AbstractPurpose:To measure shear wave velocities (SWVs) by acoustic radiation force impulse (ARFI) ultrasound elastography in normal kidneys and in hydronephrotic kidneys in young children and to compare SWVs between the hydronephrosis grades.
Methods:This study was approved by an institutional review board, and informed consent was obtained from the parents of all the children included. Children under the age of 24 months were prospectively enrolled. Hydronephrosis grade was evaluated on ultrasonography, and three valid ARFI measurements were attempted using a high-frequency transducer for both kidneys. Hydronephrosis was graded from 0 to 4, and high-grade hydronephrosis was defined as grades 3 and 4.
Results:Fifty-one children underwent ARFI measurements, and three valid measurements for both kidneys were obtained in 96% (49/51) of the patients. Nineteen children (38.8%) had no hydronephrosis. Twenty-three children (46.9%) had unilateral hydronephrosis, and seven children (14.3%) had bilateral hydronephrosis. Seven children had ureteropelvic junction obstruction (UPJO). Median SWVs in kidneys with high-grade hydronephrosis (2.02 m/sec) were higher than those in normal kidneys (1.75 m/sec; P=0.027). However, the presence of UPJO did not influence the median SWVs in hydronephrotic kidneys (P=0.362).
Numerous studies have been conducted using acoustic radiation force impulse (ARFI) velocity on ultrasound elastography to measure the stiffness of tissue [1-4]. However, to the best of our knowledge, there are few studies that have focused on ARFI measurements in pediatric kidneys, primarily due to the absence of ultrasonographic transducer for young children [1,5]. We believe that the newly developed 4-9-MHz high-frequency linear transducer with ARFI may offer a means of measuring shear wave velocities (SWVs) in subjects with a relatively small body size, such as very young children.
Intra-abdominal organs of pediatric patients are often different from organs of adults in terms of size, physiology, and stiffness. Organs growin size and their physiology changes spontaneously as pediatric patients undergo growth and development. It can be assumed that SWVs in pediatric organs are different from SWVs in adult organs, and the measurement of SWVs in pediatric kidneys has not been widely performed [1,6-8]. Establishing standard SWVs for normal kidneys in pediatric patients may help to distinguish pathologic kidneys from normal kidneys, if there is a stiffness change.
Hydronephrosis is a common pathology in pediatric kidneys. It involves the distension and dilation of the renal pelvis and calyces, usually caused by the obstruction of the free flow of urine from the kidney [9]. In obstructive hydronephrotic kidneys, interstitial fibrosis eventually develops and leads to loss of nephrons and ultimately to impaired renal function [10]. In high-grade obstructive hydronephrosis, increased pelvic pressure can cause renal parenchymal stiffness, which in turn can cause renal interstitial fibrosis. ARFI can measure the stiffness of the tissue, and we can make an assumption that the “stiff” kidney of a hydronephrotic patient will show a high ARFI value. The purpose of this study was to evaluate SWVs in young children with normal kidneys and with hydronephrosis, and to determine whether there is a difference between SWVs of each degree of hydronephrosis with or without obstruction.
This study prospectively enrolled pediatric patients under the age of 24 months who underwent abdominal ultrasonography and ARFI measurements from July to August 2011. The patients enrolled in this study either were referred to evaluate hydronephrosis or were healthy children. This study was approved by the institutional review board of our center, and informed consent was obtained from the parents of all children. Age, sex, height, and weight were recorded for every patient, and the body mass index was calculated from the height and the weight.
One pediatric radiologist with ten years of experience in pediatric ultrasonography performed all the evaluations. Abdominal ultrasonography and ARFI measurements were performed using a 4-9-MHz linear transducer (Acuson S2000, Siemens Medical Solutions, Mountain View, CA, USA). During abdominal ultrasonography, patients were excluded if they had other renal diseases besides hydronephrosis (such as cystic renal disease or parenchymal echogenicity change) or other pathologic conditions outside of the kidney but within the abdomen. If there were no pathological findings in the kidneys and other solid organs in the abdomen, then the subjects were included in the normal group. A patient with unilateral or bilateral hydronephrosis was included in the hydronephrosis group. If hydronephrosis was observed, the degree of hydronephrosis was evaluated on the basis of Onen's [11] hydronephrosis grading system. It was graded from 0 to 4 as follows: 0, no hydronephrosis; 1, dilatation of the renal pelvis alone; 2, additional calyceal dilatation; 3, dilatation plus <50% (mild-tomoderate) renal parenchymal loss; and 4, dilatation plus >50% (severe) renal parenchymal loss (cyst-like kidney with no visually significant renal parenchyma). High-grade hydronephrosis was defined as grades 3 and 4 in Onen’s grading system, which included parenchymal thinning.
The Virtual Touch Tissue Quantification software (Siemens Medical Solutions) was used to measure SWVs with a region of interest (ROI; size, 5 mm × 6 mm) in the bilateral renal parenchyma, including both the renal cortex and the medulla in the axial view (Fig. 1). Three valid measurements were obtained for each kidney at the same portion of the mid-pole, as parallel as possible to the radially arranged tubular system, while the subject breathed freely. The measurements were not completed if the child was unable to tolerate the study. We apply similar amounts of transducer pressure only when necessary to create a gray-scale image in order to avoid substantial preload. Results are expressed as meters per second (m/sec), and the mean of three SWVs were used for the statistical analysis.
We retrospectively determined the final diagnosis of each kidney on the basis of the follow-up imaging studies and medical records, including surgical findings during the two years before August 2013. We reviewed imaging studies such as follow-up ultrasonography (30 patients), Tc-99m mercaptoacetyltriglycine (Tc99mMAG3; 18 patients), dimercaptosuccinic acid scan (20 patients), and voiding cystourethrography (17 patients). Obstructive hydronephrosis was confirmed using Tc99mMAG3 with or without the operation findings. Vesicoureteral reflux (VUR) was evaluated on voiding cystourethrography, and the renal scar change was evaluated on the dimercaptosuccinic acid scan.
Statistical analyses were performed using SPSS ver.18.0 (SPSS Inc., Chicago, IL, USA). Fisher exact test was used to compare gender prevalence between the normal group and the hydronephrosis group. The Wilcoxon signed-rank test was used to compare the SWVs between the right and the left kidneys. Other continuous data were compared with the Mann-Whitney U test or Kruskal-Wallis test. Two-sided P-values of <0.05 were considered to be statistically significant. All data in the table are reported as median (range) values.
Fifty-two children underwent abdominal ultrasonography. Among these, one patient was excluded due to bilaterally increased renal cortical echogenicity. The remaining 51 pediatric subjects underwent ARFI measurements. Three valid ARFI measurements were performed for both kidneys in 49 patients; these measurements were not possible in two patients due to inability to tolerate the procedure. Only two valid SWVs were measured in the two patients. Finally, 49 patients were enrolled in this study. The overall success rate for obtaining three valid ARFI measurements was 96% (49/51).
There were 36 boys and 13 girls with a median age of 6 months (range, 0 to 23 months). Nineteen patients (eleven boys and eight girls; 38.8%) had no hydronephrosis bilaterally and were included in the normal group. There was no remarkable medical event during follow-up in any of these patients.
Twenty-three patients (18 boys and 5 girls; 46.9%) had unilateral hydronephrosis, and 7 patients (7 boys; 14.3%) had bilateral hydronephrosis. These 30 children were included in the hydronephrosis group. Age, sex, weight, height, and body mass index were not significantly different between the normal and the hydronephrosis groups (Table 1).
Among the 30 children who had hydronephrosis, 18 children with high-grade (grades 3 and 4) hydronephrosis underwent Tc99mMAG3, and 7children were diagnosed with ureteropelvic junction obstruction (UPJO). They underwent pyeloplasty with routine renal cortex biopsy in one year after ARFI measurements. On pathology, there was mild to severe interstitial fibrosis with or without an ischemic change of the medulla in all seven patients with UPJO.
Among the children in the hydronephrosis group, 16 children underwent voiding cystourethrography and 6 of them had VUR without obstructive lesions. VUR grades were grade 3 in three children, grade 4 in two children, and grade 5 in one child. Dimercaptosuccinic acid scan was performed in the six children with VUR, and three of them had renal parenchymal scar change.
Only follow-up ultrasonography was performed in five children with grade 1 hydronephrosis and one child with grade 2 hydronephrosis; it showed no remarkable change. Remarkable medial events such as urinary tract infection were not observed in these patients during follow-up.
The median SWVs measured in the kidneys of the normal group were 1.80 m/sec for the right and 1.83 m/sec for the left without any statistical difference (P=0.546). By comparing the normal kidneys in the normal group and the hydronephrosis group, we found that the median SWVs were not different in either the right (1.80 m/sec in the normal group vs. 1.74 m/sec in the hydronephrosis group; P=0.796) or the left (1.83 m/sec in the normal group vs. 1.73 m/sec in the hydronephrosis group; P=0.972) kidneys.
Considering both right and left kidneys together of both the normal group and the hydronephrosis group, we found that there were 61 normal kidneys and 37 hydronephrotic kidneys, including 7 obstructive hydronephrotic kidneys. Table 2 presents the comparison of SWVs between normal kidneys and hydronephrotic kidneys. Median SWVs were slightly higher in the hydronephrotic kidneys (1.94 m/sec) than in the normal kidneys (1.75 m/sec) without any statistical significance (P=0.079). However, the median SWVs measured in the high-grade (grades 3 and 4) hydronephrotic kidneys (2.02 m/sec) were higher than that in the normal kidneys (1.92 m/sec) (P=0.027). We also compared the median SWVs of the hydronephrotic kidneys with and without UPJO and found no significant differences (1.98 vs. 1.94 m/sec; P=0.590). The median SWVs of the six kidneys with VUR (2.15 m/sec; P=0.101) and those of the three kidneys with parenchymal scar change (1.68 m/sec; P=0.433) were not different from those of the normal kidneys.
Hydronephrosis is an obstructive or non-obstructive nephropathy that is a commonly identified disease during pediatric abdominal ultrasonography. Congenital obstructive nephropathy constitutes the single most important identifiable cause of renal impairment in infants and children [10-12]. In obstructive nephropathy, interstitial fibrosis eventually develops and leads to a loss of nephrons [10].
Numerous papers that focus on the molecular biological mechanisms associated with renal interstitial fibrosis due to obstructive nephropathy have been recently published [10,13,14]. However, there is limited radiological research on renal interstitial fibrosis in the case of hydronephrosis. This could be attributed to the difficulty of detection, evaluation, and quantification of interstitial fibrosis by radiological methods.
There are many studies that explored ARFI measurements as a means of evaluating tissue stiffness, including several studies on kidneys. Gallotti et al. [6], Eiler et al. [7], and Goertz et al. [8] measured the ARFI velocities of normal kidneys in healthy adults. Further, there have been several trials using ARFI in adult kidneys to evaluate renal masses, to assess renal allograft fibrosis, and to detect chronic kidney diseases [15-17]. However, there is a lack of studies involving ARFI measurements in young children. This could be attributed to the fact that the previously used low-frequency transducer is not effective in the case of such small patients. However, the availability of the 4-9-MHz high-frequency linear transducer makes it possible to measure SWVs in small subjects.
Recently, our group demonstrated normal values of SWVs using ARFI in pediatric abdominal organs including kidneys in 202 children with an average age of 8.1±4.7 years [1]. The mean SWVs were 2.19 m/sec for the right kidney and 2.33 m/sec for the left kidney in the above mentioned study. The previously reported mean SWVs in normal adult kidneys were 2.24-2.37 m/sec, with no significant difference between the right and the left kidney [6,8]. The median SWVs in normal kidneys in the present study were 1.75 m/sec without any difference between the right and the left ones. This value is relatively low as compared to that obtained in previous studies. However, this result is comparable with that of our previous study, which concluded that the mean ARFI SWV for the kidneys increased according to age in children less than 5 years of age [1]. In this study, we only included children under the age of 24 months.
Only one study has been performed on the evaluation of diseased kidneys in children. Bruno et al. [5] conducted a study of ARFI measurements in pediatric patients with vesicoureteral reflux. The study suggested that ARFI can provide reliable information about the severity of renal damage and maybe useful in the diagnostic workup in children with a chronic reflux renal disease. However, the patient age in the study ranged from 8 to 16 years. Therefore, our study is the first report evaluating ARFI for hydronephrotic kidneys in young children.
We aimed to correlate SWVs with the hydronephrosis grade. Even though there are hydronephrosis grading systems on ultrasonography [11,18,19], these could not definitely differentiate between obstructive and non-obstructive hydronephrosis. Further, these systems cannot suggest the grade of renal parenchymal fibrosis. If SWVs have a correlation with the renal parenchymal stiffness, its measurement would be helpful in evaluating the status of a patient’s kidney. Further, SWV can show a continuous spectrum of stiffness. On the other hand, the grading system has an ordinal scale that cannot show a continuous value. Therefore, elastography has a possibility of having an additional value to evaluate hydronephrosis. In our study, there was a significant difference in the median SWVs between normal kidneys (1.75 m/sec) and high-grade hydronephrotic kidneys (2.02 m/sec). This suggests that elasticity decreases and stiffness increases in high-grade hydronephrotic kidneys. However, ARFI measurements cannot differentiate the cause of stiffness change such as tissue fibrosis and edema. Further research with a large group of patients and pathologic correlation is needed.
We also compared SWVs for a hydronephrotic kidney with and without UPJO. Further, there were only seven patients proven to have UPJO during the study period. The mean ARFI velocities were 0.69-2.51 m/sec for hydronephrotic kidneys without UPJO and 1.54-2.72 m/sec for those with UPJO; there was no statistical difference. Kidneys with VUR and a parenchymal scar change also exhibited no remarkable difference in SWVs. This could be attributed to the small number of patients, variable interstitial fibrosis of the UPJO group, and heterogeneous parenchymal scar change in the refluxing kidneys. This needs further evaluation with a large number of patients.
This study has several limitations. Almost all previous studies performed in adults measured about 5-10 valid SWVs and used mean values. However, due to the characteristics of the pediatric patient group, only three valid SWVs were obtained in this study. Repetitive measurements over a long time while subjects hold their breath is not possible in many children, particularly young children. Although only three valid ARFI velocities were attempted, two children could not tolerate the examinations and the success rate was 96%. Moreover, subjects were allowed to breathe freely during measurements. Thiscan increase the variability of SWV. The development of a method to measure SWV without breathholding would lead to more reliable results. The second limitation is the representativeness of the ARFI value. To represent a global kidney, measurement should be performed on multiple sites of the kidney, such as the upper, mid-, and lower poles. However, if the upper and lower poles are to be imaged, it is necessary to use a similar angle of incidence in all patients relative to the tubular system to avoid anisotropy issues. It is conceivable that shear waves generated within the kidney move at different velocities depending on the angle of incidence [20]. We tried to measure SWVs at the same portion of the mid-pole from the axial view, as parallel to the tubular system as possible in order to reduce the angle effect. The variation of the depth of the ROI position should also be considered. We targeted renal parenchyma, including both the renal cortex and the medulla, from the axial view in each patient. Therefore, we might expect that the depth of the ROI position would be different between patients and could increase according to the body size. Further study is needed to evaluate the effect of the depth of the ROI position and the body size in children. The fourth limitation is that we considered the contralateral kidneys without hydronephrosis as normal in the hydronephrosis group. Even though we demonstrated no significant difference in SWVs between normal kidneys in the normal group and contralateral kidneys in the hydronephrosis group, there could have been a physiological change in the bilateral kidneys of the hydronephrosis group.
In conclusion, obtaining ARFI measurements of kidneys using a high-frequency transducer is feasible in very young pediatric patients. The median SWV of normal kidneys in children under the age of 24 months was 1.75 m/sec. These velocities increased in high-grade hydronephrotic kidneys but were not helpful in differentiating hydronephrotic kidneys with and without UPJO.
References1. Lee MJ, Kim MJ, Han KH, Yoon CS. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol 2013;82:e290–e294.
2. Hanquinet S, Rougemont AL, Courvoisier D, Rubbia-Brandt L, McLin V, Tempia M, et al. Acoustic radiation force impulse (ARFI) elastography for the noninvasive diagnosis of liver fibrosis in children. Pediatr Radiol 2013;43:545–551.
3. Hanquinet S, Courvoisier D, Kanavaki A, Dhouib A, Anooshiravani M. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol 2013;43:539–544.
4. Friedrich-Rust M, Schlueter N, Smaczny C, Eickmeier O, Rosewich M, Feifel K, et al. Non-invasive measurement of liver and pancreas fibrosis in patients with cystic fibrosis. J Cyst Fibros 2013;12:431–439.
5. Bruno C, Caliari G, Zaffanello M, Brugnara M, Zuffante M, Cecchetto M, et al. Acoustic radiation force impulse (ARFI) in the evaluation of the renal parenchymal stiffness in paediatric patients with vesicoureteral reflux: preliminary results. Eur Radiol 2013;23:3477–3484.
6. Gallotti A, D'Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med 2010;115:889–897.
7. Eiler J, Kleinholdermann U, Albers D, Dahms J, Hermann F, Behrens C, et al. Standard value of ultrasound elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents. Ultraschall Med 2012;33:474–479.
8. Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry: a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol 2011;80:e226–e230.
9. Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia, PA: Elsevier Saunders, 2005.
10. Chevalier RL, Thornhill BA, Forbes MS, Kiley SC. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol 2010;25:687–697.
11. Onen A. An alternative grading system to refine the criteria for severity of hydronephrosis and optimal treatment guidelines in neonates with primary UPJ-type hydronephrosis. J Pediatr Urol 2007;3:200–205.
13. Yao Y, Zhang J, Tan DQ, Chen XY, Ye DF, Peng JP, et al. Interferon-γ improves renal interstitial fibrosis and decreases intrarenal vascular resistance of hydronephrosis in an animal model. Urology 2011;77:761.e768-e713.
14. Yoo KH, Thornhill BA, Forbes MS, Chevalier RL. Inducible nitric oxide synthase modulates hydronephrosis following partial or complete unilateral ureteral obstruction in the neonatal mouse. Am J Physiol Renal Physiol 2010;298:F62–F71.
15. Clevert DA, Stock K, Klein B, Slotta-Huspenina J, Prantl L, Heemann U, et al. Evaluation of Acoustic Radiation Force Impulse (ARFI) imaging and contrast-enhanced ultrasound in renal tumors of unknown etiology in comparison to histological findings. Clin Hemorheol Microcirc 2009;43:95–107.
16. Stock KF, Klein BS, Vo Cong MT, Sarkar O, Romisch M, Regenbogen C, et al. ARFI-based tissue elasticity quantification in comparison to histology for the diagnosis of renal transplant fibrosis. Clin Hemorheol Microcirc 2010;46:139–148.
17. Syversveen T, Brabrand K, Midtvedt K, Strøm EH, Hartmann A, Jakobsen JA, et al. Assessment of renal allograft fibrosis by acoustic radiation force impulse quantification: a pilot study. Transpl Int 2011;24:100–105.
18. Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol 1993;23:478–480.
19. Riccabona M, Avni FE, Blickman JG, Dacher JN, Darge K, Lobo ML, et al. Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol 2008;38:138–145.
Shear wave velocity measurements in a two-month-old girl with hydronephrosis.Figures show acoustic radiation force impulse measurements with a 4-9-MHz linear transducer using a 5 mm × 6 mm region of interest including both the renal cortex and the medulla with a normal right kidney in the axial view (A) and a hydronephrotic left kidney in the axial view (B). Note that the region of interest includes a calyx in the hydronephrotic kidney, which can cause a partial volume effect.
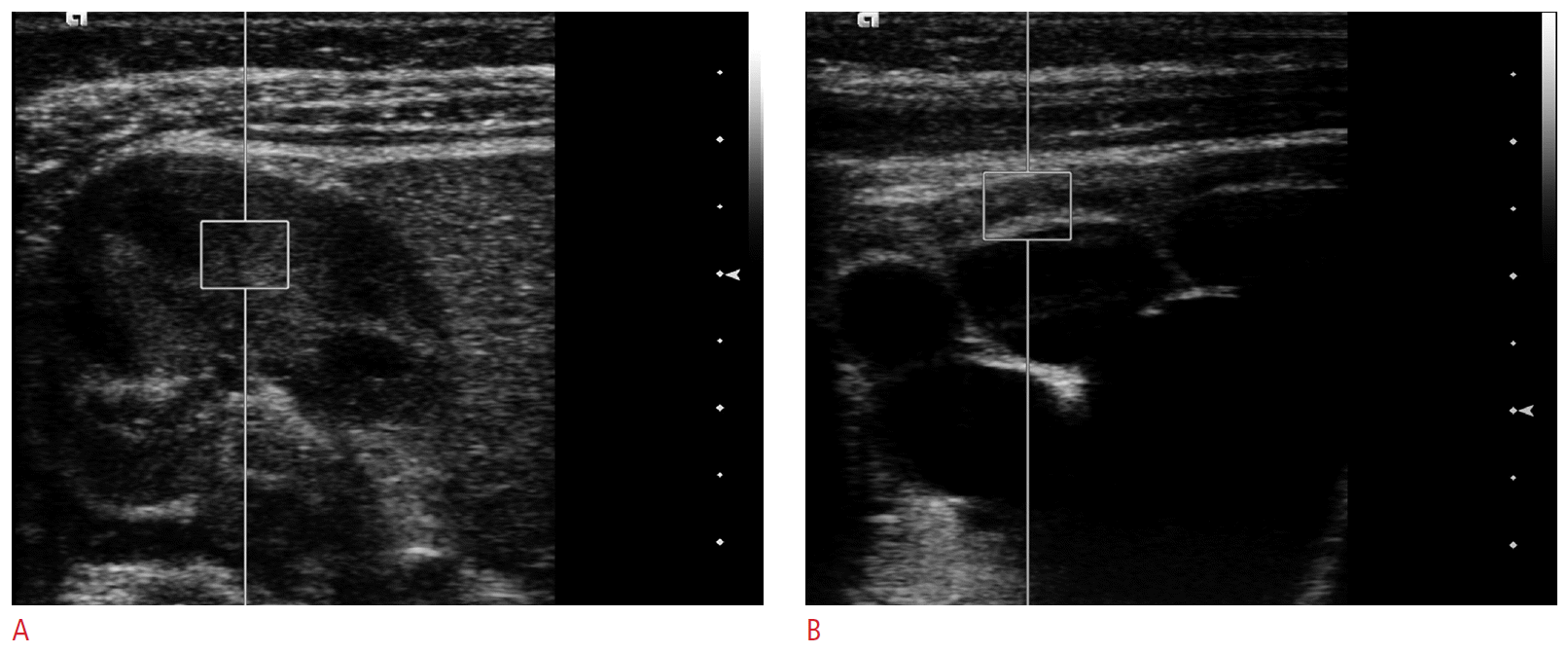 Fig. 1.Table 1.Sex, age, height, weight, and BMI in the normal and hydronephrosis groups
Table 2.Comparison of ARFI velocities (m/sec) for normal kidneys and hydronephrotic kidneys
|
|||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




