications, although pain was significantly greater in the retention group. Therefore, ethanol aspiration may be preferable for treating benign cystic and predominantly cystic thyroid nodules, because we can expect a comparable VRR with less pain. In the subgroup analysis, the retention technique showed no benefit for either cystic or predominantly cystic nodules.
Regarding the treatment of cystic and predominantly cystic nodules, previous randomized clinical trials [10,21] and guidelines [16] have suggested that EA is an effective treatment strategy that is preferable to RFA. However, the advantages and disadvantages of the retention and aspiration techniques are still under debate. Some authors prefer the retention technique due to its simplicity [12]. In a retrospective study by Kim et al. [12], the authors found no significant difference in the therapeutic success rate between the ethanol retention and aspiration techniques. Considering the higher incidence of intracystic hemorrhage on follow-up and the longer procedure time in the aspiration group, they concluded that the ethanol retention technique should be preferred. However, retained ethanol can cause extrathyroidal ethanol leakage, resulting in pain, voice change, and a feeling of intoxication [12,27-29]. Ethanol leakage into the perithyroid soft tissue can induce reactive fibrosis, which can be minimized by using the transisthmic approach [20,30]. Rare but serious complications related to extrathyroidal ethanol leakage, such as skin necrosis and venous thrombosis, have also been reported [13]. Therefore, we believe that the ethanol aspiration technique is safe and effective for treating benign cystic and predominantly cystic thyroid nodules.
It is known that the proportion of the solid component is a major predictive factor for the success of EA [11,25]. The solid component is relatively resistant to ethanol, and the vessels in the solid portion drain the ethanol, lowering the therapeutic efficacy of EA [22]. Additionally, vessels in the solid portion can cause internal bleeding during the follow-up period, which leads to treatment failure or recurrence of symptoms. When we performed a subgroup analysis according to the proportion of the solid component, no significant difference was found in the mean volume reduction between the two techniques in either the cystic or predominantly cystic group. Even though some studies have argued that the ethanol retention technique may be beneficial for predominantly cystic nodules, as the retained ethanol can diffuse into the solid portion and induce continuous ablation [14], our study did not show a statistically significant difference in the VRR or therapeutic success rate in either cystic thyroid nodules or predominantly cystic thyroid nodules.
The present study has several limitations. First, since this was a retrospective single-center study, selection bias may have been present. Further, the included population was relatively small, which could hamper the generalizability of our results.
In conclusion, ethanol aspiration may be preferable for treating benign cystic and predominantly cystic thyroid nodules, because we can expect a comparable VRR to that obtained using the ethanol retention technique, but with less pain.
Notes
No potential conflict of interest relevant to this article was reported.