AbstractUltrasonography (US)-based elastography has been introduced as a noninvasive technique for evaluating thyroid nodules that encompasses a variety of approaches such as supersonic shear imaging and acoustic radiation force impulse imaging as well as real-time tissue elastography. However, the diagnostic performances for differentiating malignant thyroid nodules from benign ones with elastography as an adjunctive tool of gray-scale US is still under debate. In this review article, diagnostic performances of conventional US and a combination of conventional US and elastography are compared according to the type of elastography. Further, the interobserver variability of elastography is presented according to the type of elastography.
Thyroid nodules are very commonly observed on thyroid ultrasonography (US), and conventional US has been widely used to determine which nodules should be biopsied. There are several suspicious US features that predict thyroid cancer, such as hypoechogenicity, marked hypoechogenicity, a microlobulated or spiculated margin, micro- or macro-calcifications, and a taller-than-wide shape [1,2]. Although conventional US can provide meaningful information in thyroid nodule diagnosis, there has been considerable variation in diagnostic performances [2-4].
On physical examination, a hard or firm nature is associated with thyroid malignancy. However, palpation is very subjective and limited in patients with multinodular goiter or small deep-seated nodules [5]. Meanwhile, US-based elastography can provide an objective evaluation of tissue stiffness [6,7]. There are two kinds of elastography (strain and shear wave elastography) that are currently used in clinical practice [8,9]. Although many reports have compared conventional US with elastography, in clinical practice, the final decision or diagnosis is usually based on a combination of conventional US and elastography. This review summarizes the current techniques, diagnostic performance, reproducibility, and limitations of thyroid elastography.
To evaluate the relationship between compression and strain, Young’s modulus, the ratio of pressure to strain, has been used. Strain (or static) elastography requires an external palpation with a probe or endogenous stress such as cardiovascular movements, resulting in an axial displacement of the tissue by mechanical stress (Fig. 1) [10]. Tissue deformation from the stress is measured and visualized in a split-screen mode with a conventional B-mode image and an elastogram on a screen. To acquire the elastographic images, compression is continuously applied by a transducer and followed by decompression. The elastic image is superimposed on the B-mode image, and tissue stiffness is displayed in a continuum of colors from red (soft tissue) to blue (hard tissue). According to the chosen machine, color scales are applied inversely.
By strain elastography, two kinds of elasticity assessments can be obtained. First, visual scoring of colors within and around the nodules can be assessed, using 4-5-scale scoring systems (Fig. 2) [6,7]. Second, two regions of interest (ROIs) are drawn over the target region and the adjacent reference region, respectively. Then, a strain ratio is automatically calculated through the machine. The likelihood of malignancy increases with an increase in the strain ratio [11].
Shear waves are the transverse components of particle displacement that are rapidly attenuated by the tissue. Their speed is closely related to Young’s modulus formula, in which tissue elasticity can be assessed from the shear wave propagation speed (Fig. 1) [12]. Shear wave elastography (SWE) provides quantitative elastic information on the basis of the acoustic pulse of a US probe that stimulates tissues, thereby producing a real-time elastogram [13,14]. As SWE is dependent on the production of radiation force by the probe, it is more operator-independent, reproducible, and quantitative. There are two applicable methods for the clinical evaluation of thyroid nodules: the supersonic shear wave and the acoustic radiation force impulse (ARFI) methods [15]. The former uses focused ultrasonic beams that propagate through the entire imaging area. A color-coded image displaying the shear wave velocity (m/sec) or elasticity (kilopascals, kPa) for each pixel in the ROI is acquired. Within a given ROI, a variety of stiffness parameters can be measured, including the mean stiffness (Emean), maximum stiffness (Emax), and standard deviation (SD). Unlike strain elastography, soft tissue is displayed in shades of blue and hard tissue is displayed in red [13,16]. ARFI uses short-duration acoustic pulses that excite the tissue within the ROI. The elasticity is expressed as in meters per second (m/sec) and does not display color-coded images for elastography [17].
Clinical application of thyroid elastography was first reported in 2007 by Rago et al. [6]; it used five-point scales based on Ueno and Itoh [18]’s study using strain elastography. A score of 1 defined elasticity that is entirely soft in the nodule, 2 as mostly soft in the nodule, 3 as peripherally soft, 4 as entirely hard in the nodule, and 5 as hard in the area under consideration as well as the entire nodule (Fig. 2). Other criteria demonstrated in 2008 by Asteria et al. [7] used four-point scales based on the study of Itoh et al. [10]. Asteria’s criteria defined a score of 1 as elasticity that is entirely soft in the nodule, 2 as mostly soft in the nodule, 3 as mostly hard in the nodule, and 4 as entirely hard in the nodule (Fig. 2) [7]. In their study, nodules with Rago scores of 4 and 5 or Asteria scores of 3 and 4 were regarded as suspicious elastographic features for malignancy. Using Rago’s criteria in a study including 92 consecutive patients with a single nodule, the researchers calculated the sensitivity to be 97% and the specificity to be 100% for predicting thyroid malignancy [6]. Using Asteria’s criteria, the researchers calculated sensitivity and specificity to be 94.1% and 81%, respectively, in 86 nodules [7]. The two investigations evaluated the diagnostic performances of each US feature on gray-scale US but did not evaluate combinations of US features. Further, they did not demonstrate the diagnostic role of a combination of conventional US and elastography. In practice, elastography is usually performed as an extension of conventional US and not as an independent test. Therefore, a comparison of conventional US with elastography can be meaningless in view of its current clinical utility. The value of elastography should be evaluated by comparing conventional US with a combination of conventional US and elastography. In 2012, Moon et al. [19] evaluated the practical role of elastography as an adjunctive tool of gray-scale US in 676 patients with 703 solid nodules. In the study, neither elastography nor the combination of elastography and grayscale US showed better performance for diagnosing thyroid cancers compared with gray-scale US.
SWE was first reported for diagnosing thyroid nodules in 2010 by Sebag et al. [13] in a study that included 146 nodules form 93 patients. Using significant US features (hypoechogenicity, microcalcifications, and intranodular vascularity) in the study, they calculated the US score by using the following equation: 42.18 (if intranodular vascularity=yes) + 6 (if hypoechogenicity=no) + 30.70 (if hypoechogenicity=yes) + 27.13 (if micro-calcification=yes). The US scores ranged from 0 to 100, with the higher values predicting malignancy. In that study, the best cut-off point for discriminating benign nodules from malignant ones was a mean elasticity index of 65 kPa on SWE and 63.60 (US score) on gray-scale US. The sensitivity and the specificity for malignancy were 51.9% and 97% in the case of gray-scale US and 81.5% and 97.0% in the case of the combination of gray-scale US and SWE, respectively. With these results, they concluded that SWE could be a promising tool for the differentiation of thyroid nodules. However, the sample size was small; further, the calculation of US scores is not commonly used in the clinical field due to its complexity. Thus far, there have been several reports regarding the use of SWE in the differentiation of benign and malignant nodules. Bhatia et al. [20] investigated the role of SWE using several parameters such as the mean elasticity index of the largest ROI within the whole lesion, the mean elasticity index of a 2-mm-diameter area in the stiffest region, the maximum and minimum elasticity index of the largest ROIs within the whole lesion, and standard deviation of the largest ROIs within the whole lesion (Fig. 3). Among these numerous parameters, the most accurate value was that of the mean elasticity index of a 2-mm-diameter area in the stiffest region with a cut-off value of 34.5 kPa. The sensitivity and the specificity for predicting malignancy were 76.9% and 71.1%, respectively. Veyrieres et al. [21] also evaluated the diagnostic performances of SWE in the case of 297 thyroid nodules. The sensitivity and the specificity had good results of 80% and 90.5%, respectively, with the best cutoff value of 66 kPa. They measured the elasticity index of ROIs covering the visually stiffer nodule regions on elastographic color mapping, which resulted in differently sized ROIs according to the nodules. Kim et al. [14] studied the role of SWE when combined with conventional US. They used several parameters of SWE, including the mean elasticity index of the stiffest portion of mass or surrounding tissue, the maximum elasticity index of the stiffest portion of mass or surrounding tissue, the minimum elasticity index of the stiffest portion of mass or surrounding tissue, the ratio of the mean elasticity score of the lesion and parenchyma, and the ratio of the mean elasticity score of the lesion and the strap muscle. Among them, the maximum elasticity index of the stiffest portion of mass or surrounding tissue had the highest areas under the ROC curves. Although the researchers concluded that the combination of quantitative SWE and conventional US had higher specificity than conventional US alone for predicting malignancy, the areas under the ROC curves were higher in conventional US alone than in the combination of quantitative SWE and conventional US [14]. The most recent report of SWE was presented by Szczepanek-Parulska et al. [22]. They evaluated the diagnostic powers of each US feature of conventional US and SWE both qualitatively and quantitatively in the case of 393 thyroid nodules. However, in their study, they did not definitely document their approach to finding the elasticity index. The best cutoff elasticity index providing the highest odds ratio was 50 kPa, and a qualitative evaluation of SWE was not inferior to a quantitative evaluation of thyroid lesions.
Thus far, the abovementioned results are considered controversial despite many reports on the diagnostic utility of thyroid elastography (Table 1) [14,19,21,23-28]. Several investigators have compared elastography with each suspicious US feature and not with a combination of suspicious US features on conventional US [6,29]. Considering that the final assessment of a thyroid nodule is usually made on the basis of variable US features, the comparison of each suspicious US feature to elastography can only provide limited information for physicians [1,2]. For elastography to be an excellent adjunctive tool to conventional US, the combination of conventional US and elastography should have higher accuracy and sensitivity than conventional US alone. Most reports have stated that a combination of conventional US and elastography showed higher sensitivity than conventional US alone. In contrast, the diagnostic accuracy, specificity, and positive predictive value were inferior to those of conventional US alone. Therefore, the clinical application of elastography should be decided by how much experience the operator has with both conventional US and elastography for the thyroid.
US-guided fine-needle aspiration (US-FNA) has been widely used to diagnose thyroid nodules with excellent performance and less invasiveness. It has been considered the standard diagnostic tool to diagnose a thyroid nodule [30,31]. However, US-FNA has major limitations, such as “indeterminate” or “non-diagnostic” cytology, which can occur for up to 30% of all aspirated thyroid nodules [32,33]. To overcome these limitations, there have been several methods such as molecular markers and intraoperative frozen section [34-36]. The former need additional costs and the latter can be performed intraoperatively, not preoperatively.
Some investigators have attempted to evaluate the role of thyroid elastography in thyroid nodules with “indeterminate” or “nondiagnostic” cytology. The first study was performed in 32 patients with thyroid nodules with “indeterminate” cytology by Rago et al. [6]; it showed better diagnostic performance for each suspicious US feature. The two subsequent studies demonstrated the usefulness of thyroid elastography to predict thyroid cancer using qualitative and quantitative methods [37,38]. However, Lippolis et al. [39]’s study showed the opposite result; the study considered 102 thyroid nodules with “indeterminate” cytology and used qualitative elastography. The above studies evaluated the diagnostic utility of conventional US in a thyroid nodule with “indeterminate” cytology using each US feature, not a combination of US features. Although there were two studies concerning the value of thyroid elastography in thyroid nodules with “non-diagnostic” cytology [27,38], conventional US also showed good diagnostic results in thyroid nodules with “non-diagnostic” cytology even without additional time and cost [33]. Therefore, further validation is needed in the field.
To overcome the subjectivity of palpation on physical examination, elastography has been developed to objectively evaluate tissue firmness or hardness. However, elastography can be affected by an operator’s degree of compression and experience. Park et al. [5] were the first to report on the interobserver agreement of strain elastography of thyroid cancers. They independently examined thyroid cancers by three operators, using conventional US and strain elastography. The agreement was very poor in the case of strain elastography, in contrast to a better interobserver agreement in the case of conventional US. Park et al. [5] speculated that different external compressions by the probe and carotid artery pulsation influenced the poor agreement. In their study, the elastographic machine did not have any objective parameters to show the compressive force generated by the probe. Since then, there have been several reports on the reproducibility of elastography (Table 2) [5,11,15,20,21,40-43]. Most of them show substantial or almost perfect agreement. The remarkable improvement in interobserver agreement from the study by Park et al. [5] can be explained as follows: First, strain elastographic machines have the ability to monitor compressive force using real-time monitors, thereby reducing the over- or under-compression by different operators that previously influenced elastographic scoring [40]. Second, SWE has different physics from strain elastography, resulting in reproducible results [13,14].
Several factors can affect the results of elastography, including nodule characteristics (calcifications and cystic components), the experience of the operator, and motion artifacts such as carotid artery pulsation [40,44]. To overcome these limitations, elastography can be selectively used in thyroid nodules without calcifications and cystic changes, and should be performed by experienced operators using objective parameters provided by elastographic machines.
With respect to SWE, there have been no definite cut-off values given for the elasticity index even when the same machine was used, and no method has yet been standardized for the measurement of the thyroid lesion areas [13,14,20-22]. Further studies are needed to provide a practical guideline for the evaluation of thyroid nodules with SWE.
While elastography is a promising technique in some organs such as the breast [45,46] and the liver [47-50], there have been conflicting results of its additional value in predicting thyroid malignancy [14,19-26]. To be a good adjunctive diagnostic tool to conventional US, additional elastography should improve the diagnostic performances of conventional US, rationalizing the consequently longer US time and the additional cost of elastographic software that comes with its use. The value of elastography may be limited in institutions that show high diagnostic performances of conventional US by highly dedicated physicians. However, further studies should continuously validate the utility of elastography, particularly in selected cases.
AcknowledgementsThe authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations.
Reference1. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 2002;178:687–691.
2. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011;12:1–14.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–1214.
4. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract 2010;16 Suppl 1:1–43.
5. Park SH, Kim SJ, Kim EK, Kim MJ, Son EJ, Kwak JY. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. AJR Am J Roentgenol 2009;193:W416–W423.
6. Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab 2007;92:2917–2922.
7. Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid 2008;18:523–531.
8. Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med 2013;34:238–253.
9. Cantisani V, Lodise P, Grazhdani H, Mancuso E, Maggini E, Di Rocco G, et al. Ultrasound elastography in the evaluation of thyroid pathology. Current status. Eur J Radiol 2013. Jun 11 [Epub]. http://dx.doi.org/10.1016/j.ejrad.2013.05.008.
10. Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006;239:341–350.
11. Cantisani V, Grazhdani H, Ricci P, Mortele K, Di Segni M, D'Andrea V, et al. Q-elastosonography of solid thyroid nodules: assessment of diagnostic efficacy and interobserver variability in a large patient cohort. Eur Radiol 2014;24:143–150.
12. Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 2013;34:169–184.
13. Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab 2010;95:5281–5258.
14. Kim H, Kim JA, Son EJ, Youk JH. Quantitative assessment of shearwave ultrasound elastography in thyroid nodules: diagnostic performance for predicting malignancy. Eur Radiol 2013;23:2532–2537.
15. Zhang YF, Xu HX, He Y, Liu C, Guo LH, Liu LN, et al. Virtual touch tissue quantification of acoustic radiation force impulse: a new ultrasound elastic imaging in the diagnosis of thyroid nodules. PLoS One 2012;7:e49094.
16. Bhatia KS, Tong CS, Cho CC, Yuen EH, Lee YY, Ahuja AT. Shear wave elastography of thyroid nodules in routine clinical practice: preliminary observations and utility for detecting malignancy. Eur Radiol 2012;22:2397–2406.
17. Bojunga J, Dauth N, Berner C, Meyer G, Holzer K, Voelkl L, et al. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PLoS One 2012;7:e42735.
18. Ueno E, Itoh A. Diagnosis of breast cancer by elasticity imaging. Eizo Joho Med 2004;36(12):2–6.
19. Moon HJ, Sung JM, Kim EK, Yoon JH, Youk JH, Kwak JY. Diagnostic performance of gray-scale US and elastography in solid thyroid nodules. Radiology 2012;262:1002–1013.
20. Bhatia K, Tong CS, Cho CC, Yuen EH, Lee J, Ahuja AT. Reliability of shear wave ultrasound elastography for neck lesions identified in routine clinical practice. Ultraschall Med 2012;33:463–468.
21. Veyrieres JB, Albarel F, Lombard JV, Berbis J, Sebag F, Oliver C, et al. A threshold value in Shear Wave elastography to rule out malignant thyroid nodules: a reality? Eur J Radiol 2012;81:3965–3972.
22. Szczepanek-Parulska E, Wolinski K, Stangierski A, Gurgul E, Biczysko M, Majewski P, et al. Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy. PLoS One 2013;8:e81532.
23. Shweel M, Mansour E. Diagnostic performance of combined elastosonography scoring and high-resolution ultrasonography for the differentiation of benign and malignant thyroid nodules. Eur J Radiol 2013;82:995–1001.
24. Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu-Perrard M, Leenhardt L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol 2013;168:649–655.
25. Trimboli P, Guglielmi R, Monti S, Misischi I, Graziano F, Nasrollah N, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab 2012;97:4524–4530.
26. Ragazzoni F, Deandrea M, Mormile A, Ramunni MJ, Garino F, Magliona G, et al. High diagnostic accuracy and interobserver reliability of real-time elastography in the evaluation of thyroid nodules. Ultrasound Med Biol 2012;38:1154–1162.
27. Cappelli C, Pirola I, Gandossi E, Agosti B, Cimino E, Casella C, et al. Real-time elastography: a useful tool for predicting malignancy in thyroid nodules with nondiagnostic cytologic findings. J Ultrasound Med 2012;31:1777–1782.
28. Unluturk U, Erdogan MF, Demir O, Gullu S, Baskal N. Ultrasound elastography is not superior to grayscale ultrasound in predicting malignancy in thyroid nodules. Thyroid 2012;22:1031–1038.
29. Razavi SA, Hadduck TA, Sadigh G, Dwamena BA. Comparative effectiveness of elastographic and B-mode ultrasound criteria for diagnostic discrimination of thyroid nodules: a meta-analysis. AJR Am J Roentgenol 2013;200:1317–1326.
30. Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fineneedle aspiration biopsy of thyroid nodules. Thyroid 1998;8:15–21.
31. Kim SJ, Kim EK, Park CS, Chung WY, Oh KK, Yoo HS. Ultrasoundguided fine-needle aspiration biopsy in nonpalpable thyroid nodules: is it useful in infracentimetric nodules? Yonsei Med J 2003;44:635–640.
32. Yoon JH, Kwak JY, Kim EK, Moon HJ, Kim MJ, Kim JY, et al. How to approach thyroid nodules with indeterminate cytology. Ann Surg Oncol 2010;17:2147–2155.
33. Yoon JH, Moon HJ, Kim EK, Kwak JY. Inadequate cytology in thyroid nodules: should we repeat aspiration or follow-up? Ann Surg Oncol 2011;18:1282–1289.
34. Park SJ, Sun JY, Hong K, Kwak JY, Kim EK, Chung WY, et al. Application of BRAF, NRAS, KRAS mutations as markers for the detection of papillary thyroid cancer from FNAB specimens by pyrosequencing analysis. Clin Chem Lab Med 2013;51:1673–1680.
35. Kwak JY, Kim EK, Kim JK, Han JH, Hong SW, Park TS, et al. Dual priming oligonucleotide-based multiplex PCR analysis for detection of BRAFV600E mutation in FNAB samples of thyroid nodules in BRAFV600E mutation-prevalent area. Head Neck 2010;32:490–498.
36. Caraci P, Aversa S, Mussa A, Pancani G, Ondolo C, Conticello S. Role of fine-needle aspiration biopsy and frozen-section evaluation in the surgical management of thyroid nodules. Br J Surg 2002;89:797–801.
37. Cantisani V, Ulisse S, Guaitoli E, De Vito C, Caruso R, Mocini R, et al. Q-elastography in the presurgical diagnosis of thyroid nodules with indeterminate cytology. PLoS One 2012;7:e50725.
38. Rago T, Scutari M, Santini F, Loiacono V, Piaggi P, Di Coscio G, et al. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab 2010;95:5274–5280.
39. Lippolis PV, Tognini S, Materazzi G, Polini A, Mancini R, Ambrosini CE, et al. Is elastography actually useful in the presurgical selection of thyroid nodules with indeterminate cytology? J Clin Endocrinol Metab 2011;96:E1826–E1830.
40. Kim JK, Baek JH, Lee JH, Kim JL, Ha EJ, Kim TY, et al. Ultrasound elastography for thyroid nodules: a reliable study? Ultrasound Med Biol 2012;38:1508–1513.
41. Calvete AC, Rodriguez JM, Rios A, Abellan-Rivero D, Reus M. Interobserver agreement for thyroid elastography: value of the quality factor. J Ultrasound Med 2013;32:495–504.
42. Merino S, Arrazola J, Cardenas A, Mendoza M, De Miguel P, Fernandez C, et al. Utility and interobserver agreement of ultrasound elastography in the detection of malignant thyroid nodules in clinical care. AJNR Am J Neuroradiol 2011;32:2142–2148.
43. Lim DJ, Luo S, Kim MH, Ko SH, Kim Y. Interobserver agreement and intraobserver reproducibility in thyroid ultrasound elastography. AJR Am J Roentgenol 2012;198:896–901.
44. Bhatia KS, Rasalkar DP, Lee YP, Wong KT, King AD, Yuen HY, et al. Cystic change in thyroid nodules: a confounding factor for realtime qualitative thyroid ultrasound elastography. Clin Radiol 2011;66:799–807.
45. Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, et al. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography 2014;33:3–10.
46. Youk JH, Son EJ, Park AY, Kim JA. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography 2014;33:34–39.
47. Chang JM, Won JK, Lee KB, Park IA, Yi A, Moon WK. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. AJR Am J Roentgenol 2013;201:W347–W356.
48. Kim JE, Lee JY, Bae KS, Han JK, Choi BI. Acoustic radiation force impulse elastography for focal hepatic tumors: usefulness for differentiating hemangiomas from malignant tumors. Korean J Radiol 2013;14:743–753.
Principle of ultrasound elastography.A. Strain elastography evaluates elasticity through tissue displacement caused by compression, with the degree of displacement being larger in soft tissue than in hard tissue. B. Shear wave elastography evaluates elasticity through the propagation speed of transverse-oriented shear waves, with the wave speed being faster in hard tissue than in soft tissue.
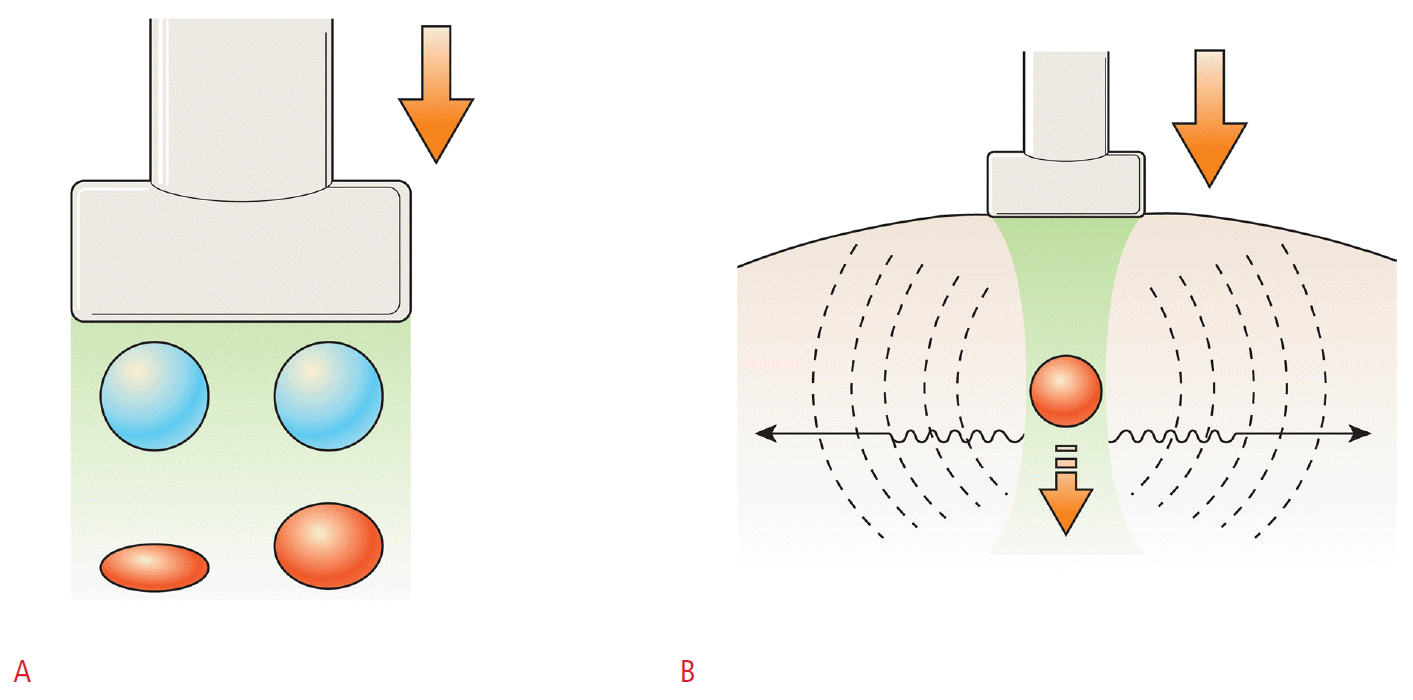 Fig. 1.Qualitative assessment of strain elastography.A. Strain elastographic scores by Rago et al. [6]. A score of 1 indicated even elasticity in the whole nodule. A score of 2 indicated elasticity in a large part of the nodule. A score of 3 indicated elasticity only at the peripheral part of the nodule. A score of 4 indicated no elasticity in the nodule. A score of 5 indicated no elasticity in the nodule or in the area showing posterior shadowing. B. Strain elastographic scores by Asteria et al. [7]. A score of 1 indicated elasticity in the entire examined area. A score of 2 indicated elasticity in a large part of the examined area. A score of 3 indicated stiffness in a large part of the examined area. A score of 4 indicated a nodule without elasticity.
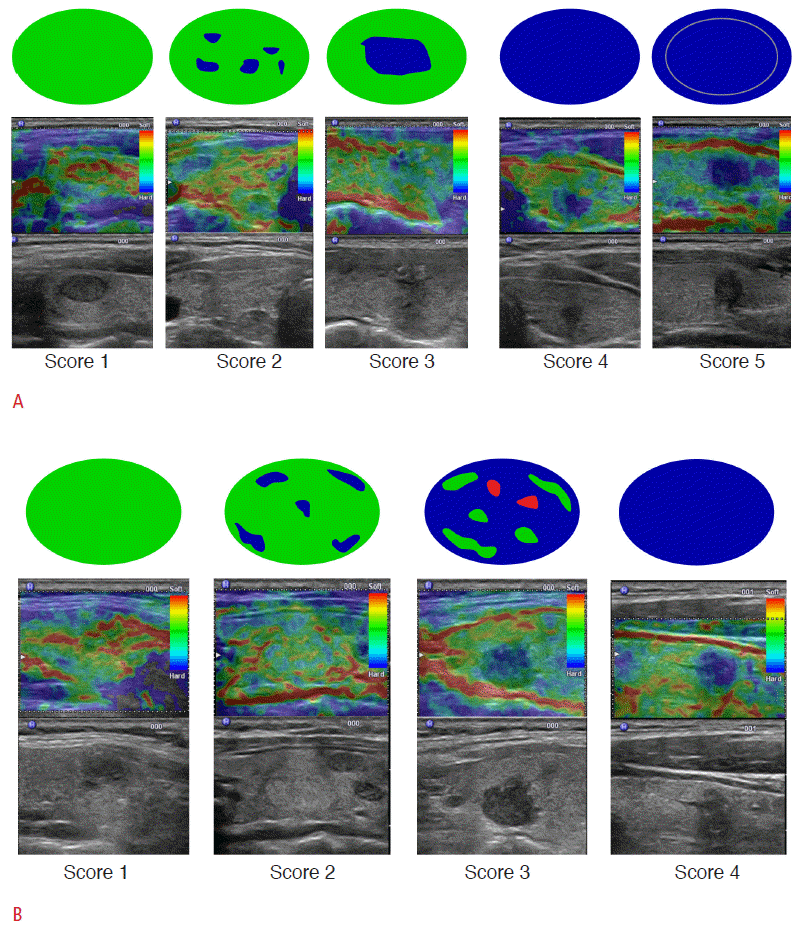 Fig. 2.Quantitative assessment of shear wave elastography.Elasticity of the nodule is expressed in the unit of kilopascals (kPa) in the right Q-Box.
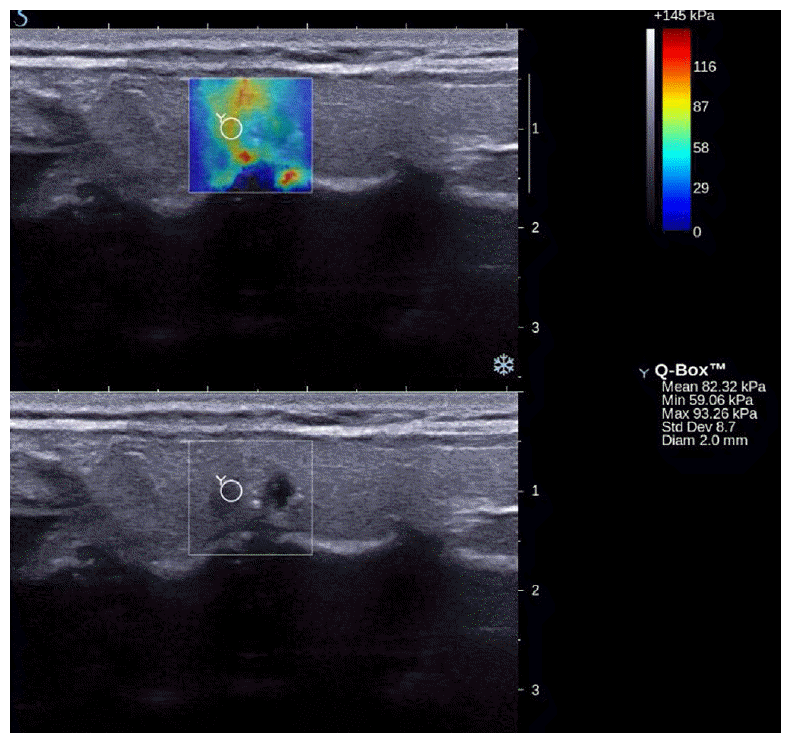 Fig. 3.Table 1Diagnostic performance of conventional US and a combination of conventional US and elastography for diagnosing thyroid malignancy according to the type of elastography
Table 2Interobserver variability of elastography at a thyroid nodule according to the type of elastography
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




