Introduction
Chronic liver injury of various etiologies may cause the excessive extracellular matrix accumulation, resulting in liver fibrosis [1]. It has generally been accepted that the development of liver cirrhosis, the advanced stage of liver fibrosis, is predictive of increased risks for various complications, as well as liver-associated morbidity and mortality [2]. Moreover, it has been suggested that these risks begin to increase in the very early stages of liver fibrosis [3]. The prognosis of chronic liver diseases, including chronic hepatitis B (CHB) infections, may strongly depend on the degree of liver fibrosis.
CHB is a major causative factor of liver disease globally, affecting approximately 240 million patients and causing 600,000 deaths annually [4]. However, CHB shows a variable spectrum of disease, ranging from inactive hepatitis to progressive hepatitis leading to liver cirrhosis in up to 20% of patients [5]. The management of CHB depends on the clinical phase of the disease. The severity of liver fibrosis and the extent of liver inflammation are essential factors guiding the treatment of the disease. Although liver biopsy has been the conventional means of evaluating liver inflammation and fibrosis, much progress has been made in the development of noninvasive methods. In this study, we examine transient elastography (TE) and other noninvasive methods for assessing liver fibrosis, and also discuss the usefulness of TE for follow-up in patients with CHB.
Stages of Chronic Hepatitis B
The progression of CHB may exhibit several clinical phases: the immune tolerant phase, the immune active phase, the immune control phase, the immune escape phase, and the hepatitis B surface antigen-clearance phase. Individual patients do not necessarily experience these clinical phases in a continuous manner, and these clinical phases are not always correlated with criteria or indications for antiviral therapy [6,7]. Hepatitis B virus (HBV) DNA positivity indicates ongoing HBV infection, and negativity indicates resolution of the infection. For this reason, the World Health Organization decided to discontinue usage of the term inactive carrier, replacing it with inactive CHB.
Immune Tolerant Phase
The immune tolerant phase is characterized by hepatitis B envelope antigen (HBeAg) positivity, high levels of serum HBV DNA (generally Ōēź107 IU/mL), normal levels of aspartate transaminase (AST) and alanine transaminase (ALT), and mild or no liver inflammation [8-11]. The phase is especially predominant in cases of perinatal infection. This phase may continue for more than three decades in patients infected with HBV genotype C, which is common among Korean patients, and the rate of spontaneous HBeAg loss is very low [12]. No or mild histologic liver damage is presence despite high levels of HBV DNA due to patientsŌĆÖ immune tolerance to HBV [13].
Immune Active HBeAg-Positive CHB
Most patients in the immune tolerant phase will experience immune responses to HBV as they grow older. The immune active phase is characterized by HBeAg, lower serum HBV DNA levels, and increased or fluctuating ALT levels [14,15]. Histologic findings in this phase include moderate to severe liver inflammation and, in some patients, rapid progression of fibrosis [16]. Such changes are due to the enhancement of hepatitis B core antigen or HBeAg-specific cytotoxic T-lymphocyte activity and the resulting destruction of infected hepatocytes [17]. Sustained HBV DNA suppression occasionally accompanies HBeAg seroconversion. However, in many cases, antiviral therapy is needed to suppress viral activity in patients with elevated AST and ALT levels.
Immune Escape HBeAg-Negative CHB
Approximately 20% of patients who experience HBeAg seroconversion during their immune active phase maintain HBeAg negativity and hepatitis B envelope antibody positivity, resulting in immune escape HBeAg-negative CHB. Immune escape HBeAg-negative CHB involves HBV DNA levels Ōēź2,000 IU/mL, increased ALT levels, and active liver inflammation [18]. These patients show HBeAg negativity since they harbor HBV variants in the precore or basal core promoter regions of the HBV DNA, resulting in failure to produce HBeAg [19-21]. HBeAg-negative CHB is associated with low rates of prolonged spontaneous disease remission, and often leads to persistent hepatocellular inflammation, resulting in hepatic fibrosis and cirrhosis [21-23]. Therefore, these patients need antiviral therapy to prevent the progression of hepatic inflammation and fibrosis.
Immune Control Inactive CHB
Inactive CHB is defined as having persistently normal ALT levels and undetectable levels of serum HBV DNA (Ōēż2,000 IU/mL). The typical histologic findings in this phase are minimal or no liver inflammation, theoretically indicating that no further liver injury can be expected [24].
Liver Biopsy: The Classical Means of Staging Liver Disease
Although serum biochemical markers such as AST and ALT are very commonly used in assessing the severity of liver inflammation, several recent clinical studies have found that 12%-43% of patients with persistently normal ALT levels had histologic evidence of significant fibrosis or inflammation in a liver biopsy [25-31]. Another retrospective study of the relationship between ALT levels and fibrosis in CHB patients reported similar results: of the 59 patients with persistently normal ALT levels, 18% had stage 2 fibrosis and 34% had grade 2 or 3 inflammation, with 37% of all patients with persistent normal ALT levels having significant fibrosis (stage 2-4) and inflammation [32]. Liver biopsy may not be required in patients with clinical evidence including indicators for the initiation of antiviral therapy irrespective of the grade of inflammation activity or the stage of fibrosis. However, in some cases, it may be necessary to assess the severity of liver disease using means other than biochemical markers.
Liver biopsy has been considered the gold standard method for evaluating the degree of necroinflammation and fibrosis in patients with CHB. It provides information crucial for determining the management of HBV patients, including whether antiviral therapy should be commenced. Treatment should be considered if a liver biopsy reveals fibrosis at stage 2 or greater and/or necroinflammation. Although liver biopsy is an invasive procedure, the rate of serious complications is very low, ranging from 1/4,000 to 1/10,000 [33]. However, the diagnostic accuracy of liver biopsies is often limited by sampling variability, since the average length of the biopsy core is 15 mm, which represents approximately 1/50,000 of the size of the entire liver [34]. In addition, it has been reported that significant interobserver variability may take place in histologic assessments, even among very specialized pathologists [33].
Transient Elastography
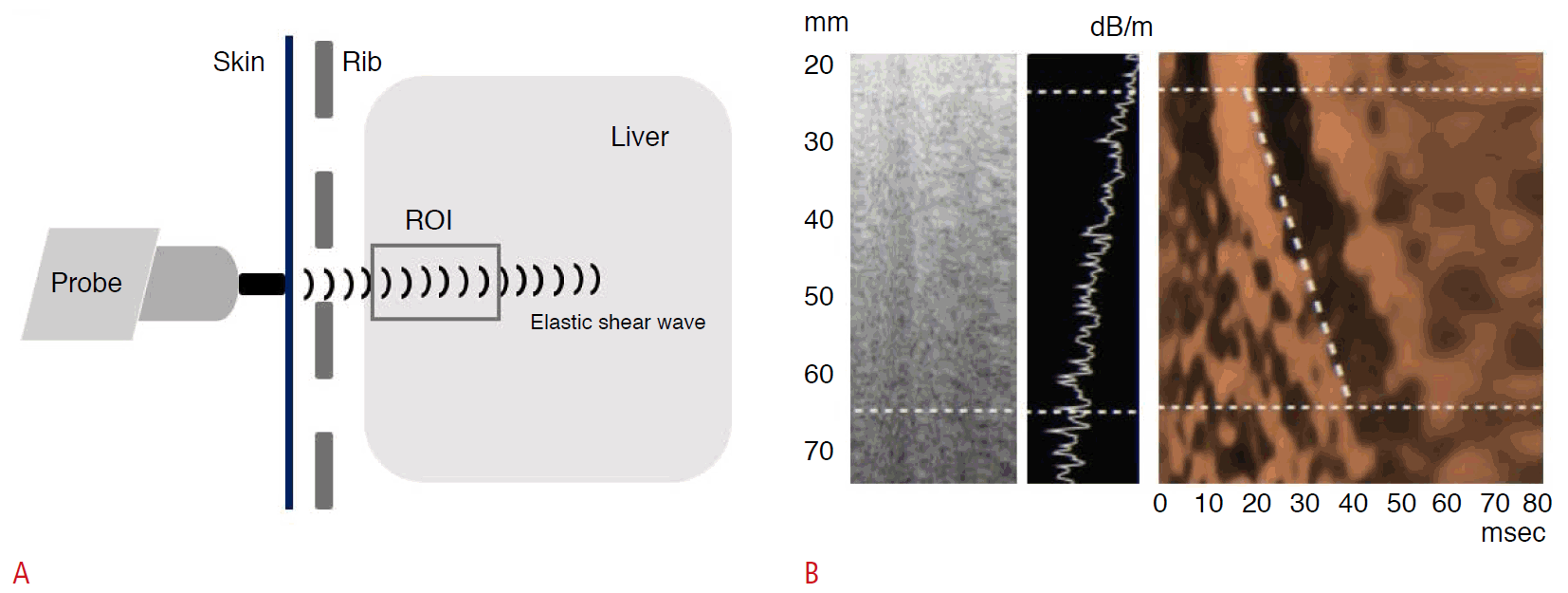
TE using FibroScan (EchoSens, Paris, France) is a representative and noninvasive method of assessing liver fibrosis with the advantages of acceptable accuracy and reproducibility. It is performed with an ultrasound transducer probe that produces vibrations of mild amplitude and low frequency. This induces an elastic shear wave that propagates through the liver tissue. The velocity of the shear wave is directly related to liver tissue stiffness (LS); the harder the tissue is, the faster the shear wave propagates (Fig. 1) [35]. The outcome is expressed as a pressure in kilopascals (kPa). The examination is painless, rapid, and easy to perform even in outpatient settings [36]. TE has been demonstrated to be a highly reproducible technique in terms of interobserver and intraobserver agreement, with an intraclass correlation coefficient of 0.98 [37]. It has been suggested by the manufacturer and confirmed in many studies that successful measurements should be validated using the following three criteria: (1) at least 10 valid shots, (2) a success rate (ratio of valid shots to the total number of shots) of at least 60%, and (3) an interquartile range (reflecting the variability of measurements) of less than 30% of the median LS measurement value. LS measurement using TE is considered a failure when no value is obtained after 10 shots of measurement or more [38].
Supported by several validations in cross-sectional studies, TE is regarded as a reliable means of evaluating liver fibrosis [39-41]. More recent longitudinal studies assessing changes in liver fibrosis over time or before and after antiviral therapy have indicated that TE can be used to differentiate inactive CHB from active CHB with improved accuracy [42-44].
TE in Inactive CHB
Inactive CHB is defined as undetectable serum HBV DNA (Ōēż2,000 IU/mL) with persistently normal ALT levels, indicating that no liver inflammation persists in this setting [47]. However, distinguishing true inactive CHB from active CHB with relatively normal ALT levels is not always straightforward. A study reported that 37% of all patients with persistently normal ALT levels had significant fibrosis and inflammation [32]. TE may be helpful in identifying inactive CHB patients with relatively normal livers. Several studies reported that inactive CHB patients with preserved liver parenchyma showed LS values comparable with those of healthy subjects. In a prospective study by Oliveri et al. [48], the mean LS value of inactive CHB patients was 4.3┬▒1.0 kPa, whereas that of the healthy subjects was 4.6┬▒1.2 kPa. Maimone et al. [49] performed TE on 125 inactive CHB patients and obtained a mean LS value of 4.8┬▒1.2 kPa, which was significantly lower than that of active CHB patients.
A recent longitudinal study reported liver fibrosis progression, detected by increased LS, in only 2.9% of inactive CHB patients, supporting the definition of inactive CHB [43].
TE in Active CHB and Liver Cirrhosis
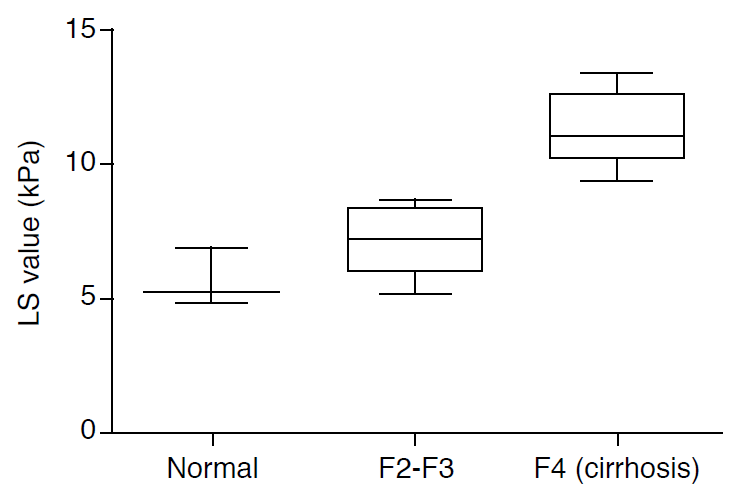
The extent of fibrosis is important, especially in active CHB, given the presence of ambiguous biochemical markers, including mildly elevated ALT levels, which are used to make therapeutic decisions. Several studies have evaluated the diagnostic accuracy of TE in predicting the fibrosis stage in CHB patients. Studies have suggested LS cut-offs ranging from 5.2 to 8.7 kPa in diagnosing significant fibrosis (Ōēźgrade 2) (Fig. 2) [48,50-55]. The sensitivity estimates ranged from 70% to 93%, and the specificity estimates ranged from 38% to 92%. For liver cirrhosis (fibrosis grade 4) without significant complications involving portal hypertension, the proposed LS cutoff values varied from 10.3 to 13.4 kPa, with sensitivity estimates ranging from 59% to 100% and specificity estimates ranging from 79% to 94% [48,50,51,53,55].
Although the definition of LS cut-off values that reliably classify patients in a given fibrosis stage is crucial in order to use this technique in practice, a single definition with consistent diagnostic accuracy has not been proposed. Usually, the cut-off value is derived from the receiver operating characteristic curves, and the value optimizing both sensitivity and specificity is chosen. Vigano et al. [53] validated the positive and negative prediction of significant fibrosis and cirrhosis in treatment-na├»ve patients with CHB. Patients were examined by percutaneous liver biopsy, and the LS values of >13.1 kPa and Ōēż9.4 kPa as positive and negative cut-offs for cirrhosis had >90% sensitivity and specificity, with an overall predictive power of 94%, independent of ALT values [53].
The LS cut-off may vary according to the etiology of the underlying disease. While most studies have investigated patients with chronic hepatitis C (CHC), the few studies that have compared CHC and CHB patients have suggested that LS cut-offs tend to be lower in CHB patients than in CHC patients [54-56].
TE in Monitoring CHB Patients under Antiviral Therapy
It is beginning to be accepted that fibrosis regression can be achieved in CHB patients by efficiently suppressing the replication of HBV [57,58]. Since it is the major prognosis-determining factor in CHB, assessing longitudinal changes in liver fibrosis during antiviral therapy would be very important for establishing CHB treatment strategies. With the introduction of noninvasive means of fibrosis evaluation such as TE, several studies have investigated longitudinal changes in liver fibrosis before, during, and after antiviral therapy [42,59,60].
A study that followed patients treated with either entecavir or lamivudine for 3 years showed that LS values decreased significantly from baseline to 1, 2, and 3 years after treatment (medians, 12.9 kPa, 7.5 kPa, 6.5 kPa, and 4.7 kPa, respectively; all P<0.05) [59].
TE seems to be useful for monitoring changes in fibrosis in patients with CHB receiving antiviral therapy.
Usefulness of TE in CHB Patients with Liver Cirrhosis
For patients with CHB and liver cirrhosis, the major complications of concern are portal hypertension-related complications, such as esophageal varices, and hepatocellular carcinoma. Many studies have attempted to use TE to predict the probability of these complications. Based on a review of recent papers, higher LS values in TE may be associated with an increased incidence of these complications. Although some discrepancies are present among the papers, a grade can be assigned for the prediction of esophageal varices based on LS values in TE, and it is also possible to predict the extent of portal hypertension and the hepatic venous pressure gradient using LS values from TE (Table 1).
Therefore, it is thought that CHB patients showing high LS values require more careful follow-up, since they are at a higher risk of complications. Nevertheless, LS values are only for reference, and they should only be used in combination with the results of endoscopy and abdominal ultrasound.
Considerations in Interpreting TE
Although LS values from TE are reproducible and relatively reliable, the measured values can be influenced by several factors. Since LS is a physical parameter related to fibrosis, factors that might modify liver elasticity, such as variations in inflammatory infiltrates, parenchymal edema, vascular congestion, cholestasis, and hepatic steatosis can affect the LS values [61-64].
Liver inflammation in CHB patients during hepatitis flare-ups or viral reactivation is known to lead to elevated LS values. It has been demonstrated that the presence of elevated ALT levels can increase LS values [65].
It is difficult to obtain LS measurements in obese patients, especially those with a body mass index >28 kg/m2, and in patients with narrow intercostal spaces, ascites, extrahepatic cholestasis, or congestion [61,66].
Eating before TE may compromise the accuracy of the LS measurements. Theoretically, eating a meal can increase portal blood flow and the hepatic venous pressure gradient, thus increasing liver stiffness [67].
Other Noninvasive Instrumental Techniques
Magnetic Resonance Elastography
Magnetic resonance elastography (MRE) is a magnetic resonance imaging (MRI)-based technique that uses a modified phase-contrast method to image the propagative characteristics of a shear wave travelling through the liver [68]. A sound driver is placed on the right upper quadrant of the patient instead of a probe, and the measurements are obtained from the anterior segment of the right lobe. Low-frequency vibrations are emitted, and the MRI spin echo sequence is used to gather data. Mean liver stiffness is expressed in kPa.
MRE has been reported to show excellent accuracy in differentiating significant fibrosis from mild or no fibrosis. The optimally discriminating cutoff MRE value was 4.33 kPa for the diagnosis of cirrhosis in CHB patients, with a sensitivity of 100% and a specificity of 100% [69]. The positive predictive value was 91.3%, and the negative predictive value was 100%.
MRE scans cover practically the entire liver and have no limitations in evaluating obese patients or those with ascites. Liver inflammation and elevated ALT do not appear to be associated with MRE values [70]. However, MRE is an expensive and more time-consuming means of evaluation. In addition, patients with claustrophobia or heart pacemakers cannot be evaluated with MRE.
Acoustic Radiation Force Impulse Imaging
Acoustic radiation force impulse imaging (ARFI) is a sonography-based technique that is an alternative to TE. ARFI mechanically excites tissue for a brief period by delivering a high-intensity acoustic pulse to the region of interest (ROI) [71]. The ROI can be chosen by the operator, and patients with lesions that could interfere with TE measurements can be evaluated using ARFI. The shear-wave velocity (SWV) is recorded in meters per second (m/sec), and increases with stiffness. ARFI SWV diagnosis cutoff values for CHB patients have not been definitively established, although several studies have reported cutoff values for cirrhosis of 1.75-2.00 m/sec [72,73].
ARFI may be useful in patients with lesions that would interfere with LS measurements. However, not many validation studies have been performed and the range of the reported cut-off values is wide (0.5-4.4 m/sec), limiting the clinical usefulness of ARFI. It is also less efficient in obese patients [74].
Real-Time Elastography
Real-time elastography (RTE) uses a modified version of standard ultrasound equipment and assesses the extent of liver fibrosis. RTE propagates a shear wave through the liver and echo signals are captured in real time [82]. Not many studies have been performed of the usefulness of RTE in CHB patients. A study of 111 CHB patients suggested a liver fibrosis index of >3.25 and a % area of >28.83% for the diagnosis of cirrhosis, with 100% sensitivity and 85.9% specificity [83]. RTE can be used in patients with ascites or severe obesity. However, RTE requires further clinical validation in order to have practical value in evaluating CHB patients.
Conclusion
TE is a noninvasive means of evaluating liver fibrosis with high accuracy and reproducibility for the diagnosis of severe fibrosis and cirrhosis. This enables the identification of CHB patients who need immediate interventional management and helps establish practice guidelines.
TE can also effectively assess dynamic changes in CHB patients receiving antiviral therapy and provide useful means of monitoring and assessing the effects of antiviral therapy.
However, the proposed cut-off values for stages of fibrosis overlap for some stages. LS values can be influenced by the degree of liver inflammation, high ALT levels, and hepatic congestion. Furthermore, TE has limited applicability in obese patients or patients with ascites. Therefore, other noninvasive means of evaluating liver fibrosis complementary to TE are expected to be in use in the future.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




