Contrast-enhanced ultrasonography of the carotids
Article information
Abstract
Contrast-enhanced ultrasonography of the carotids has recently emerged as a complementary examination to conventional carotid Doppler ultrasonography. It is an examination providing improved visualization of the vascular lumen, more accurate and detailed delineation of the vascular wall, and identification of atherosclerotic plaques. Moreover, contrast-enhanced ultrasonography has specific advantages over conventional ultrasonography and plays an important role in the diagnosis of the vulnerable carotid plaque, as it can identify intraplaque neovascularization and carotid plaque ulceration. Given the specific advantages and improved imaging of the carotids provided by this method, radiologists should be familiar with it. This pictorial essay illustrates the advantages of this technique and discusses its value in the imaging of carotid arteries.
Introduction
Ultrasonography (US) is a well-established imaging modality for evaluating carotid atherosclerotic disease, which may cause approximately a third of brain infarcts [1,2]. Contrast-enhanced ultrasonography (CEUS) has emerged as an improved imaging technique to detect and evaluate atherosclerotic plaques with the use of a contrast agent based on microbubbles [3]. CEUS has been proven to more accurately visualize the arterial lumen than the color Doppler technique [4]. In addition to this, CEUS has been shown to reliably evaluate intraplaque neovascularization (IPN) and identify carotid plaque ulcerations with superior diagnostic accuracy as compared to the color Doppler technique. Both neovascularization and ulcerations are considered to increase the plaque’s vulnerability and predict cerebrovascular events [5-7].
In this pictorial essay, we present selected images from the carotid US and CEUS examinations of patients with important abnormal findings. Where appropriate, we provide comparative multidetector computed tomographic angiography (MDCTA) or contrast-enhanced magnetic resonance angiography (CEMRA) images. Our purpose is to illustrate the value of a CEUS examination.
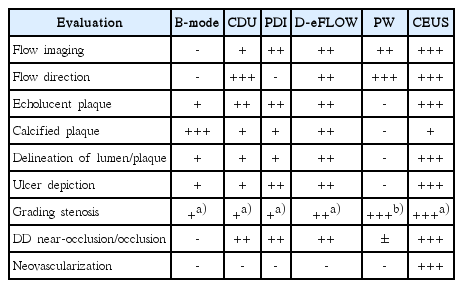
Imaging Technique
The standard protocol for carotid US in our Radiology Department includes gray-scale imaging (B-mode), color Doppler ultrasonography (CDU), power Doppler imaging (PDI), pulsed-wave (PW) Doppler, and the directional-eFLOW (D-eFLOW) technique of the common, internal, external carotids and the vertebral arteries. Gray-scale imaging provides information about morphological structures within the vascular lumen, the vascular walls, and the surrounding tissues. The color Doppler technique estimates and displays the mean velocity of red blood cells in a scanned area as a color map superimposed on the gray-scale image. It thus reveals echolucent plaques, which are undetected in the gray-scale technique, and demonstrates the blood flow pattern, highlighting stenotic areas. PW Doppler is used to measure prestenotic and poststenotic blood flow velocities and grade stenosis. PDI is used as a technique to better delineate the plaque surface and the slow flow as it is more sensitive than color Doppler and free of the aliasing artifact. The D-eFLOW technique is a new high-definition blood flow imaging technique that is characterized by increased spatial and temporal resolution and is available in some US devices. It is used to more accurately delineate the borders of the plaque and to image highly stenotic parts, avoiding the overwriting artifact of the conventional color Doppler technique. All the examinations were performed with an Aloka Prosound alpha 7 device (Aloka GmbH, Meerbusch, Germany), with a 5-13 MHz linear array transducer. During the last year, we performed a complementary CEUS examination of selected patients where the conventional techniques could not fully evaluate the extent of atherosclerotic disease. The CEUS examination was performed after intravenous administration of the SonoVue (Bracco SpA, Milan, Italy) contrast agent and was mainly used to either accurately define the stenosis grade, identify plaque ulcerations or neovascularization. US contrast agents are based on microbubbles measuring approximately 1-8 μm, which are filled with a perfluorinated gas and covered with a phospholipid or protein shell. The contrast agent dose used was 2.4 mL administered with an intravenous fast bolus injection and followed by normal saline infusion through a three-way stopcock. When necessary, the dose was modified according to the body type of the patient’s neck and was increased to as much as 4.8 mL. The microbubbles can be seen filling the arterial lumen 30 seconds after the injection. Their concentration is maximal at approximately 60 seconds and then gradually decreases, lasting for up to 5 minutes. The use of microbubble agents is contraindicated in patients with unstable angina, acute cardiac failure, acute endocarditis, known right-to-left shunts, and known allergy for these agents. In our case series, we did not encounter any side effects of the US contrast agent. Patients who needed interventional treatment were further investigated with MDCTA or CEMRA according to the guidelines of the Department of Vascular Surgery.
Stenosis Evaluation with CEUS in Comparison with Other Techniques
A CEUS examination provides better imaging of the blood flow and better delineation of the vessel wall, particularly in highly stenotic parts of the carotids (Fig. 1). In these parts, the echogenicity reflected by the moving microbubbles is higher than that of normal vessels due to their increased concentration. Thus, imaging of the highly stenotic parts is achieved without flow artifacts (Figs. 1, 2). The use of contrast agents greatly improves the delineation of the plaque’s surface and grading of stenosis. Thus, the identification of plaque ulcerations is also facilitated by this technique. When the ulcer is rounded in shape, the microbubbles may be seen moving in a turbulent pattern, which is equivalent to the flow reversal in CDU. In an early experimental study, Sirlin et al. [8] compared CEUS with CDU and PDI in terms of the evaluation of the atherosclerotic arterial lumen. They concluded that the use of an US contrast agent enables rapid and precise evaluation of the vessel lumen and wall with its irregularities, grading of stenosis, and detection of plaque ulcerations [8]. Subsequently, Kono et al. [9] reported that CEUS accurately identifies carotid stenoses greater than 70%. Further, it was shown that the diametric stenosis estimated by CEUS is strongly correlated with that estimated with conventional angiography [9].
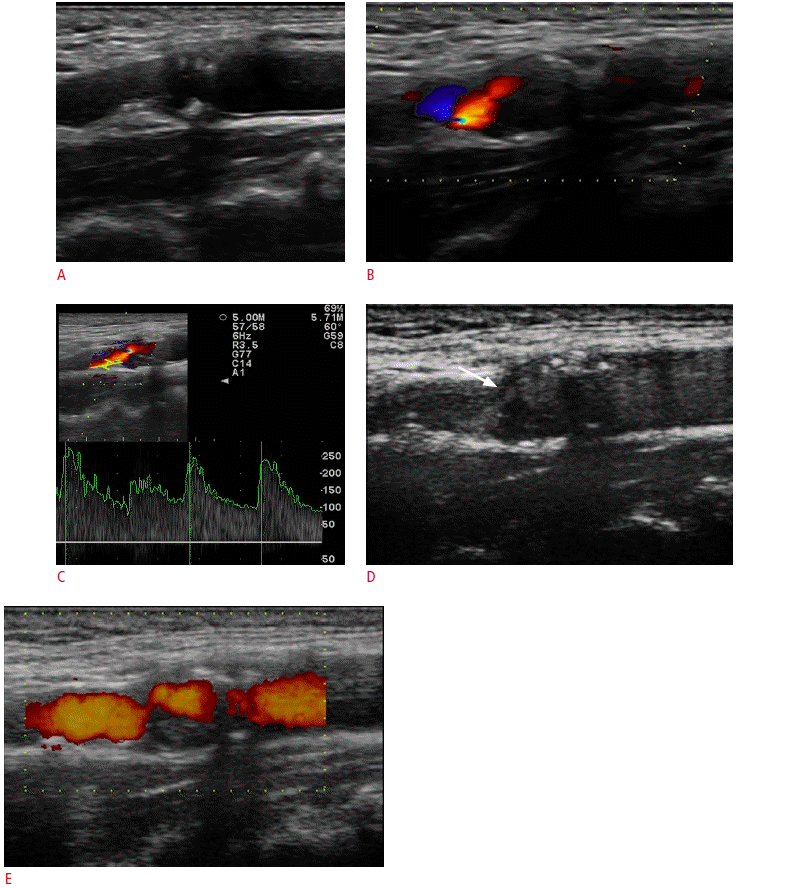
A 65-year-old male with stroke having significant stenosis at proximal internal carotid artery (ICA).
A. The gray-scale sonogram shows an atherosclerotic mixed plaque (inhomogeneous echogenicity) with significant stenosis (>50%) at proximal ICA. B, C. Color Doppler (B) and pulsed-wave Doppler (C) sonograms show intraluminal turbulent flow with increased peak systolic velocity (250 cm/sec) with spectral widening. D. Contrast-enhanced ultrasonography provides a more accurate delineation of atherosclerotic plaques and detailed visualization of the perfused stenotic lumen (arrow) because it can reveal the full length of the lumen without overwriting the vessel walls. E. Power Doppler after injection of the microbubbles shows the overamplification of the Doppler signal inside the highly stenotic part of the vessel, which is achieved with the use of microbubbles.
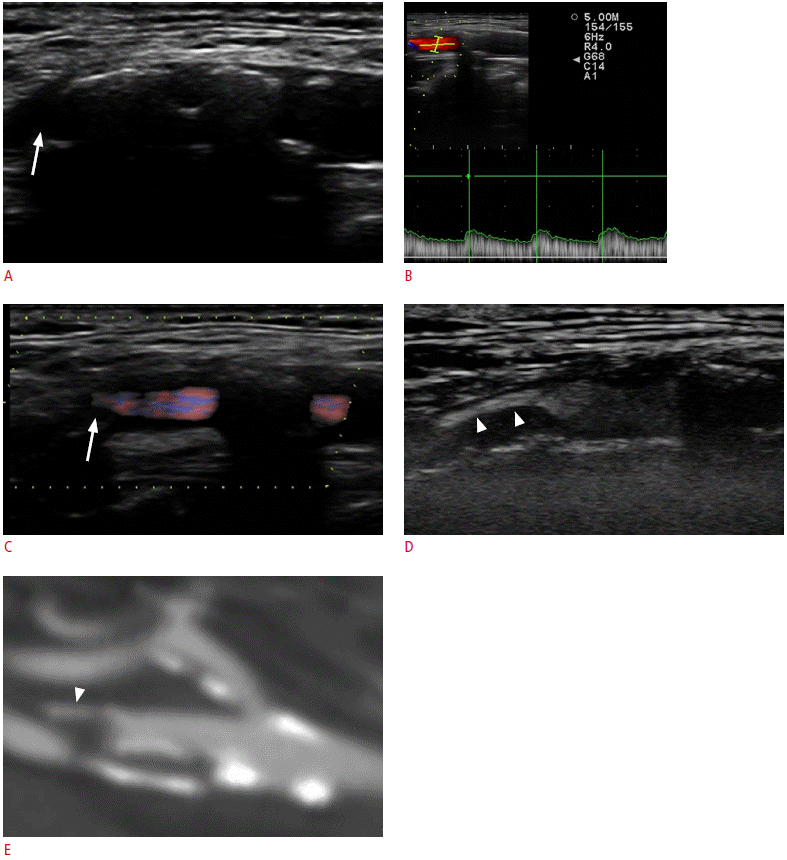
A 77-year-old male with TIA due to distal internal carotid artery (ICA) atherosclerosis.
A. The gray-scale sonogram shows heavily calcified plaques in the bifurcation and the origin of ICA, which hinder a complete evaluation of the plaques (arrow). B. The color pulsed-wave Doppler technique was falsely considered to show no significant stenosis because the imaging of the origin of the ICA and the flow velocities measured in this part of the vessel, distal to heavily calcified plaques, were within normal limits. C. The directional-eFLOW technique was not more reliable to image the peripheral part of the vessel due to the defective filling of the lumen with flow signals (arrow). D. A contrast-enhanced ultrasonography examination was able to adequately evaluate more distal parts of the internal carotid, which were highly stenotic due to the presence of an anechoic plaque (arrowheads). This is facilitated by the increased concentration of the microbubbles in the stenotic lumen. The stenotic parts in this case could not be fully evaluated with the previous methods. E. Multidetector computed tomographic angiography after contrast agent injection confirmed the distal stenosis (arrowhead). This image was produced after 90° rotation of the computed tomography image, which was done in order to produce an image that resembled the sonogram. TIA, transient ischemic attack.
The CEUS technique is currently considered more reliable than conventional Doppler US techniques in depicting a slow blood flow or a flow in severe stenosis, even without the use of specialized equipment. When the latter is available, low mechanical index CEUS provides an improved visualization of the blood flow and delineation of the vascular wall in prestenotic, intrastenotic, or poststenotic parts even in elongated vessels. Improved depiction of both the blood flow and the vessel wall by the CEUS technique is greatly facilitated by the fact that neither is CEUS affected by aliasing and blooming artifacts nor does it depend on the Doppler angle. All these three technical parameters may hinder a detailed evaluation of the vascular lumen in severely stenotic carotids (Fig. 3) [10]. CEUS was found to better evaluate subclinical atherosclerosis as it detected more plaques than conventional US. A majority of the plaques detected only by CEUS were hypoechoic, thus eluding detection by conventional techniques (Fig. 4) [11]. Thanks to its higher spatial resolution, the CEUS technique can also effectively identify the dissection of the carotids with a depiction of the true and the false lumen or even fistulous communications between the common carotid artery and the internal jugular vein. In the latter case, aliasing, overwriting artifacts and “visible color bruit” may hamper the precise imaging of the communication with conventional Doppler techniques [12].
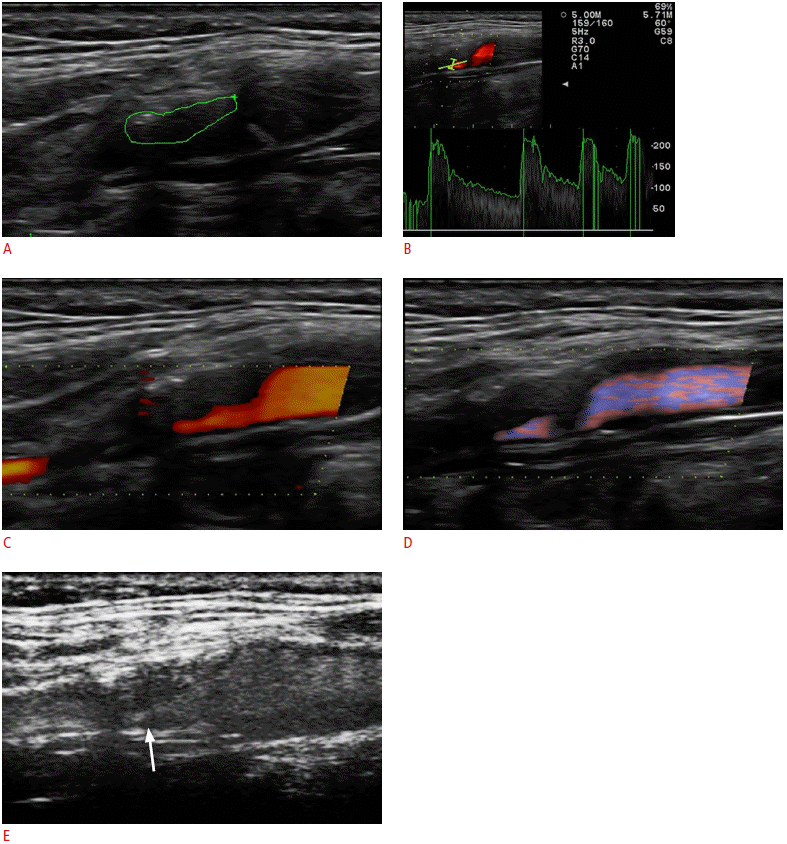
A 67-year-old male with recent TIA.
A. The gray-scale sonogram shows a predominantly hypoechoic plaque in the internal carotid artery, which is circulated by an ROI created to produce an echogenicity histogram (green line). B. The color pulsed-wave Doppler technique measured high velocities in the stenotic part of the internal carotid artery (peak systolic velocity, 220 cm/sec). C, D. The power Doppler imaging (C) and directional-eFLOW technique (D) show parts of the lumen with no detectable flow, which could be caused by the narrowing of the blood flow through the stenotic vessel. E. Contrast-enhanced ultrasonography accurately depicts the blood flow in the stenotic part of the vessel (arrow). The surface of the plaque is more accurately delineated, without any flow artifacts. TIA, transient ischemic attack; ROI, region of interest.
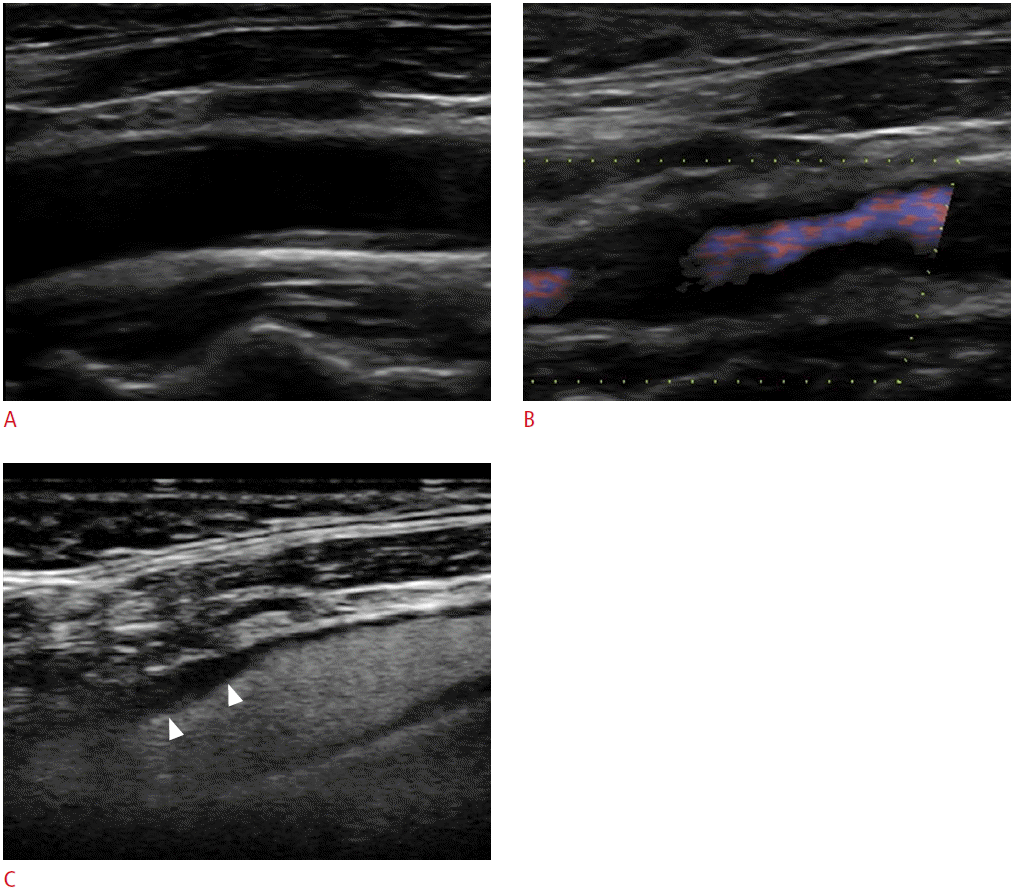
A 63-year-old male with TIA.
A. The gray-scale sonogram of the origin of the internal carotid artery fails to detect a completely echolucent plaque. B. Directional-eFLOW imaging raises suspicion of filling defects in both sides of the vascular lumen. C. The respective contrast-enhanced ultrasonography image clearly depicts the complete filling of the lumen with microbubbles except for a part of the proximal wall, which is occupied by a homogeneously echolucent plaque (arrowheads). TIA, transient ischemic attack.
CEUS is also currently indicated for the differentiation of carotid occlusion from near-occlusion stenosis (Figs. 5, 6) [10]. This differential diagnosis is greatly assisted by the higher spatial and time resolution produced by CEUS [4,12]. CEUS is thus very important for treatment decision making; specifically, patients with preocclusive stenosis should be treated surgically whereas patients with the occlusion of the carotid should not be.

A 68-year-old female with near-occlusion stenosis of the internal carotid artery (ICA).
A. The gray-scale sonogram demonstrates an ICA, which is filled with a hypoechoic material (green line represents an ROI for an echogenicity histogram). ECA, external carotid artery. B. The color Doppler technique reveals limited flow signals in the origin of the vessel, which are absent more distally, showing evidence of occlusion. C. Contrast-enhanced ultrasonography demonstrates normal contrast uptake of the common and external carotid artery, while there are moving microbubbles inside the residual lumen towards the periphery of the ICA, posing the diagnosis of its near-occlusion stenosis (arrow). D. Contrast-enhanced magnetic resonance angiography proves the presence of a contrast agent inside the internal carotid, confirming the diagnosis of near-occlusion stenosis (arrow). ROI, region of interest.
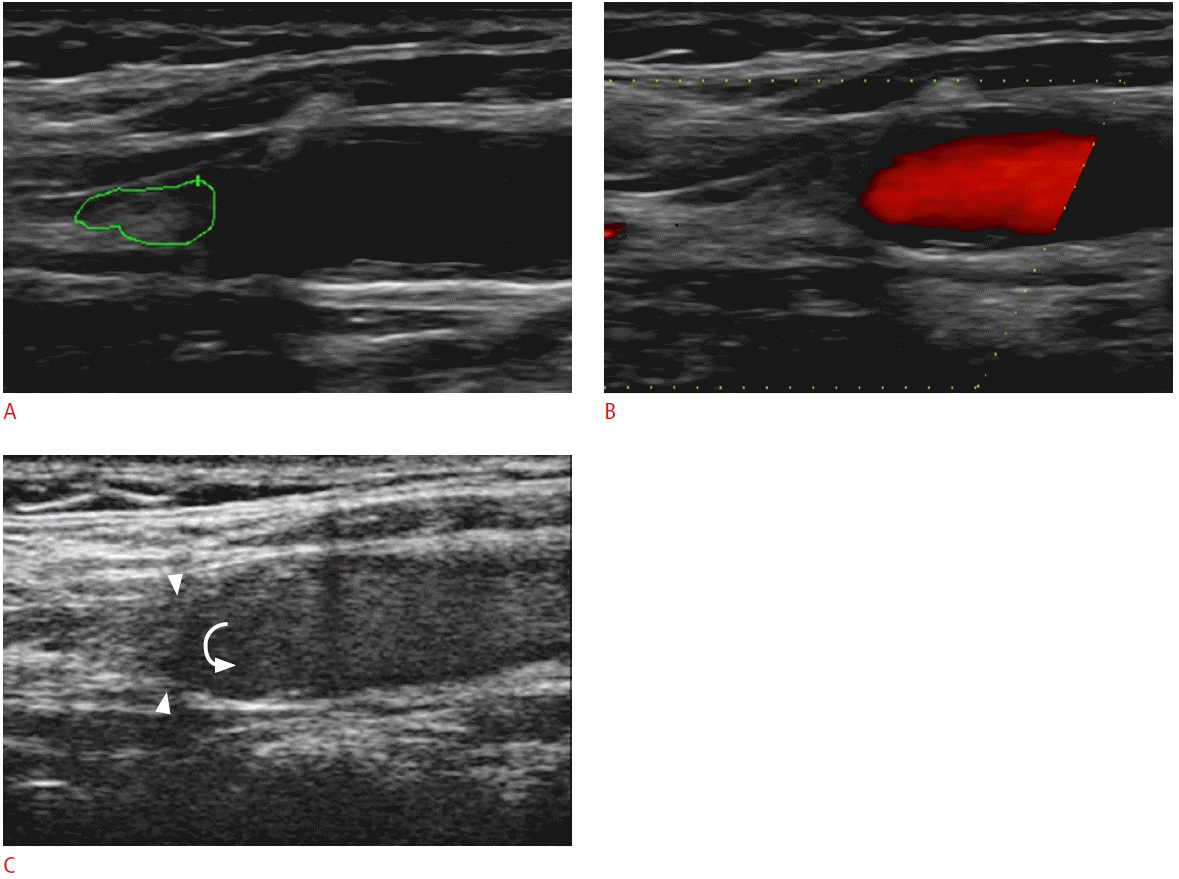
An 82-year-old male with the occlusion of the right internal carotid artery (ICA).
A. The gray-scale sonogram shows the echogenic material filling the lumen of the ICA (green line represents an ROI for an echogenicity histogram). B. The color Doppler technique reveals no color flow signals inside the internal carotid showing evidence of obstruction. C. Contrast-enhanced ultrasonography confirms the diagnosis with greater confidence as there are no microbubbles detected inside the obstructed vessel (arrowheads). The microbubbles could be seen moving in a revolving pattern at the site of obstruction (curved arrow). ROI, region of interest.
Plaque Assessment with CEUS in Comparison with Other Techniques
Carotid Plaque Ulcerations
The carotid plaque surface may be smooth, irregular, or ulcerated and constitutes a very important determinant of vulnerability [13]. That is, it was shown that ulcerated plaques are highly vulnerable and increase the likelihood of cerebrovascular events [6,7,14]. Research has shown that conventional US techniques detect plaque ulcerations with a sensitivity ranging from 30% to 80% with histology as a reference standard [13]. Saba et al. [14] investigated the diagnostic accuracy of CDU and MDCTA in identifying carotid plaque ulcerations. Having used surgical confirmation as their reference standard, they concluded that MDCTA was superior to US. These results were in agreement with previous studies as well [14]. However, the CEUS technique was not evaluated in these studies. This method was recently studied by ten Kate et al. [7], who compared it with CDU and used MDCTA as the reference standard. They defined carotid plaque ulceration as the presence of at least one disruption of the plaque/lumen border measuring more than 1×1 mm and concluded that CEUS was superior to the conventional color Doppler technique in detecting plaque ulcerations (Fig. 7) [7].
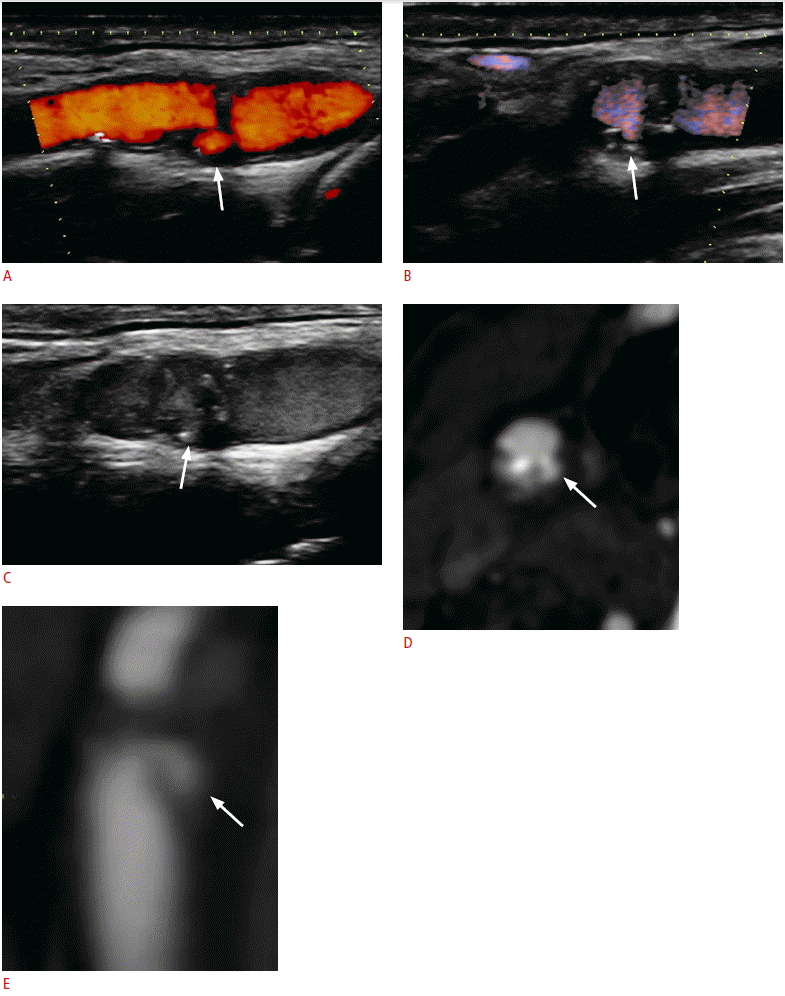
A 77-year-old male who has plaque ulceration.
A, B. Power Doppler imaging (A) and directional-eFLOW imaging (B) adequately identify an ulcer in the carotid plaque located in the distal carotid wall (arrows). However, there are interruptions in the color flow signals due to calcifications. C. The contrast-enhanced ultrasonography examination provided better delineation of the carotid plaque surface, the blood flow, and better filling of the ulcer (arrow). D, E. Contrast-enhanced multidetector computed tomographic angiography confirmed the presence of an ulcerated plaque (arrows) in both transverse (D) and sagittal reformatted planes (E).
IPN and Hemorrhage
IPN is regarded as a marker of the vulnerability of carotid plaque as it increases the risk of rupture [6]. IPN is also considered to be associated with the presence of intraplaque hemorrhage. It is suggested that the rupture of intraplaque neovessels may represent a cause of hemorrhage formation [6]. IPN can be seen on US as echogenic microbubbles moving inside atherosclerotic plaque after the administration of the contrast medium. On the other hand, intraplaque hemorrhage is related to US characteristics of uniformly or partially echolucent plaques or abruptly enlarged plaques.
There has been a series of studies investigating the identification of intraplaque neovessels by CEUS with histologic confirmation as the reference standard. This hypothesis was based on the grounds that microbubbles remain only in the vascular lumen. They thus enable the identification of intraplaque neovessels when seen moving inside an atherosclerotic plaque [5]. It was concluded that the contrast enhancement of a carotid plaque on CEUS correlates with the extent of IPN as the higher the grade of enhancement, the higher is the density of microvessels on histology (Fig. 8). IPN was evaluated on histology by using specific vascular and angiogenic markers like CD31, hemosiderin, and vascular endothelial growth factor (Fig. 9) [3].
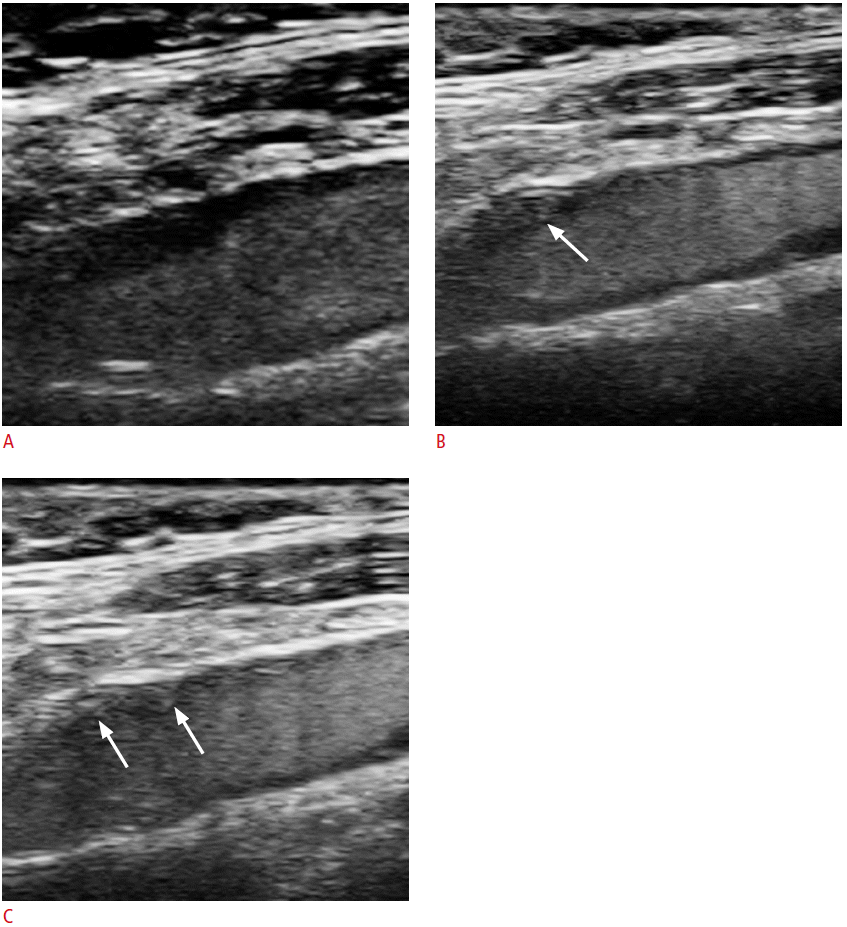
A 63-year-old male with TIA and a carotid plaque with intraplaque neovascularisation.
A-C. Images of this patient are also presented in Fig. 4. This figure presents a series of images with contrast-enhanced ultrasonography, which were captured at time intervals of several seconds. Of note are the contrast microbubbles, which are seen entering the plaque and represent intraplaque neovascularisation (arrows). TIA, transient ischemic attack.

A 77-year-old male with a carotid plaque with intraplaque neovascularisation.
A. Power Doppler imaging as well as the other conventional ultrasonographic techniques (not presented) identified a hypoechoic plaque in the proximal part of the internal carotid artery (asterisk). B. Contrast-enhanced ultrasonography reveals the presence of moving linear echoes inside the plaque, which represent moving microbubbles inside the neovessels and thus neovascularisation (arrows). The identification of neovascularisation is more prominent in the proximal wall of the vessel than the distal one. C. The corresponding histologic image with immunohistochemical staining with CD34 shows the presence of intraplaque neovessels (×10).
Recent studies have tested quantitative methods of evaluating the contrast enhancement of carotid plaques. It was reported that such methods as the calculation of the IPN area, IPN area ratio, and the neovessel count were significantly correlated with the visual assessment and had a good-to-excellent intraobserver and interobserver agreement [15]. Different studies using quantitative methods confirmed that the enhancement is significantly associated with histologic neovascularization and is higher in ruptured plaques and in symptomatic patients [16]. Therefore, CEUS-detected neovascularization may indeed play an important role in the risk stratification of patients with carotid atherosclerosis. The performance of various US techniques in the above-discussed aspects according to our experience can be seen in Table 1.
Limitations of CEUS
Regarding limitations of the technique, we should mention the need of an US device with specialized software and presets to better image the microbubbles. Heavily calcified plaques that produce acoustic shadows hinder the examination of the lumen and plaques, as with the other US techniques (Fig. 1). As the concentration of the microbubbles decreases after some minutes, the CEUS examination has a time limitation. Finally, CEUS does not provide information on the blood flow profile. Such information is obtained using the PW Doppler technique.
Conclusion
In conclusion, CEUS of the carotids represents a recently introduced application of this well-established US method, which is characterized by notable advantages as compared to conventional US. Beyond the improved wall delineation and the detailed identification of carotid atherosclerotic plaques, this technique plays an important role in diagnosing vulnerable carotid plaque, as it has the potential to identify IPN and carotid plaque ulcerations.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The first author (V.R.) has received a scholarship for his master thesis on “Imaging of the Carotid Atherosclerotic Disease” from the Onassis Foundation.
We would like to thank A. Papanikolaou, MD, PhD, Director of the Department of Pathology of the G. Gennimatas General Hospital of Thessaloniki and C. Theocharides, MD, resident of the same department for providing the histologic image with immunohistochemical staining of the patient presented in Fig. 9C.