Ultrasonographic findings of type IIIa biliary atresia
Article information
Abstract
Purpose:
To describe the ultrasonographic (US) findings of type IIIa biliary atresia.
Methods:
We retrospectively reviewed a medical database of patients pathologically confirmed to have biliary atresia, Kasai type IIIa, between January 2002 and May 2013 (n=18). We evaluated US findings including the visible common bile duct (CBD), triangular cord thickness, gallbladder size and shape, and subcapsular flow on color Doppler US; laboratory data; and pathological hepatic fibrosis grades. We divided them into two groups-those with visible (group A) and invisible (group B) CBD on US-and compared all parameters between the two groups.
Results:
CBD was visible on US in five cases (27.8%; group A) and invisible in 13 cases (72.2%; group B). US was performed at an earlier age in group A than in group B (median, 27 days vs. 60 days; P=0.027) with the maximal age of 51 days. A comparison of the US findings revealed that the triangular cord thickness was smaller (4.1 mm vs. 4.9 mm; P=0.004) and the gallbladder length was larger (20.0 mm vs. 11.7 mm; P=0.021) in group A. The gallbladder shape did not differ between the two groups, and the subcapsular flow was positive in all cases of both groups. There was no significant difference in the laboratory data between the two groups. Upon pathological analysis, group A showed low-grade and group B showed low- to high-grade hepatic fibrosis.
Conclusion:
When CBD is visible on US in patients diagnosed with type IIIa biliary atresia, other US features could have a false negative status. A subcapsular flow on the color Doppler US would be noted in the type IIIa biliary atresia patients.
Introduction
Neonatal cholestasis has a variety of underlying causes. However, the two major causes accounting for 60%-90% of all neonatal cholestasis are neonatal hepatitis and biliary atresia [1]. Discrimination between these two entities is very important, since they require completely different treatment approaches-medical treatment for hepatitis and surgery for biliary atresia.
Among the three Kasai types of biliary atresia, type III (porta hepatis obstruction) is the most common-accounting for more than 90% of all biliary atresia patients, and has the worst prognosis [2-4]. The concept that a younger age at the diagnosis of and surgery for biliary atresia leads to a better outcome has been generally accepted [1,5-14], while some of the published data have revealed that patient age has no significance or sometimes even a negative effect on the prognosis [15,16]. However, when it comes to type III biliary atresia, the negative effect of older age seems more obvious [9].
Among non-invasive imaging tools, it is well known that ultrasonography (US) has the highest diagnostic accuracy, which makes it possible to avoid unnecessary invasive procedures in many cases [1,17,18]. Further, many previous studies have reported several US findings to diagnose biliary atresia, including a triangular cord sign, abnormal gallbladder length and shape, invisible common bile duct (CBD), and subcapsular flow on color Doppler US [1,18- 32]. However, some patients show equivocal US findings, requiring additional invasive diagnostic methods or leading to a delayed diagnosis. In particular, while visible CBD on US is generally regarded as a finding suggestive of the disease other than biliary atresia, it can be a possible finding of Kasai subtype IIIa (patent distal CBD) biliary atresia. Further, there have been only a few reports on the US findings of biliary atresia based on its types [33,34]. Therefore, the purpose of this study was to determine the US features of type IIIa biliary atresia.
Materials and Methods
Patients
The Institutional Review Board approved this retrospective study and waived the requirement of written informed consent from all patients.
We retrospectively collected data on patients who were confirmed as having biliary atresia by surgical pathology between January 2002 and May 2013. Further, one pediatric radiologist (MJK) reviewed the operative cholecystocholangiography performed in these patients, analyzed the Kasai type of biliary atresia, and selected type IIIa. A total of 18 patients (10 boys and 8 girls) were diagnosed with Kasai type IIIa biliary atresia. We reviewed their US findings along with the laboratory and pathological data. Patient ages at the US examination were recorded.
Ultrasonography
In our institution, all patients with neonatal cholestasis undergo US without being fed for at least 4 hours. A visualization of the CBD is recorded, and the triangular cord thickness is measured as the thickness of the echogenic anterior wall of the right portal vein just proximal to the right portal vein bifurcation site [26]. The maximal gallbladder length is measured, and the gallbladder shape is evaluated using a high-frequency transducer. When considering the gallbladder shape, a discontinuous or absent mucosal lining, an indistinct wall, and an irregular outer margin are regarded as abnormal. On color Doppler US, a hepatic subcapsular flow is evaluated using the color box (height, 1 cm; width, 3-4 cm) positioned on the anterior surface around the falciform ligament [23]. In some patients, whether normal gallbladder contraction occurs after an oral feeding is also evaluated.
Images and Data Review
US findings of invisible CBD, triangular cord thickness of more than 4 mm, gallbladder length of less than 15 mm, abnormal gallbladder shape, and subcapsular flow on color Doppler US are regarded as the diagnostic criteria of biliary atresia. We retrospectively reviewed and recorded these US findings.
Laboratory data at the time of US were reviewed including total and direct serum bilirubin levels, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transpeptidase (γ-GT), and alkaline phosphatase (ALP).
Liver wedge biopsy specimens were routinely obtained from all the patients at the time of operation. Further, the liver fibrosis grades were determined according to the Metavir scoring system as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa; F3, numerous septa without cirrhosis; F4, cirrhosis [35,36].
Statistical Analyses
We divided the patients into two groups on the basis of the US finding of CBD-those with visible CBD (group A) and those with invisible CBD (group B). All the parameters were compared between these two groups.
Categorical parameters such as sex, gallbladder shape on US, and subcapsular flow on color Doppler US were compared using a Fisher exact test. A Mann-Whitney U test was used to compare the continuous variables. Liver fibrosis grades were compared using Pearson chi-squared test. Statistical analyses were performed using the IBM SPSS ver. 20 (IBM Co., Armonk, NY, USA).
Results
US was performed at the age of 11-100 days with a median of 50 days in the 18 patients with Kasai type IIIa biliary atresia. CBD was visible on US in five cases (27.8%; group A) and invisible in 13 cases (72.2%; group B). There were four boys and one girl in group A and six boys and seven girls in group B without any difference between the two groups (P=0.314). US was performed at an earlier age in group A than in group B (median, 27 days vs. 60 days; P=0.027) with the maximal age of 51 days (Table 1).
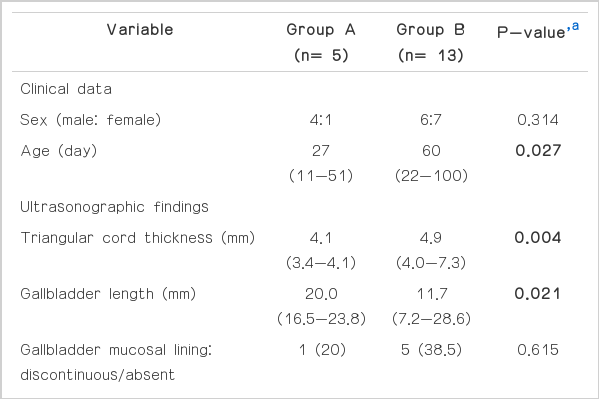
Comparison between group A (visible CBD) and group B (invisible CBD) of clinical data, ultrasonographic findings, laboratory findings, and pathological results
Representative images of US and operative cholangiogram are illustrated in Fig. 1 for group A patients and Fig. 2 for group B patients. On the US findings, the triangular cord thickness was significantly smaller in group A than in group B (median, 4.1 mm vs. 4.9 mm; P=0.004). When considering the cut-off value of the triangular cord sign to be 4 mm [26], two patients (2/5, 40%) showed a negative triangular cord sign in group A, and all patients showed a positive triangular cord sign in group B.
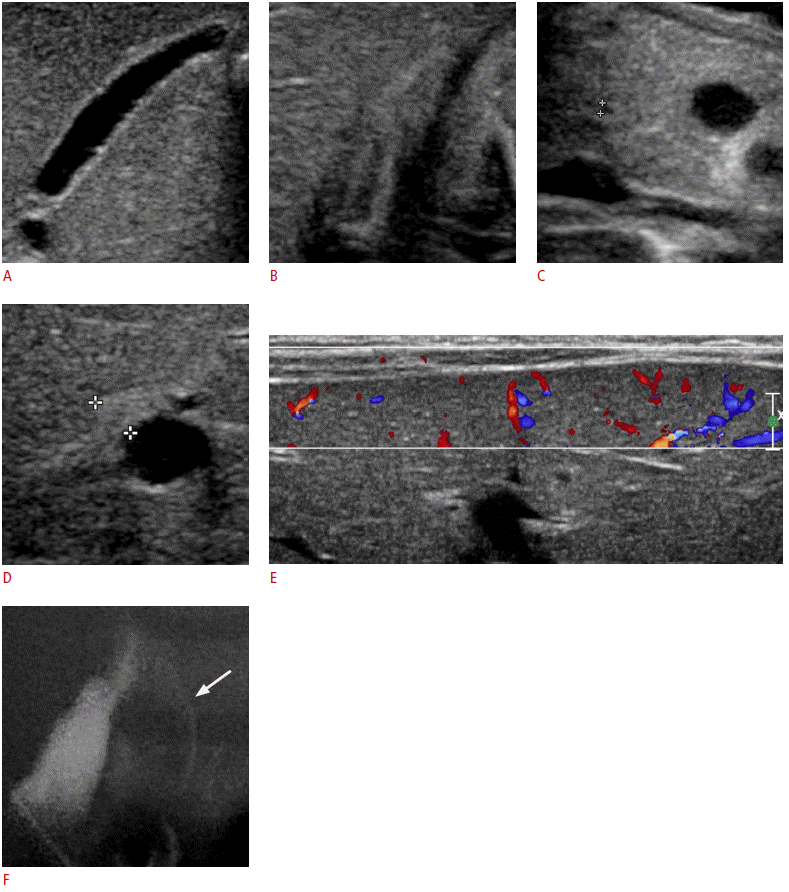
A 27-day-old boy with visible common bile duct (CBD) on ultrasonography (US) (group A).
A. Mucosa, wall, and outer margin of the gallbladder look normal. Gallbladder length was measured about 20.3 mm. B. After an oral feeding, the gallbladder contracts. C. CBD is visible, and its diameter is being measured (crosshairs). D. Triangular cord thickness is 3.4 mm (crosshairs), which is less than the cut-off value of 4 mm. E. Hepatic subcapsular flow is evident on the color Doppler US. F. Operative cholangiogram shows patent CBD (arrow) without the filling of the common hepatic duct, confirming type IIIa biliary atresia.

A 49-day-old boy with invisible common bile duct (CBD) on ultrasonography (US) (group B).
A. Gallbladder length is 12.8 mm (crosshairs). The mucosal lining and the outer margin of the gallbladder are relatively smooth with a distinct wall. Postprandial gallbladder evaluation was not done. B. CBD is invisible in the pancreatic head portion. C. Triangular cord thickness is 5.3 mm (crosshairs), which is larger than the cut-off value of 4 mm. D. Hepatic subcapsular flow is evident on the color Doppler US. E. Operative cholangiogram shows a patent CBD (arrow) without a visible common hepatic duct, confirming type IIIa biliary atresia.
The gallbladder length was larger in group A than that in group B (median, 20.0 mm vs. 11.7 mm; P=0.021). When we use the cutoff value of an atretic gallbladder as 15 mm in length [22], all five patients (5/5, 100%) in group A and three patients (3/13, 23%) in group B were false negative.
With respect to the gallbladder shape, four cases had a normal gallbladder, and only one (1/5, 20%) demonstrated a discontinuous mucosal lining in group A. In group B, five cases (5/13, 38%) showed an abnormal gallbladder with a discontinuous or absent mucosal lining including one case combined with an indistinct wall. All gallbladders revealed a smooth outer margin in both groups. However, the gallbladder shape was not significantly different between the two groups (P=0.615).
Three patients in group A and one patient in group B showed normal gallbladder contraction after an oral feeding, while whether the gallbladder contracted normally after an oral feeding was not evaluated in the remaining 14 patients (not shown in the tables).
On color Doppler US, all patients demonstrated a positive subcapsular flow in both groups.
Laboratory and pathological data in the two groups are also summarized in Table 1. The application of the upper normal limit values used in our institution (total bilirubin, 0.8 mg/dL; direct bilirubin, 0.4 mg/dL; γ-GT, 54.0 IU/L; and alkaline phosphatase, 341 IU/L) increased the total bilirubin, direct bilirubin, and γ-GT in all patients of both groups and increased alkaline phosphatase in three patients of group A and ten patients of group B. None of the laboratory data were significantly different between the two groups. In the pathological analysis of liver fibrosis, there were one case of F1 and four cases of F2 in group A, while there were nine cases of F2, two cases of F3, and two cases of F4 in group B, showing a greater tendency toward the advanced grades of liver fibrosis in group B. However, this distinction was not statistically significant (P=0.246).
Discussion
There have been many studies on the diagnostic US findings of biliary atresia. Tan Kendrick et al. [27] suggested a “gallbladder ghost triad” for the diagnostic US findings of biliary atresiagallbladder length of <19 mm, indistinct gallbladder wall with a lack of a smooth or complete echogenic mucosal lining, and an irregular or lobular outer contour. Azuma et al. [21] reported that the absence of an extrahepatic bile duct on the US exam can further increase the diagnostic accuracy of biliary atresia. Both the triangular cord sign and the hepatic subcapsular flow are also known as one of the diagnostic US findings of biliary atresia [19,23]. However, not all these US findings are always present in patients with biliary atresia.
Mittal et al. [1] stated that CBD can potentially be visible on US in certain types of biliary atresia with distal CBD sparing. According to the proposal by Kasai et al. [37], biliary atresia with a porta hepatis obstruction and patent distal CBD is classified into type IIIa. Therefore, patients with type IIIa biliary atresia might show visible CBD on US.
There have been only a few reports on the US findings of biliary atresia on the basis of its types [33,34]. Further, no study has compared the US findings, including the visibility of CBD on US.
Among the 18 patients with type IIIa biliary atresia, CBD was not visible in 13 patients even though patent CBD was confirmed on operative cholangiography. In our study, one patient showed visible CBD at the age of 11 days and was included in group A. However, follow-up US, which was not included in this study, was performed 10 days later, and revealed that CBD was not visible. Therefore, one possible explanation for the invisible CBD is that the CBD started being affected by the disease process to become too small to be seen on US, and still insufficient to cause complete luminal obstruction. Another possible explanation is the decreased bile flow from the liver caused by the progression of the porta hepatis obstruction. This decreased bile flow subsequently causes the luminal collapse of CBD, making it difficult to be identified on US.
The triangular cord sign is defined as the triangular cord thickness of more than 4 mm [26] and has been proven to be highly specific for the diagnosis of biliary atresia by many other authors [1,17,18,22,24-26,29]. In contrast, the sensitivity of the triangular cord sign has been reported to vary from 14% to 100% [1,17,22,24-26,29]. Li et al. [28] suggested the following three possible explanations for a negative triangular cord sign and its low sensitivity: (1) no triangular cord at the porta hepatis, (2) triangular cord at the porta hepatis, but too small to be seen, and (3) insufficient experience of the US operator. In addition, Saxena et al. [38] pointed out that the triangular cord sign may initially be negative if the patient is very young and possibly become positive later. He reached this conclusion from the observation of a 22-dayold patient who showed a negative triangular cord sign at the initial US and then developed a positive triangular cord sign 2 weeks later.
In our study, group A showed a significantly smaller triangular cord thickness than group B, and two patients (2/5, 40%) in group A had a negative triangular cord sign, while all patients in group B had a positive triangular cord sign. This may be related to the suggestion of Saxena et al. [38] since the patient age at the time of US was significantly lower in group A than in group B. In particular, the two patients with a negative triangular cord sign were of the age of 20 days and 27 days, respectively, at the time of US, and were considerably younger than the others with a positive triangular cord sign.
According to Tan Kendrick et al. [27], all three findings of the “gallbladder ghost triad” were constantly positive in 97% of the infants with biliary atresia and showed no false positives. Recently, several studies have also demonstrated that the small size and the abnormal morphology of the gallbladder on US were useful in distinguishing biliary atresia from neonatal hepatitis [1,18,30-32]. According to our results, the gallbladder length was significantly greater in group A than in group B. The use of the cut-off value of the atretic gallbladder of 15 mm (length) [22] revealed that all five patients (5/5, 100%) in group A and three patients (3/13, 23%) in group B were false negative. The evaluation of the gallbladder shape was helpful in only one patient (1/5, 20%) of group A and five patients (5/13, 38%) of group B. These results are quite inconsistent with those of the previous studies on the US features of the gallbladder, particularly in group A. One possible explanation is that the gallbladder length and shape might be within normal limits at an early stage of the disease and become affected as the disease progresses, considering the progressive nature of biliary atresia and the younger age of group A.
If the gallbladder contracts after an oral feeding, biliary atresia is generally considered unlikely [1,39]. However, several authors have already reported that normal gallbladder contraction was seen after an oral feeding in patients with type IIIa biliary atresia [33,34]. In our study, three patients in group A and one patient in group B showed normal gallbladder contraction after an oral feeding. In the remaining 14 patients, whether the gallbladder contracted normally after an oral feeding was not evaluated.
Lee et al. [23] defined a hepatic subcapsular flow as a hepatic arterial flow signal continuing to the liver capsular surface on color Doppler US and suggested it to be a diagnostic US finding of biliary atresia with high sensitivity (100%) and relatively low specificity (86%). Recently, El-Guindi et al. [29] supported their findings with a sensitivity of 96.3% and a specificity of 96.3%. In our study, the subcapsular flow was positive in all patients of both groups and was the only finding suggestive of biliary atresia in one patient of group A. Therefore, a hepatic subcapsular flow can be used to predict biliary atresia even when CBD is visible on US. Furthermore, when a hepatic subcapsular flow is not evident, the possibility of biliary atresia might be greatly lowered.
It is well known that conjugated hyperbilirubinemia cannot reliably discriminate between biliary atresia and neonatal hepatitis [40]. Total bilirubin, direct bilirubin, and γ-GT were increased in all patients of both groups, and alkaline phosphatase was increased in three patients of group A and ten patients of group B. However, laboratory data in our study were not statistically different between the two groups.
Although pathological liver fibrosis grades were not statistically different between the two groups, a tendency towards the advanced grades of liver fibrosis was noted in group B. This result is consistent with the progressive nature of biliary atresia in causing hepatic fibrosis, liver cirrhosis, and eventual liver failure sequentially unless appropriate treatment is given [2].
Several limitations of our study should be acknowledged. First, the sample size was small, and the sample distribution was uneven (group A: n=5 vs. group B: n=13), lowering the reliability of our data. Further studies with larger samples are needed to support our results. Second, since it was retrospective study, the pathological correlation of the gallbladder and the CBD with their US findings could not be established. In order to understand the exact underlying mechanisms of the abnormal gallbladder morphology and the visibility of the CBD on the US, a correlation between the US images and the pathological results should be established in the future.
In conclusion, CBD can be either visible or invisible on US in patients diagnosed with type IIIa biliary atresia. When CBD was visible, other US features with respect to the triangular cord thickness and the gallbladder length and shape tended to more often show false negative results than when it was invisible. As the subcapsular flow on the color Doppler US was always noted in patients diagnosed with biliary atresia, Kasai type IIIa, a color Doppler study should be included in the routine US examination of neonatal cholestasis patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.
