Local ablation therapy with contrast-enhanced ultrasonography for hepatocellular carcinoma: a practical review
Article information
Abstract
A successful program for local ablation therapy for hepatocellular carcinoma (HCC) requires extensive imaging support for diagnosis and localization of HCC, imaging guidance for the ablation procedures, and post-treatment monitoring. Contrast-enhanced ultrasonography (CEUS) has several advantages over computed tomography/magnetic resonance imaging (CT/MRI), including real-time imaging capability, sensitive detection of arterial-phase hypervascularity and washout, no renal excretion, no ionizing radiation, repeatability, excellent patient compliance, and relatively low cost. CEUS is useful for image guidance for isoechoic lesions. While contrast-enhanced CT/MRI is the standard method for the diagnosis of HCC and post-ablation monitoring, CEUS is useful when CT/MRI findings are indeterminate or CT/MRI is contraindicated. This article provides a practical review of the role of CEUS in imaging algorithms for pre- and post-ablation therapy for HCC.
Introduction
Contrast-enhanced ultrasonography (CEUS) for liver imaging has been extensively studied in the last few decades and is now established as one of the routine imaging tests for imaging hepatocellular carcinoma (HCC) in many institutions [1,2]. According to recent practice guidelines for HCC management support for a non-invasive diagnosis of typical HCC on imaging without biopsy [3-5], the management of HCC largely depends on imaging diagnosis and post-treatment monitoring. Local ablation therapy including radiofrequency ablation (RFA) has been increasingly used for the treatment of HCC as a first-line curative therapy or a bridge to liver transplantation [6]. Ultrasonography (US) is most commonly used for image guidance for local ablation therapy; however, it is occasionally difficult to visualize lesions due to isoechoic HCC or lesions with ill-defined margins. CEUS can help localize these lesions and provide real-time guidance for the ablation procedure. Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are most commonly used for post-RFA monitoring, but the findings are often inconclusive. CEUS is useful for further characterization of indeterminate findings on CT or MRI by utilizing advantages such as its real-time imaging capability and sensitive detection of arterial-phase hypervascularity and washout. CEUS can be safely performed in patients with renal failure when CT or MRI cannot be performed with the use of a purely intravascular contrast agent without renal excretion. CEUS has been used as a routine clinical test for more than 10 years at our institution and has become an essential component in the imaging algorithms related to local ablation therapy for HCC.
CEUS Techniques
A contrast-specific imaging mode, such as pulse inversion or amplitude modulation, with the use of low-mechanical index (MI) imaging enables a real-time evaluation of the enhancement. Continuous low-MI (0.07-0.2) imaging is performed during the arterial phase and the portal venous phase, for 4-5 minutes after injection of the microbubbles. The currently used US contrast agents are perfluorocarbon microbubbles stabilized by a protein, lipid, or polymer shell. Microbubbles cannot move through the vascular endothelium into the interstitium; therefore, they are true blood pool agents. A variety of microbubble-based contrast agents are now available for clinical use in different parts of the world. The microbubble contrast agent used at our institution is Definity (perflutren lipid microspheres; Lantheus Medical Imaging, North Billerica, MA, USA). Typically, a bolus of 0.2- or 0.3-mL of Definity is injected manually through a three-way stopcock, followed by 5 mL of saline flush. The first injection usually includes a stationary field of view to include the lesion of interest and adjacent liver, both observed continuously for 4-5 minutes. Subsequent injections concentrate on the arterial-phase enhancement pattern and vessel morphology as well as the sweeps of the entire liver in the portal venous phase to look for any further abnormalities. Injections are typically repeated 2-5 times to obtain images for the same lesion or to evaluate different lesions. The injections are administered at intervals of 3-5 minutes. A simultaneous dual-imaging mode, which displays grayscale imaging and contrast-specific imaging side-by-side, is available in most of the updated US scanners. The dual-imaging mode is particularly useful for evaluating small liver lesions.
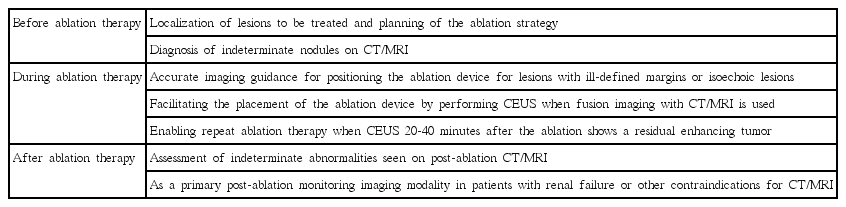
Before and during Ablation Therapy
Local ablation therapy for HCC is commonly performed without biopsy confirmation when the lesion shows the characteristic imaging patterns of HCC on dynamic contrast-enhanced images (i.e., hypervascularity in the arterial phase and later washout) according to the practice guidelines [3-5]. The detection of hypervascularity is essential for a non-invasive diagnosis of HCC. CEUS with a real-time evaluation of the dynamic enhancement of the liver nodule has an advantage over CT or MRI with respect to the detection of hypervascularity, eliminating the issue of inappropriate arterial-phase timing [7]. One of the most common uses of CEUS is to evaluate small, indeterminate, non-hypervascular lesions seen on CT or MRI. CEUS is often able to diagnose HCC by detecting hypervascularity in some of these lesions, avoiding an invasive biopsy (Fig. 1) [7-9]. The use of CEUS is controversial because intrahepatic cholangiocarcinoma can be misdiagnosed as HCC, and CEUS has thus been subsequently excluded from the list of diagnostic tests for HCC in the updated (American Association for the Study of Liver Diseases [AASLD]) practice guidelines [3]. However, it has been argued that intrahepatic cholangiocarcinoma is relatively rare in liver cirrhosis patients and that CEUS can depict findings suggestive of cholangiocarcinoma [10]. As the imaging diagnosis of small nodules 1-2 cm in size can be particularly challenging, a multi-modality approach is often needed. CEUS is actively used as one of the diagnostic tests for HCC at our institution. Transient hypovascularity followed by isovascularity in the arterial phase of CEUS indicates a borderline lesion including a dysplastic nodule or early HCC [1,11,12], usually necessitating a biopsy before treatment decisions. A real-time evaluation using CEUS also enables a detailed assessment of the arterial-phase filling pattern and vascular morphology within the liver lesion, which is often critical for the differential diagnosis of rapidly enhancing hypervascular liver lesions [13,14]. Washout, which is one of the two essential findings for characterizing malignant liver lesions, is also consistently seen on CEUS because of the purely intravascular nature of microbubbles without a leak to the interstitium (Fig. 2) [7]. Tumor thrombosis in the portal or hepatic vein is usually considered a contraindication for local ablation therapy for HCC. CEUS is useful for differentiating between benign and malignant venous thrombosis [15].
A 64-year-old man with hepatocellular carcinoma (HCC).
A. Unenhanced T1-weighted magnetic resonance image shows a slightly hyperintense nodule (arrow) in the liver. B. The nodule (arrow) is hyperintense in the arterial phase; however, it is not clear if the nodule is hypervascular or not as the nodule was hyperintense on an unenhanced scan. C. The nodule is not seen in the delayed phase due to isointensity to the liver. Therefore, the magnetic resonance imaging appearance is indeterminate. D. The nodule (arrow) is slightly hypoechoic on grayscale ultrasonography. E, F. The nodule (arrow) is clearly hypervascular in the arterial phase of contrast-enhanced ultrasonography (E) and shows washout at 195 seconds (F), confirming the diagnosis of recurrent HCC. The nodule was then treated with radiofrequency ablation (not shown).

A 90-year-old man for post-radiofrequency ablation (RFA) follow-up.
A. An magnetic resonance scan in the arterial phase shows a nodular hypervascular lesion (arrow) in the dome of the right lobe of the liver. B. The lesion is not seen due to isointensity to the liver in the delayed phase. There is a markedly hypointense ablation zone (asterisks in A and B) posteriorly. C. The nodule (arrows) is slightly hypoechoic on grayscale ultrasonography. D. Contrast-enhanced ultrasonography (CEUS) in the arterial phase shows hypervascularity in the lesion (arrow). E. CEUS at 240 seconds shows clear washout (arrow), confirming the diagnosis of recurrent hepatocellular carcinoma. The lesion was treated with RFA (not shown).
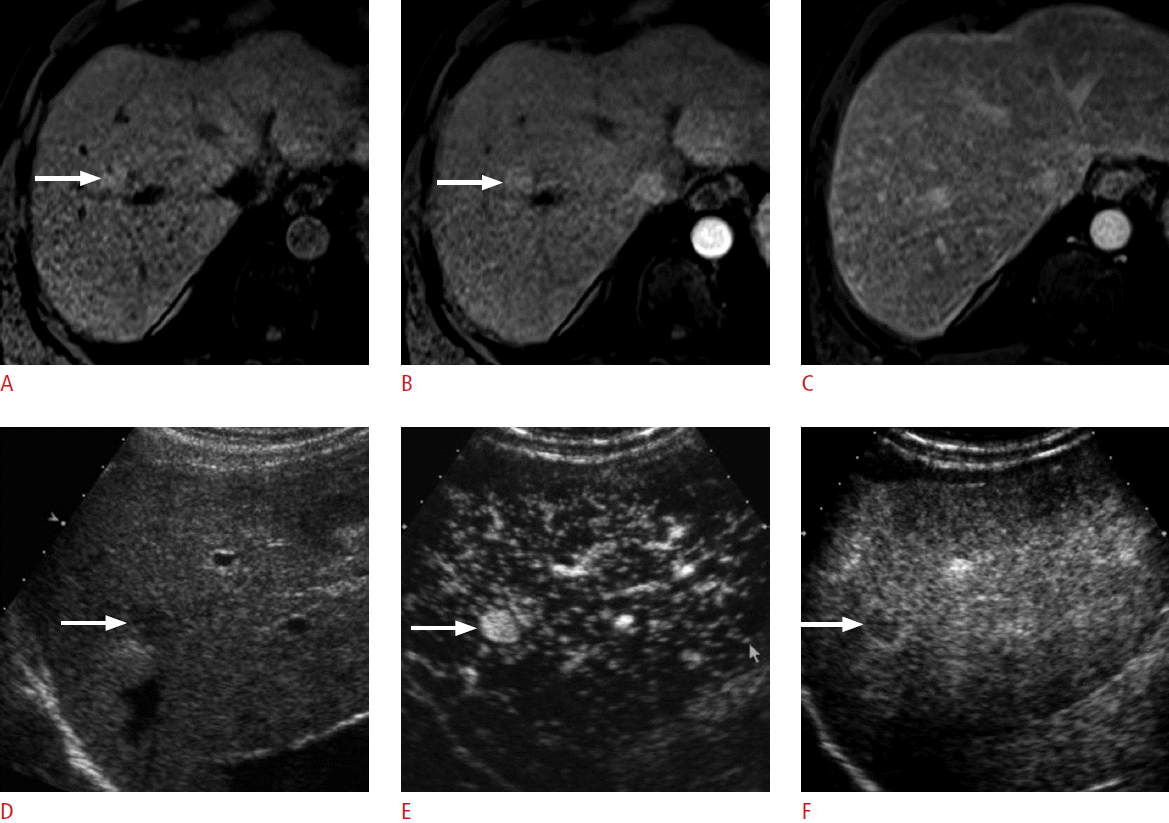
US is most commonly used as the imaging guidance modality for local ablation therapy for HCC. However, there are occasional HCC cases with ill-defined margins or isoechogenicity on grayscale US, which make the ablation procedure challenging. Most of the current US scanners provide a dual-imaging mode, which simultaneously displays a contrast-specific mode and a grayscale mode side-by-side (Fig. 3). The dual-imaging mode is essential for localizing the target lesion and for an appropriate evaluation of the dynamic enhancement of the lesion. The performance of CEUS before a US-guided RFA procedure can support reliable localization of HCC, reducing the number of failed RFA procedures [16]. Fusion imaging techniques that can coordinate the CEUS images with CT/MRI images are also helpful for the successful localization of difficult lesions and reducing the overall procedure time [17].
A 47-year-old woman with chronic hepatitis B.
A. Grayscale sonogram shows a subtle hypoechoic lesion (arrows) in the liver. The visibility of the lesion on grayscale ultrasonography is not satisfactory enough to perform ultrasonography-guided radiofrequency ablation. B. Dual-imaging mode contrast-enhanced ultrasonography (contrast mode on the left and grayscale mode on the right) in the arterial phase demonstrates diffuse hypervascularity (left arrow) within the subtle hypoechoic lesion (right arrow) on the grayscale sonogram. C. The nodule (arrow) shows washout at 220 seconds, a typical late washout pattern of hepatocellular carcinoma.
One of the important advantages of CEUS is that the contrast can be repeatedly injected at short intervals. CEUS can be performed just before the placement of the ablation needles and repeated after the needle placement to ensure the appropriate placement. CEUS can also be repeated after the ablation to identify any residual enhancing tumor. This should be performed approximately 20-40 minutes after the ablation because gas-related artifacts secondary to cavitation within the heated tissues can misdirect the assessment [18]. Repeat ablation therapy can be immediately performed if there is any residual enhancing tumor within the ablated lesion.
Evaluation of Post-Ablation Therapeutic Response
Multiphasic contrast-enhanced CT or MRI is most commonly used for evaluating the treatment response to local ablation therapy for HCC because CT or MRI not only shows the findings for the completeness of the treatment and any residual viable tumors around the ablation zone, but also provides appropriate restaging of HCC, including the rest of the liver, vascular invasion, lymph nodes, and any extrahepatic metastasis. CT or MRI is typically performed within 30 days of ablation. However, the interpretation of the post-ablation CT or magnetic resonance images is often challenging because of benign perfusion changes related to the ablation procedures and occasional non-hypervascular abnormalities near the ablation zone [19].
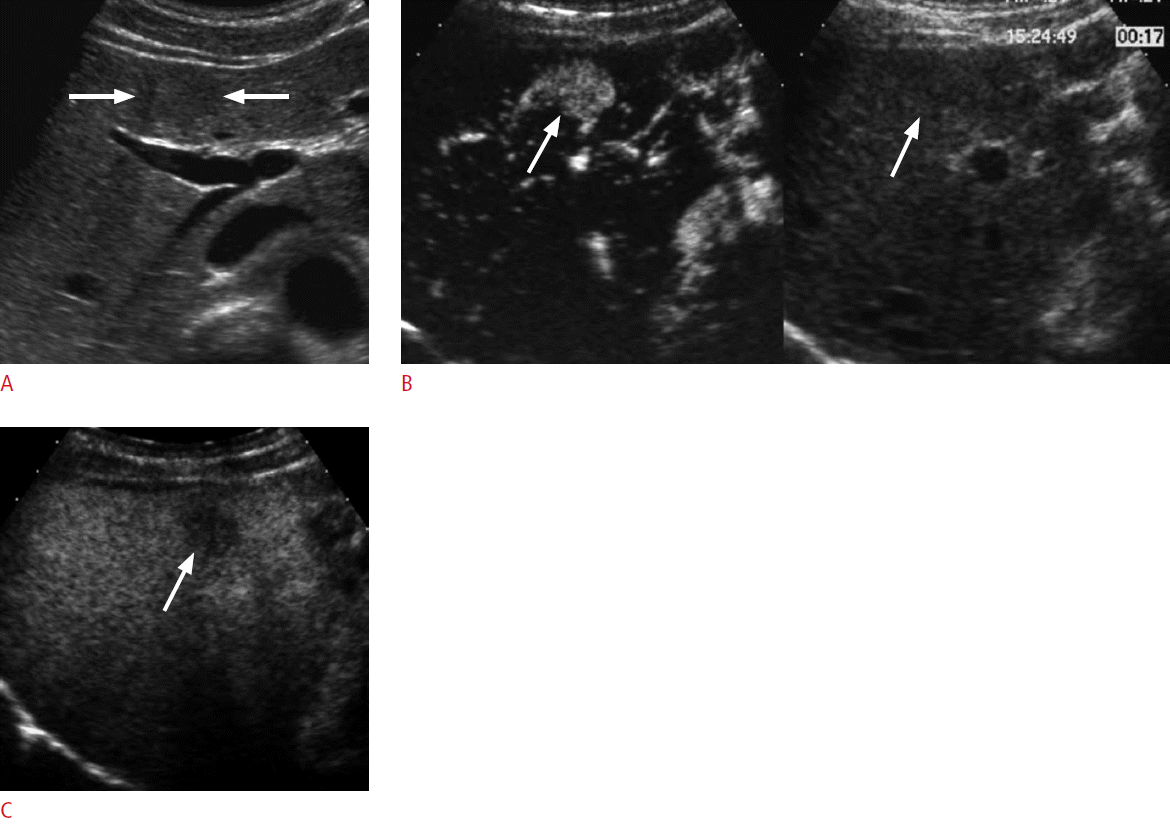
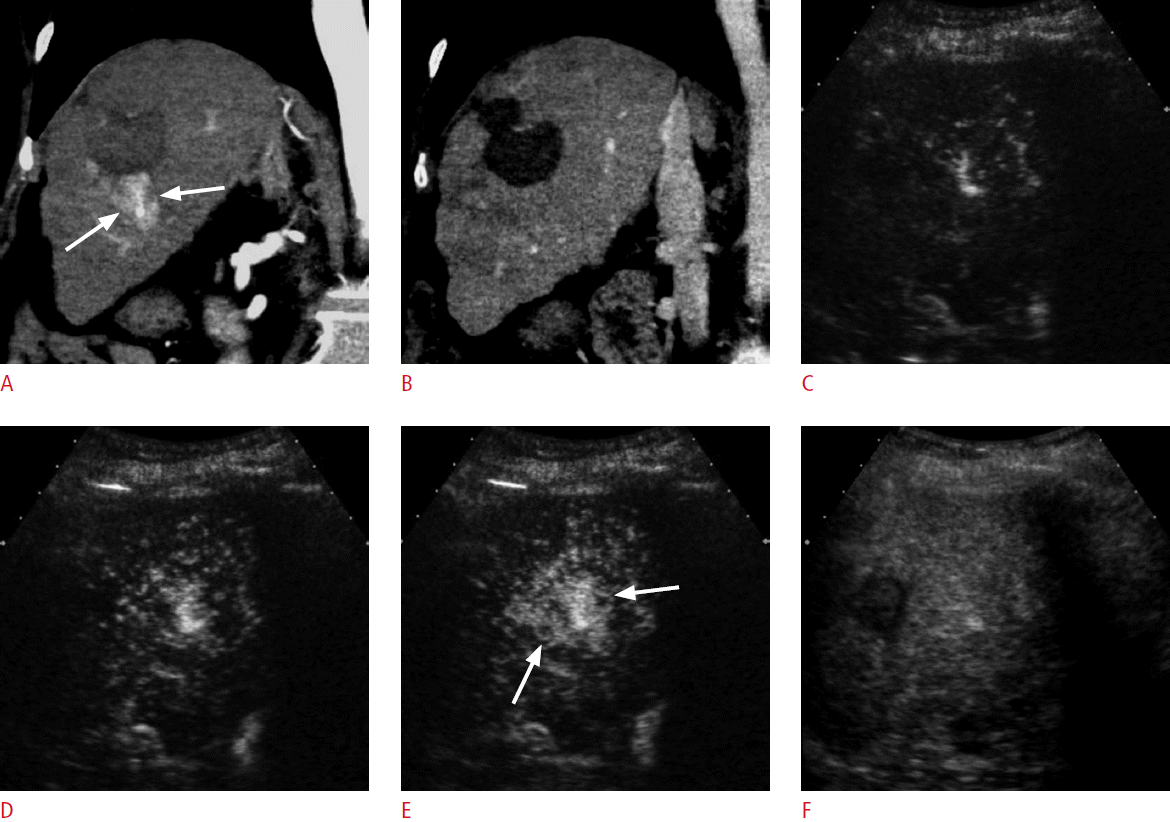
Hypervascular abnormalities surrounding the ablation zone are commonly seen after ablation therapy. This can be residual or recurrent HCC, but often represents benign perfusion abnormalities such as arterioportal shunting (Fig. 4). The differentiation between the two can be difficult when washout is not clearly seen. The challenge is often the difficulty in outlining the treated tumor because the pre-existing HCC lesion is obscured by the ablation zone on CT or MRI. On the other hand, grayscale US usually shows the outline of the original HCC lesion even after ablation. Therefore, CEUS has an advantage in the detection of any residual enhancing tumor within the ablated HCC (Fig. 5). Marginal recurrence of HCC is usually seen as a focal grayscale abnormality adjacent to the ablation zone on unenhanced US. A subsequent evaluation of the dynamic enhancement of the lesion with CEUS can show hypervascularity followed by washout, confirming the presence of recurrent HCC and its location. It is important to document the grayscale appearance of the recurrent HCC, which will help the US-guided repeat ablation therapy (Figs. 6, 7).
A 56-year-old woman for post-radiofrequency ablation follow-up.
A. A coronal computed tomography scan in the arterial phase shows a wedge-shaped hypervascular abnormality (arrows) below the ablation zone in the right lobe of the liver. B. The lesion is not seen due to isoattenuation to the liver in the delayed phase. C-E. Three successive contrast-enhanced ultrasonography images in the arterial phase show an initial enhancement of a small portal vein branch and subsequent wedge-shaped enhancement (arrows in E) that are typical for arterioportal shunting. F. The lesion is not seen in the late phase due to isoechogenicity at 180 seconds.
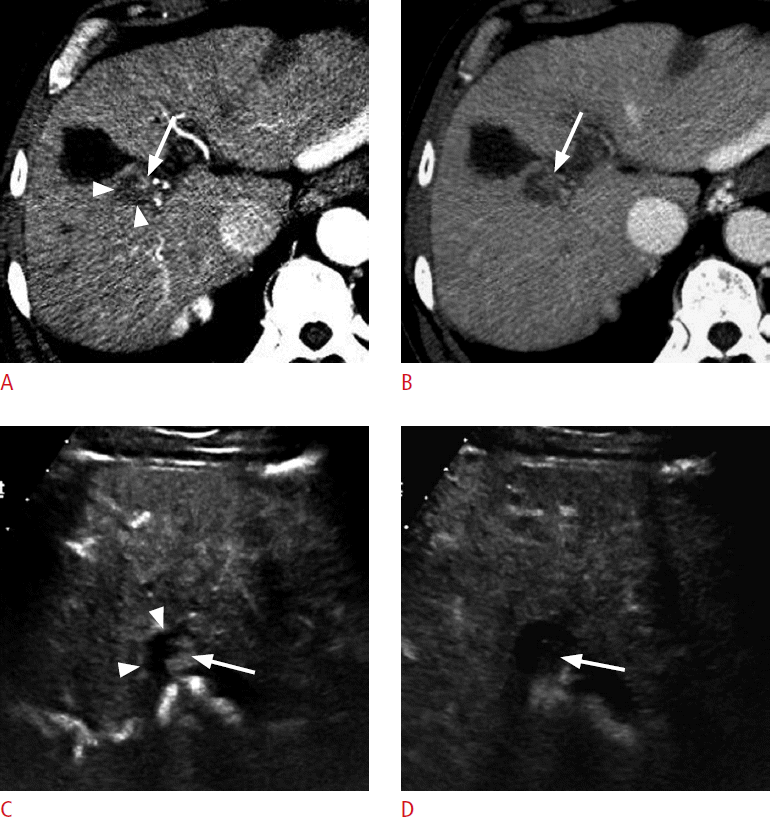
A 68-year-old man with hepatocellular carcinoma (HCC).
A. A computed tomography (CT) scan in the arterial phase shows a hypoattenuating ablation zone (arrowheads) near the gallbladder. There is a subtle enhancing focus (arrow) near the ablation zone. B. The enhancing focus (arrow) becomes hypoattenuating to the liver in the delayed phase. CT findings suggest local recurrence, but are not definitive. C, D. On contrast-enhanced ultrasonography, the enhancing focus is clearly seen within the treated nodular lesion (arrowheads). The enhancing lesion (arrow) is hypervascular in the arterial phase (C) and shows washout (arrow) at 65 seconds (D), confirming the diagnosis of residual viable HCC.

An 80-year-old man for post-radiofrequency ablation (RFA) follow-up.
A. A computed tomography scan in the arterial phase shows a hypervascular lesion (arrow) adjacent to the ablation zone in the liver. B. The lesion becomes isoattenuating to the liver in the delayed phase. The abnormality is indeterminate because of the absence of washout. C. Grayscale sonogram shows a crescent hypoechoic lesion (arrows) adjacent to the ablation zone with mixed echogenicity (asterisk). D, E. The lesion (arrows) is hypervascular in the arterial phase of contrast-enhanced ultrasonography (D) followed by washout in the late phase at 206 seconds (E). Repeat RFA was performed targeting the hypoechoic lesion under grayscale ultrasonography guidance (not shown).
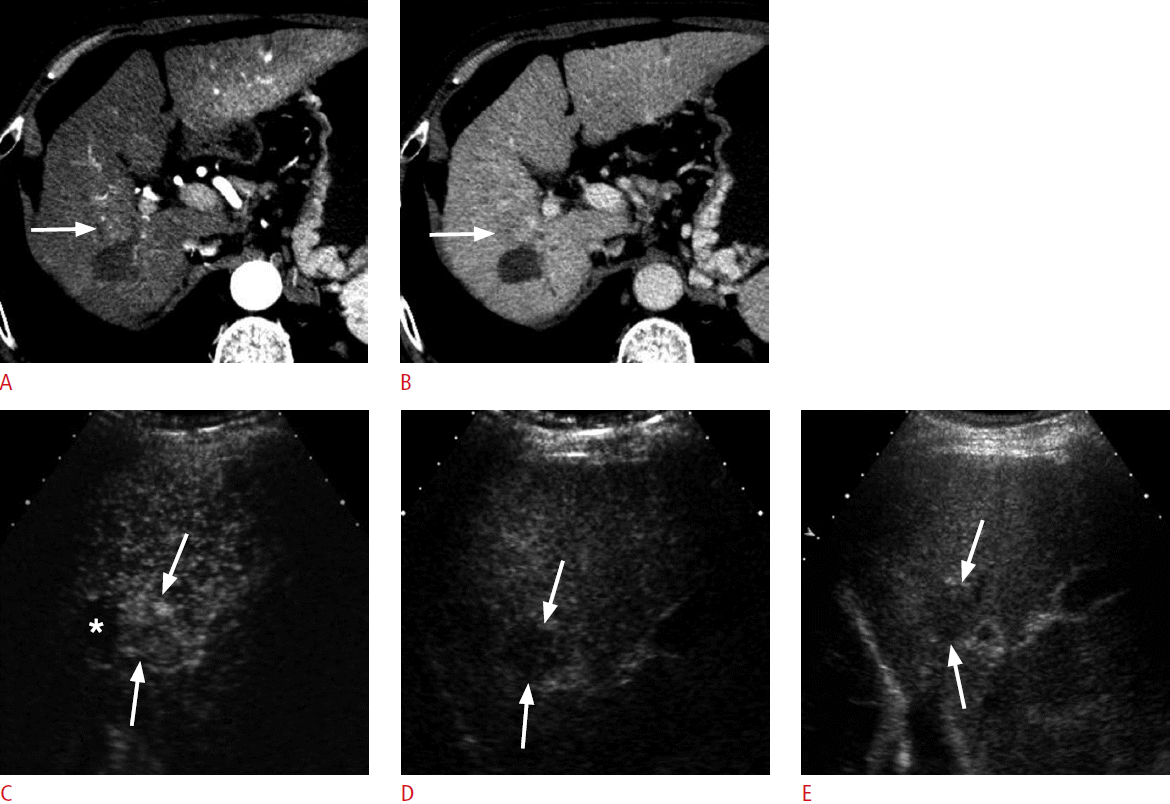
A 70-year-old man for post-radiofrequency ablation (RFA) follow-up.
A, B. A computed tomography (CT) scan in the arterial phase shows a hypoattenuating ablation zone in the right hepatic lobe. The enhancement of the liver is heterogeneous. There is a subtle area of slight hyperenhancement (arrow in A) anterior to the ablation zone, which shows slight hypoattenuation (arrow in B) in the delayed phase. The CT findings are indeterminate. C, D. Contrast-enhanced ultrasonography in the arterial phase (C) shows hypervascularity in the lesion (arrows) adjacent to the ablation zone (asterisk) and washout at 210 seconds (D), confirming the diagnosis of recurrent hepatocellular carcinoma. E. The recurrent tumor (arrows) is hypoechoic on grayscale ultrasonography, which served as an excellent imaging guidance for repeat RFA procedures.
Recurrent HCC adjacent to the ablation zone is occasionally non-hypervascular on CT or MRI due to the missed timing of the appropriate arterial phase. Subtle hypervascularity of recurrent HCC can also be obscured by adjacent perfusion changes (Fig. 7). CEUS is useful for further assessing hypoattenuating/hypointense abnormalities adjacent to the ablation zone on CT or MRI. CEUS can often diagnose the presence of lesion hypervascularity, utilizing the advantage of a real-time assessment of dynamic enhancement (Figs. 8, 9). However, one of the limitations of CEUS is that it is not possible to scan the whole liver in the arterial phase. Repeated sweepings through the ablation zone in the arterial phase and the delayed phase should be routinely performed to evaluate the entire ablation zone and its surroundings.

A 53-year-old man for post-radiofrequency ablation follow-up.
A. An magnetic resonance (MR) scan in the arterial phase shows a hypointense ablation zone (asterisk) with an irregular shape. B. There is a nodular and slightly hypointense lesion adjacent to the ablation zone (arrow) in the delayed phase. MR imaging findings are indeterminate. C. Contrast-enhanced ultrasonography (CEUS) in the arterial phase shows hypervascularity in the lesion (arrow). D. CEUS at 240 seconds shows clear washout (arrow), confirming the diagnosis of recurrent hepatocellular carcinoma.
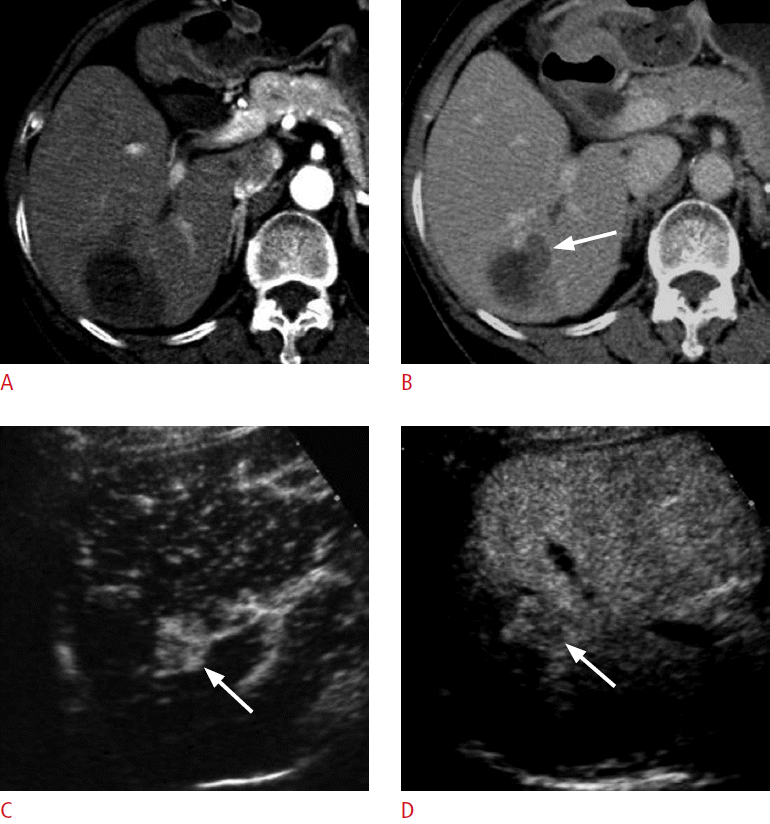
An 85-year-old woman for post-radiofrequency ablation follow-up.
A. A computed tomography scan in the arterial phase shows an ablation zone in the right lobe of the liver. No definite hypervascular lesion is seen. B. There is a hypoattenuating lesion (arrow) medial to the ablation zone in the delayed phase, which was not hyperattenuating in the arterial phase. C. On the arterial-phase contrast-enhanced ultrasonography scan, the lesion (arrow) is clearly hypervascular. D. The lesion (arrow) shows washout at 125 seconds, confirming the diagnosis of recurrent hepatocellular carcinoma.
It is important to understand that a pseudoenhancement from an echogenic ablation zone can occur on CEUS, particularly in deep-seated echogenic lesions. The pseudoenhancement probably results from a nonlinear propagation of a US beam within the liver parenchyma with microbubbles. The pseudoenhancement has distinctive imaging features including late initiation of enhancement in the late phase, progression of the degree of enhancement over time, non-marginal location, and an appearance exactly matching the echogenic region on the grayscale images (Fig. 10); these characteristic features are different from the patterns typical of tumor recurrence, which show early enhancement and later washout at the margin of the ablation zone [20].
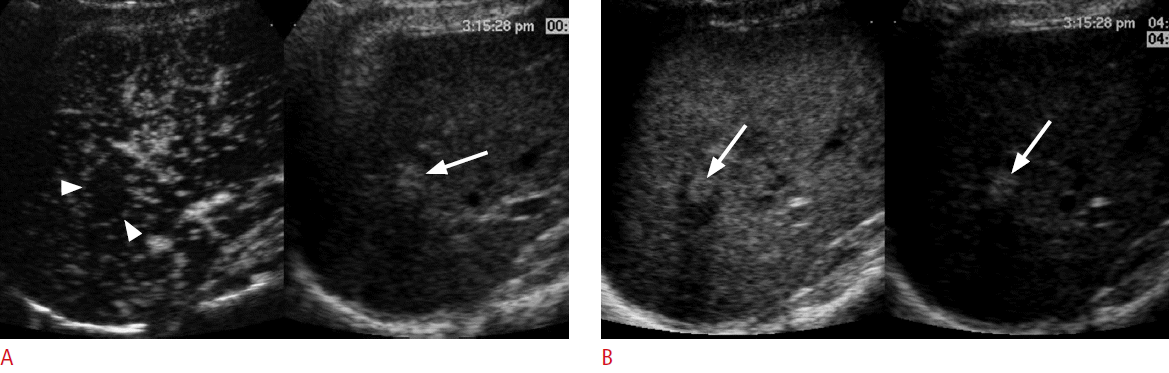
A 75-year-old man for post-radiofrequency ablation follow-up.
A. A contrast-enhanced ultrasonography scan in the arterial phase with the dual-imaging mode (contrast-mode on the left and grayscale-mode on the right) shows an avascular ablation zone (arrowheads) in the liver. There is a brightly hyperechoic area (arrow) within the ablation zone in the grayscale ultrasonography. B. In the late phase at 126 seconds, there is a focal echogenic area (left arrow) within the ablation zone on the contrast mode, which exactly matches the brightly echogenic area (right arrow) on the grayscale mode, representing a pseudoenhancement due to nonlinear artifacts that can be misdiagnosed as recurrent hepatocellular carcinoma.
Liver cirrhosis is often associated with renal failure, which contraindicates the use of a contrast agent for CT or MRI. CEUS is an excellent problem-solving method because microbubbles can be safely used in the case of renal failure. These patients can undergo CEUS in 3-6-month intervals as an alternative to CT or MRI. It is always important to carefully evaluate the whole liver with grayscale US before performing CEUS. Then CEUS can be performed focusing on the ablation zones and any new grayscale abnormalities (Fig. 11).
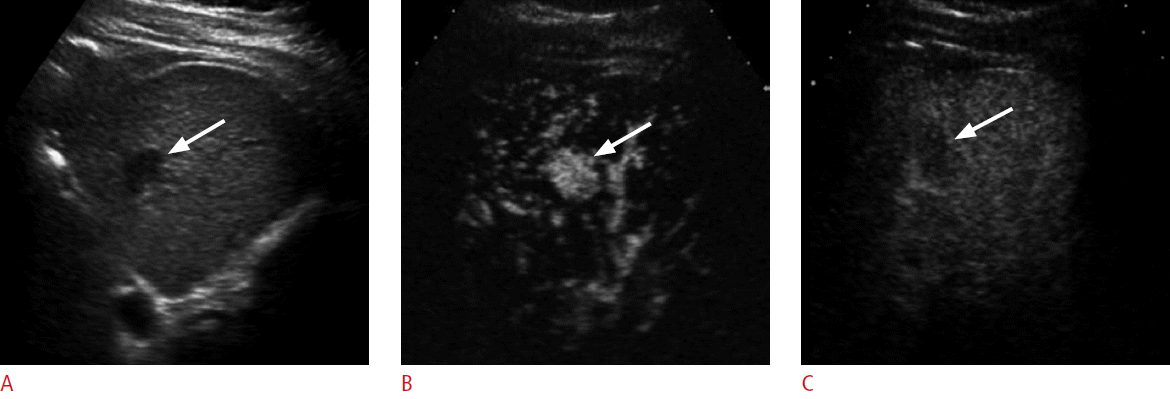
An 80-year-old man with renal failure and previous history of radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC).
A. A grayscale sonogram shows a new hypoechoic nodule (arrow) in the left lobe. As computed tomography or magnetic resonance imaging could not be performed because of renal failure, contrast-enhanced ultrasonography was performed for the diagnosis of the nodule. B, C. The nodule (arrow) shows hypervascularity in the arterial phase (B) followed by washout at 100 seconds (C), confirming the diagnosis of recurrent HCC. RFA was performed as a bridge therapy for liver transplantation (not shown).
Conclusion
While CT and MRI are the primary imaging modalities for the diagnosis/staging of HCC as well as monitoring of post-local ablation therapy for HCC, CEUS can be effectively used as a problem solver in the imaging algorithms for pre- and post-ablation therapy for HCC (Table 1).
Notes
No potential conflict of interest relevant to this article was reported.