A comparison of lymphocytic thyroiditis with papillary thyroid carcinoma showing suspicious ultrasonographic findings in a background of heterogeneous parenchyma
Article information
Abstract
Purpose:
The aim of this study was to compare ultrasonographic features in patients with lymphocytic thyroiditis (LT) and papillary thyroid carcinoma (PTC) having suspicious thyroid nodule(s) in a background of heterogeneous parenchyma and to determine the clinical and radiological predictors of malignancy.
Methods:
We reviewed the cases of 100 patients who underwent ultrasonography between April 2011 and October 2012, and showed suspicious thyroid nodule(s) in a background of heterogeneous parenchyma. Eight patients who did not undergo ultrasonography-guided fineneedle aspiration cytology (FNAC) and 34 cases of follow-up ultrasonography after initial FNAC were excluded. We compared the benign and malignant nodules in terms of their clinical and radiological factors.
Results:
For the 58 nodules including 31 LTs (53.4%) and 27 PTCs (46.6%), the mean tumor sizes of the two groups were 0.96 cm for LT and 0.97 cm for PTC. A univariate analysis revealed that PTCs were more frequent in patients younger than 45 years and having microcalcifications than was LT. An independent predictor of PTC after adjustment was an age of <45 years.
Conclusion:
LT mimics malignancy in a background of heterogeneous parenchyma on ultrasonography. A young age of <45 years is the most important predictor of malignancy in this condition.
Introduction
Lymphocytic thyroiditis (LT), also known as Hashimoto thyroiditis, is an autoimmune inflammatory disease characterized by lymphocyte infiltration, fibrosis, and gradual destruction of the thyroid gland. The typical appearance of LT on ultrasonography is described as a diffusely enlarged, heterogeneous, decreased echogenicity [1-3] or a fine micronodular pattern [4,5]. The specific ultrasonographic findings of papillary thyroid carcinoma (PTC) are well known [6-9]. There are suspicious nodules on ultrasonography diagnosed as focal LT in patients with diffuse LT who underwent fine-needle aspiration cytology (FNAC). It is true that a heterogeneously decreased parenchymal echogenicity may cause nodules to seem less hypoechoic than they would in a normal gland. Therefore, the ultrasonographic appearance of the thyroid gland can make it difficult to differentiate true nodules from the heterogeneous background, and benign nodules from malignant nodules.
Few studies have investigated the ultrasonographic findings of focal LT and PTC in patients with diffuse LT [10-13]. Wang et al. [10] reported that PTC nodules were more frequently solitary, were markedly hypoechoic or hypoechoic, and had microcalcifications. On the other hand, Takashima et al. [11] described focal LT as hypoechoic or markedly hypoechoic (64.0%). In a recent study, Anderson et al. [12] showed that the ultrasonographic features and vascularity of focal LT were extremely variable.
The purpose of this study was to compare the ultrasonographic features of LT and PTC in patients with suspicious thyroid nodule(s) and a background of heterogeneous parenchyma and to determine the clinical and radiological predictors of malignancy.
Materials and Methods
Patients
Our Institutional Review Board approved this retrospective study, and the requirement for written informed patient consent was waived. However, informed consent for ultrasonography-guided FNAC was obtained. It was found that 2,926 patients who underwent thyroid ultrasonography at our institution between April 2011 and October 2012 had heterogeneous parenchyma. Among them, 100 patients had suspicious thyroid nodules. All of these 100 patients were diagnosed with diffuse LT before or after performing thyroid ultrasonography and had a different clinical status including hypothyroidism, hyperthyroidism, or euthyroid. Eight patients who did not undergo ultrasonography-guided FNAC at the initial evaluation and 34 patients who did not undergo follow-up ultrasonography after the initial FNAC were excluded. Finally, 58 patients (mean age, 48 years; range, 21 to 73 years) who underwent both FNAC at the initial evaluation and follow-up ultrasonography after the initial FNAC were included in this study for the statistical analysis. Of these 58 patients, only five patients had a palpable mass and the others did not have any symptoms. There were 56 female and 2 male patients. Twenty-seven patients underwent surgery, 14 patients underwent follow-up ultrasonography-guided FNAC or ultrasonography-guided core needle biopsy (CNB) within 18 months, and 17 patients underwent follow-up ultrasonography (mean, 11 months; range, 6 to 18 months). We retrospectively reviewed the clinical records, histological reports, and initial and follow-up ultrasonography images of the index tumors.
Image Analysis
Ultrasonography was performed using high-resolution ultrasonography equipment (iU22, Philips Advanced Technology Laboratories, Bothell, WA, USA) with 12-5-MHz linear-array transducers. Thyroid ultrasonography, ultrasonography-guided FNAC, or ultrasonographyguided CNB was performed by a board-certified radiologist (JHS) with 9 years of experience in thyroid imaging and intervention. Both longitudinal and transverse scans were obtained in grayscale. All ultrasonography images were retrospectively reviewed by two board-certified radiologists until consensus was reached; one (JHS) of them was involved in the original ultrasonography studies, while the other (SYN) was not. At the time of the review, the readers were blinded to the histological results. Initial inclusion of suspicious nodules was defined as the inclusion of nodules showing at least 1 accepted ultrasonographic criterion: taller-than-wide shape, marked hypoechogenicity, microcalcifications, or an infiltrative border in the background of heterogeneous parenchyma [6-9]. All nodules were evaluated according to the following ultrasonographic features: shape (taller-than-wide or wider-than-tall), echogenicity (iso/hypoechoic or markedly hypoechoic), calcification (none, microcalcification, or macrocalcification), margin (infiltrative border or well-defined border), and vascularity (increased or not increased). Patients with benign and malignant nodules were then compared in terms of patient age, patient sex, tumor size, multifocality, and thyroid functional status. Histopathology was evaluated on the basis of the pathological reports.
Statistical Analysis
Patient age and tumor size were compared using a Mann-Whitney test. Patient sex, ultrasonographic features, multifocality, and clinical diagnosis were compared using the chi-squared test or Fisher’s exact test. A logistic regression analysis was performed to determine the independent predictors of malignancy. Receiver operating characteristic curve values were calculated for predicting malignancy. All statistical analyses were performed using the open-source statistical language and platform, R, ver. 2.15.0 (R Development Core Team, 2012) and a P-value of <0.05 was considered to indicate a statistically significant difference.
Results
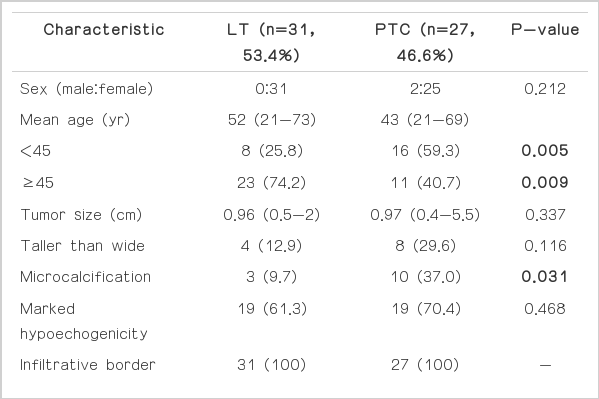
Thirty-one of the 58 nodules (53.4%) were finally diagnosed as LT (Fig. 1) and 27 (46.6%) as PTCs (Fig. 2). The clinical and imaging characteristics of LT and PTC are presented in Table 1. Patients with LT underwent surgery (n=3) because of contralateral cancer, follow-up ultrasonography-guided FNAC or CNB (n=14; interval decrease, 4; no change, 10), or follow-up ultrasonography (n=14; interval disappearance, 3; interval decrease, 2; no change, 9). Although in most patients the nodules decreased in size upon follow-up ultrasonography, four patients underwent follow-up ultrasonography-guided FNAC or CNB because of an unchanged, suspicious ultrasonographic feature of the nodule. There was no patient who was proven to have PTC on follow-up FNAC or CNB. Patients with PTC underwent surgery (n=24) or follow-up ultrasonography (n=3). The mean tumor sizes of the two groups were similar with 0.96 cm for LT and 0.97 cm for PTC (P=0.337). PTC showed a higher incidence in patients younger than 45 years and in those having microcalcifications than did LT (59.3% vs. 25.8%, respectively, P=0.009; 37.0% vs. 9.7%, respectively, P=0.031). Shape, nodule echogenicity, margin, multifocality, and thyroid functional status were not significantly different between the two groups. Of the 28 patients who underwent a color Doppler scan, 10 (35.7%) showed nodules with increased vascularity, whereas the remaining (64.3%) showed nodules with no vascularity. The vascularity of nodules was similar between the two groups.

A 63-year-old woman with lymphocytic thyroiditis (LT).
Transverse (A) and longitudinal (B) ultrasonograms show a 2.0-cm mass with a taller-than-wide shape, irregular border, and marked hypoechogenicity with heterogeneous parenchyma in the left thyroid gland (arrows). Ultrasonography-guided fine-needle aspiration cytology revealed LT. The mass had disappeared on follow-up transverse (C) and longitudinal (D) ultrasonography after one year.
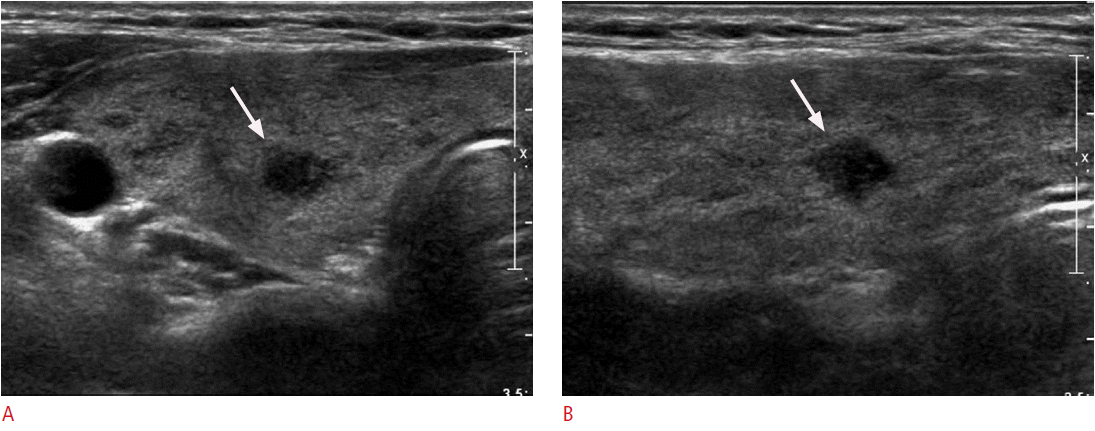
A 40-year-old woman with papillary thyroid carcinoma (PTC).
Transverse (A) and longitudinal (B) ultrasonograms show a 0.8-cm mass with an infiltrative border, marked hypoechogenicity, and microcalcifications with heterogeneous parenchyma in the right thyroid gland (arrows). Ultrasonography-guided fine-needle aspiration cytology revealed PTC. After total thyroidectomy, the final diagnosis was PTC without extrathyroidal extension or lymph node metastasis.
Table 2 presents the results of a multivariate analysis of the clinical and imaging findings of LT and PTC patients. The variables analyzed after adjustment included age, ultrasonographic findings (tallerthan- wide shape, microcalcification, and marked hypoechogenicity), multifocality, and clinical diagnosis. A young age, that is, less than 45 years (odds ratio [OR], 4.769; 95% confidence interval [CI], 1.055 to 21.549; P=0.042) was a significant predictor of PTC based on the multivariate analysis. For predicting malignancies, the area under the curve (AUC) for young age was 72% (95% CI, 0.578 to 0.853), and the AUC of microcalcifications was 67% (95% CI, 0.544 to 0.798).
Discussion
Hashimoto thyroiditis, also known as chronic LT or chronic autoimmune thyroiditis, which is characterized by the presence of high serum thyroid antibody concentrations and goiter, is the most common cause of hypothyroidism [14]. About eight or nine times more women are diagnosed with this medical condition than men, with the peak incidence occurring between the ages of 30 and 50 years [14]. Around 90% of the patients have a symmetrical, firm, painless, diffusely enlarged thyroid gland, whereas the remaining (around 10%) have an atrophic thyroid gland, which may represent the final stage of thyroid failure in LT patients. Levothyroxine sodium is the treatment of choice for LT.
It is well known that there is a strong association between LT and primary thyroid lymphoma [11,15,16]. LT is also associated, although less strongly, with PTC [17-20]. Anderson et al. [21] reported that the incidence of malignancy was slightly higher in patients with LT (16%) than in the general population (9%-13%) [22]. It is important to evaluate thyroid nodules in patients with LT because of the higher incidence of malignancy. Fortunately, the prognosis of PTC is better in patients with LT than in those without [20].
There have been several studies on the evaluation of the ultrasonographic features of both diffuse LT and focal LT [1-5,10-13]. The typical ultrasonographic appearance of diffuse LT is well established, and these features were used in our daily practice to identify patients with diffuse LT. In addition to diffuse LT, focal LT is occasionally seen on ultrasonography. Langer et al. [13] reported that focal LT has an incidence of approximately 5% among the nodules biopsied. Focal LT is a clinically less severe form of the disease than diffuse LT [12]. Although several authors have described the ultrasonographic findings of focal nodules that were proven to be LT, specific ultrasonographic findings are not yet well established [10-13].
Among nodules having an infiltrative border in the background of heterogeneous parenchyma, 53.4% of the considered cases were proven to be LT, and the remaining cases were diagnosed as PTC. Thus, an ultrasonography exam for the diagnosis of PTC in patients with heterogeneous parenchyma showed low accuracy. Further, this study revealed that an infiltrative border is a common overlapping feature of thyroiditis and malignancy. Langer et al. [13] found that the margins of LT were irregular in 17 of 21 nodules (80.9%), and Anderson et al. [21] found that 35.8% of the benign nodules had illdefined margins in patients with diffuse LT. Therefore, we needed to determine the predisposing factor for malignancy in these nodules.
The univariate analysis conducted as part of this study revealed that PTCs had a higher rate in patients younger than 45 years and with microcalcifications than did LT. An independent predictor of PTC after adjustment was age less than 45 years. Moon et al. [23] found that among nodules with focal LT on ultrasonography-guided FNAC, 20% (8 of 40) of the suspicious malignant nodules on initial ultrasonography proved to be PTC after second FNAB. Therefore, the results of this study revealed that repeated FNAC is more strongly recommended for suspicious malignant nodules on ultrasonography, which was confirmed as LT in young patients and in those having microcalcifications in the background of heterogeneous parenchyma. For predicting malignancies, the AUC of young age was 72%.
Microcalcifications are a more predictable ultrasonographic features of PTC than of LT. For predicting malignancies, the AUC of microcalcifications was 67% in this study. These results confirm those reported by other authors [10,21]. In particular, Anderson et al. [21] found that all types of calcifications were more prevalent among malignant nodules within a background of diffuse LT. The differences were statistically significant for microcalcifications (39% vs. 0%) and for tiny nonspecific bright reflectors. More recently, Wang et al. [10] reported that none of the cases of focal LT had microcalcifications, whereas PTC more frequently had microcalcifications, documented in up to 70% of the considered cases. These study results were consistent with the results of the present study.
Our study had several weaknesses. First, the present study was mainly based on ultrasonography performed by only one radiologist, and thus, a bias of patient selection may affect the results. Second, given the small number of cases owing to the short period of this study, it was difficult to calculate the predictive values for each ultrasonographic feature. A large series study should be conducted in the future. Third, imaging findings were retrospectively evaluated by two radiologists, and therefore, interobserver variability remains a concern. Finally, benignity of some nodules may also be overestimated because some patients had relatively short follow-up periods with a mean of 11 months.
In conclusion, LT mimics malignancy in the background of heterogeneous parenchyma on ultrasonography. We demonstrated that a young age in this condition is the most important predictor of malignancy. PTCs in the background of heterogeneous parenchyma have microcalcifications more frequently than LT. Performing FNAC is more actively recommended for thyroid nodules in a young patient with microcalcifications and a background of heterogeneous parenchyma on ultrasonography.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.