Usefulness of resistive index on spectral Doppler ultrasonography in the detection of renal cell carcinoma in patients with end-stage renal disease
Article information
Abstract
Purpose:
The aim of this study was to explore the usefulness of the resistive index (RI) on spectral Doppler ultrasonography (US) in the detection of renal cell carcinoma (RCC) in patients with end-stage renal disease (ESRD).
Methods:
Seventeen ESRD patients with kidneys in which renal masses were suspected in routine US were subjected. They underwent computed tomography scans and additional Doppler US for the characterization of the detected lesions. All underwent radical nephrectomy with the suspicion of RCC. Fourteen patients finally were included. RI measurements were conducted in the region of the suspected renal mass and the background renal parenchyma. The intraclass correlation coefficient was used to assess the reproducibility of the RI measurement. A paired t-test was used to compare the RI values between the renal mass and the background renal parenchyma (P<0.05).
Results:
The RI values measured at the RCCs were significantly lower than those measured at the background renal parenchyma (0.41-0.65 vs. 0.75-0.89; P<0.001). The intrareader reproducibility proved to be excellent and good for the renal masses and the parenchyma, respectively (P<0.001).
Conclusion:
RI on spectral Doppler US is useful in detecting RCC in patients with ESRD. The RI values measured at the RCCs were significantly lower than those measured at the background renal parenchyma.
Introduction
Incidence of renal cell carcinoma (RCC) in patients with end-stage renal disease (ESRD) is higher than that in the general population [1,2]. In general, ultrasonography (US) is the most widely used screening tool for RCC in patients with ESRD. In the case of a suspicion of a renal mass in an ESRD kidney upon US, a further examination using contrast enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) may be necessary to clarify the detected lesion.
As the ESRD kidney shows heterogeneous and hyperechoic parenchymal echo-texture and irregular parenchymal contour associated with uneven parenchymal atrophy and compensatory hypertrophy, it may be more difficult to detect RCC in ESRD kidneys using US than in non-ESRD kidneys. A compensatory hypertrophy may sometimes produce a mass effect or compress the pelvocalyceal systems and thus closely mimic renal neoplasm [3]. It is important not only to be able to detect a renal mass in the ESRD kidney but also to be aware of the tumor mimicking compensatory hypertrophy so as to avoid unnecessary surgical procedures to preserve what is remaining of the functioning parenchyma.
Previously, investigators have assessed the value of CECT and diffusion-weighted image (DWI) for detecting RCC in ESRD kidneys [4,5]. Despite the excellent results, there are some concerns regarding the use of CECT because iodinated contrast agents have the risk of contrast-induced nephropathy. Furthermore, although DWI does not have the risks caused by ionizing radiation or contrast agents, it is limited in terms of accessibility.
Among the several diagnostic parameters of US, the resistive index (RI) can be used in the detection of RCC in ESRD kidneys. RI values tend to increase in proportion to the progression of glomerular sclerosis, tubulointerstitial changes and arteriosclerosis in ESRD kidneys. In contrast, the neovascularized arterial wall of a malignant tumor such as RCC is known to be deficient in the muscular medial layer which results in an increase of the diastolic flow and correspondingly lower RI values [6,7]. Considering the relatively high RI of the background parenchyma of ESRD kidneys, we speculated that the RI measured in the intra-tumoral artery of RCC would be lower than that of the background renal parenchyma. This study was undertaken to assess the usefulness of the RI on spectral Doppler US in the detection of RCC in patients with ESRD.
Materials and Methods
Patient Selection
Our institutional review board approved this retrospective study and waived the requirement for informed consent. From February 2007 to September 2013, we identified 17 ESRD patients with kidneys in which renal masses were suspected in routine US. They underwent computed tomography (CT) scans and additional renal Doppler US for the characterization of the detected masses. All underwent radical nephrectomy with the suspicion of RCC in CT scans and additional renal Doppler US. Three of these patients were excluded from this study because their RI values could not be measured due to excessively small sizes or a lack of vascularity of the renal mass and background renal parenchyma, or because acquiring spectral Doppler US was difficult due to a lack of respiratory cooperation. This study was a retrospective evaluation of the collected data, and all clinical information including patient demographics and histopathological findings was reviewed by one author (SYK).
US Examination and Measurement of RI
Real-time US devices with color Doppler capacity (Aplio XG, Toshiba, Japan; iU22 Xmatrix, Philips, Netherland) and 3-5-MHz convex transducers were used. Renal US procedures including color and spectral Doppler were performed by a genitourinary radiologist (JYC with 17 years of experience).
For stable image acquisition, all patients were set in the decubitus position with an upward location of the involved kidney and rested for 5 minutes prior to the procedure. All patients underwent gray scaled, color and spectral Doppler US, successively. All images were acquired when the patients held their breath after inspiration. After observation of the intrarenal arteries by color Doppler US, the spectral Doppler waveforms were obtained from the intratumoral arteries (those of the renal masses) and from segmental arteries (those of the renal parenchyma) with the inspection of three continuous Doppler waveforms. The Doppler sample volume was set at 5 mm, and the Doppler scale was maximized by using the smallest possible frequency range (minimum pulse repetition frequency) that did not produce aliasing. The wall filter was set to low in order to detect low velocity. The penetration mode (lower megahertz, MHz) was also used in order to correct the poor vascularity on the ESRD kidney.
RI was calculated as (peak systolic velocity - end-diastolic velocity) / peak-systolic velocity. The RI of the suspected renal mass lesion on the gray scaled US or previous CT scans was measured twice and that of the renal parenchyma was measured three times. The mean RI values of the mass and the renal parenchyma were calculated and analyzed.
Histopathological analyses
All patients underwent radical nephrectomy under the impression of RCC in the ESRD kidney. The histopathological analyses were performed by the genitourinary pathologist.
Statistical analysis
A paired t-test was used to compare the RI values between the renal mass and the background renal parenchyma. The intraclass correlation coefficient (ICC) was used to assess the reproducibility of the RI measurements. All statistical analyses were performed with Medcalc (ver. 12.0, Medcalc Software, Mariakerke, Belgium). A twotailed P-value of <0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of Patients and Renal Lesions
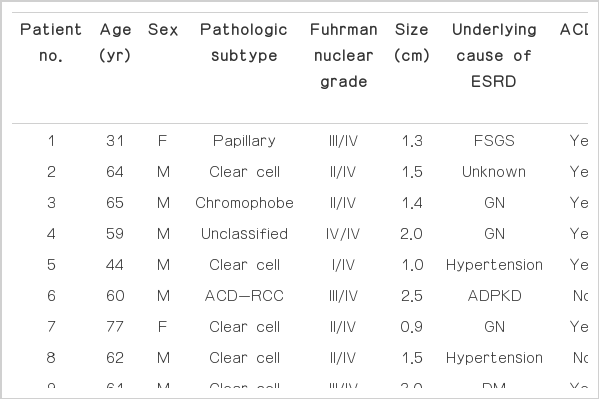
The characteristics of the patients and focal renal lesions are described in Table 1. In all, 14 patients (12 men and 2 women; mean age, 58.6±13.5 years; age range, 31 to 85 years) were included in our study.
Fourteen patients had RCC (mean size, 1.73±0.80 cm; range, 0.90 to 2.50 cm). The histopathological subtypes included the clear cell (n=8), papillary (n=2), chromophobe (n=1), acquired cystic kidney disease-associated (n=2), and unclassified (n=1) types. The underlying causes of ESRD were as follows: glomerulonephropathy (n=4), hypertension (n=4), diabetes mellitus (n=3), autosomal dominant polycystic kidney disease (ADPKD) (n=1), and unknown (n=2). The mean serum creatinine level was 7.09±4.42 mg/dL (range, 1.16 to 14.59 mg/dL). The mean estimated glomerular filtration rate (GFR) level was 16.19±17.50 mL/mg/1.73 m2 (range, 3.4 to 63.4 mL/mg/1.73 m2). Eight patients showed acquired cystic renal disease. Nine patients underwent dialysis. The mean period of the dialysis was 3.49±5.80 years (range, 0 to 20.0 years).
Gray Scaled and Color Doppler US Findings of the Renal Masses
Among the 14 RCC lesions, 10 lesions showed mainly solid features and 4 lesions showed complex cystic (solid and cystic) features in the gray scaled US. The echogenicity of the mass in the gray scaled US was as follows: hyperechoic (n=3), isoechoic (n=7), and hypoechoic (n=4). Most of the RCCs (n=10) showed increased intra-tumoral vascularity with peripherally hypervascular patterns, although the ESRD kidneys showed decreased vascularity.
RI of the Renal Masses and Background Renal Parenchyma
The RI of the renal masses and background renal parenchyma are shown in Fig. 1. In patients with RCC, the mean RI of the RCCs was 0.56±0.06 (range, 0.41 to 0.65). The mean RI of the background renal parenchyma was 0.81±0.04 (range, 0.75 to 0.89). The RI values measured at RCCs were significantly lower than those measured at the background renal parenchyma (0.41-0.65 vs. 0.75-0.89; P<0.001). Since there was no overlap between the RI values in the RCCs and the renal parenchyma, it is possible to assign cut-off values in the range of 0.65-0.75 for the discrimination of the RCCs from the background renal parenchyma (Fig. 2).

The resistive index (RI) of the renal masses and the background renal parenchyma.
In patients with renal cell carcinoma (RCC), the mean RI of the RCCs is 0.56±0.06 (range, 0.41 to 0.65). The mean RI of the background renal parenchyma is 0.81±0.04 (range, 0.75 to 0.89). The RI values measured at RCCs are significantly lower than those measured at the background renal parenchyma (0.41-0.65 vs. 0.75-0.89; P<0.001). There is no overlap between the RI values in the RCCs and renal parenchyma. In contrast with patients with RCC, the pseudo-tumor shows a higher RI value (0.92) than the renal parenchyma (0.86).

A 62-year-old patient with a transplanted kidney.
A. On gray scale ultrasonography, an exophytic low echoic lesion (annotated as "A") is observed in the right native kidney with a peripherally hypervascular feature (RK, right kidney). B. The resistive index (RI) value of the lesion is 0.63. The mean RI value of the lesion is 0.65. C. The RI value of the renal parenchyma is 0.82. The mean RI value of the lesion is 0.85. D. In the corticomedullary phased computed tomography images, the lesion shows early heterogeneous enhancement (arrows). After radical nephrectomy, this lesion was confirmed as clear cell renal cell carcinoma in the end-stage renal disease kidney.
Reproducibility of RI Values
For the 14 renal mass lesions, the RI values showed excellent reproducibility with ICC of 0.96 (P<0.001). The RI values of the background parenchyma also demonstrated good reproducibility with ICC of 0.80 (P<0.001).
Discussion
It is well known that the incidence of malignancy in patients with ESRD is higher than that in the general population, possibly due to their immunosuppressed condition [8]. The most common malignant neoplasm is RCC. In general, RCC in patients with ESRD is known to grow at a rate of approximately 0.5-1.0 cm yearly [1,9]. Ninety percent of patients treated with dialysis for more than 10 years develop acquired cystic renal disease, and the risk of RCC increases considerably in patients undergoing dialysis for more than 10 years. Gulanikar et al. [10] reported that the sensitivity of screening US in ESRD patients was good and that the positive predictive value of a solid mass was 100%. Screening CT is more sensitive than US but CT is not as cost-effective as US. Repeated follow-up imaging to screen for RCC including US is important in ESRD patients [2].
However, it is difficult to detect RCC in an ESRD kidney using the conventional gray scaled or color Doppler US because the ESRD kidney shows a heterogeneous hyperechoic parenchymal echotexture and diffuse parenchymal thinning with uneven thickness. Furthermore, long-term dialysis in patients with ESRD almost invariably results in acquired renal cystic disease in ESRD kidneys. The numerous acquired cysts that develop may interrupt the appropriate imaging acquisition for the diagnosis of renal neoplasm in ESRD kidneys. Sometimes, segmental or regional sparing and subsequent hypertrophy in non-uniform parenchymal atrophy known as compensatory hypertrophy can mimic renal tumorous conditions [3].
Previously, there have been efforts to detect RCC in patients with ESRD kidneys using several modalities. Among them, early CECT and DWI have shown promising results. Takebayashi et al. [4] demonstrated that early enhanced helical CT was able to detect more RCCs than delayed enhanced helical CT in ESRD patients with and without acquired cystic kidney disease because the cortex of the ESRD kidneys shows minimal enhancement in the early phase, rendering higher differences in the attenuation values between the RCC and the atrophic parenchyma. However, as ESRD patients require life-long follow-up, screening with CECT may be burdensome in that it uses ionizing radiation and poses a risk for contrast-induced nephropathy. Goyal et al. [5] reported that DWI could successfully differentiate between RCCs and pseudotumors. However, as all but one of the cases of RCCs in their study did not have ESRD, the true value of DWI in differentiating RCCs and pseudo-tumors in ESRD patients remains in question. Moreover, although MRI is increasingly being used for the detection and characterization of renal masses, it is still limited in terms of accessibility compared with CT or US.
In our study, we found that RCCs arising in ESRD kidneys showed significantly lower RI values than the background renal parenchyma. There was no overlap at all in the measured RI values between the two. Glomerulosclerosis, tubulointerstitial damage and vascular lesions have been reported to correlate with an increase of RI. In chronic renal disease, an RI value of more than 0.70 is an independent risk factor for worsening renal function [11-13]. RI is thought to be a complex integration of arterial compliance, pulsatility and peripheral resistance. RI correlates well with renal arteriolosclerosis, indicating that RI can reflect renal vascular resistance. On the other hand, RI correlates with extrarenal markers of vascular stiffness, such as the intima-medial thickness of the femoral and carotid arteries [13-15].
In an angiographic study, 92% of the RCCs demonstrated tumor neovascularity. Histopathologically, neovascularized vessels are primitive, avascular channels that lack smooth muscle and often consist of an endothelial layer and connective tissue alone [6]. Since the neovascularized arterial walls of malignant tumors such as RCCs lack normal arteriolar smooth muscle, particularly the muscular medial layer, low resistance to flow in such vessels can be expected. The lack or paucity of the medial layer results in an increase of the diastolic flow of the tumor vessel. The RI measured from a neovascularized intra-tumoral artery is expected to be lower than that from the adjacent parenchymal artery. As the RI of the renal parenchyma of an ESRD kidney is high (more than 0.70), the increase of diastolic flow (and decrease of RI) in a suspected renal mass in an ESRD kidney is suggestive of RCC [6,7].
There was one patient with histopathologically proven com- pensatory hypertrophy that manifested as a pseudo-tumor in US. The mean RI value was 0.92 and that of the background renal parenchyma was 0.86. Because there was only one such case, we could not draw a statistically meaningful strong conclusion regarding the value of RI in differentiating between RCCs and pseudo-tumors in patients with underlying ESRD. However, we believe that this preliminary comparison brings insight and interest for further investigation. Compensatory hypertrophy consists of focal tissue hypertrophy of the relatively preserved renal parenchyma in chronic kidney disease, but unlike RCC, it does not contain vessels that lack the normal arteriolar smooth muscle. Therefore, we may speculate that in comparison with RCC, compensatory hypertrophy may not demonstrate significantly decreased RI compared to the background ESRD kidney. Due to our limitation of a small number of cases with compensatory hypertrophy, further investigation with a larger study population is warranted to confirm our speculation.
With respect to the use of RI, we expect that concerns such as the following could be raised: (1) reproducibility in measuring RI and (2) the possibility that RI may be affected by other extrinsic factors. First, although we did not perform an interreader reproducibility study, we performed repeated measurements for both the renal masses and the background renal parenchyma. We used the mean value as the representative RI value for each entity and performed an intrareader reproducibility study as well. The intrareader reproducibility using ICC within subjects proved to be excellent and good for the renal masses and the parenchyma, respectively. Second, RI is well known to be affected by various factors such as kidney compression, breath holding during the Valsalva maneuver, extreme bradycardia and extrarenal markers of vascular stiffness [16]. However, we propose that the presence of a significant difference in RI between RCCs and the background parenchyma may be useful. This is more likely to be less dependent on the effect of external variables.
Our study has several limitations. First, the sample size was relatively small considering the long study period. Still, we were able to demonstrate a significant difference in RI values between RCCs and the background renal parenchyma of ESRD kidneys. Second, we had only one case of compensatory hypertrophy that manifested as a pseudo-tumor. Although this case of compensatory hypertrophy, unlike the RCC case, showed a high RI value as the background renal parenchyma of the ESRD kidney, we recognize that it is premature to derive a strong conclusion regarding the discrimination between RCCs and compensatory hypertrophy from our study results. Third, we did not perform an interreader reproducibility study. US itself is a highly operator-dependent modality. Moreover, it may be even more difficult to measure RI in ESRD kidneys, which are commonly atrophied and decreased in internal perfusion. A further problem is posed when the suspicious renal mass is small.
Despite its limitations, the RI on spectral Doppler US is useful in detecting RCC in patients with ESRD. The RI values measured at RCCs were significantly lower than those measured at the background parenchyma. Further studies are warranted to assess its value for differentiation between RCCs and pseudo-tumors.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.