AbstractPurposeThis study aimed to predict a heavy nodal burden (≥3 metastatic axillary lymph nodes [LNs]) using axillary ultrasonography (US) and US-guided fine-needle aspiration biopsy (FNAB) in patients with early-stage breast cancer.
MethodsWe retrospectively reviewed the medical records of 403 women (404 cancers) who underwent US-guided FNAB for axillary LN staging from January 2006 to December 2015. US findings and US-guided FNAB results were reviewed and compared using pathology results as the reference. Diagnostic performance was analyzed, and clinicopathological and radiological findings were compared between patients with <3 metastatic LNs and ≥3 metastatic LNs.
ResultsThe final pathology results revealed that 20.5% of cancers had heavy nodal metastases. US-guided FNAB showed significantly higher sensitivity (79.0% vs. 63.0%, P=0.009) and specificity (84.8% vs. 79.3%, P=0.036) in predicting heavy nodal metastases than did US. The presence of a larger number of suspicious LNs (two or more) on axillary US and positive FNAB results were significantly correlated with a heavy nodal burden in the multivariate analysis. The odds ratios were 4.20 (95% confidence interval [CI], 1.90 to 9.39) for two suspicious LNs, 9.40 (95% CI, 2.99 to 29.54) for three or more suspicious LNs, and 14.22 (95% CI, 6.78 to 29.82) for positive FNAB results.
Axillary lymph node (LN) metastasis is an important prognostic index of overall and disease-free survival in patients with breast cancer, and the number of metastatic LNs is a significant factor that helps oncologists decide whether to administer chemotherapy [1,2]. The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial suggested that patients with fewer than three positive sentinel LNs and a clinical T1-2 tumor undergoing lumpectomy and breast radiation therapy followed by systemic therapy did not benefit from axillary lymph node dissection (ALND) in terms of local control, disease-free survival, and overall survival [3]. According to this trial [3], only patients with heavy involvement (i.e., three or more nodes) were recommended to undergo ALND.
Axillary ultrasonography (US) and US-guided fine-needle aspiration biopsy (FNAB) are the standard diagnostic tools for axillary staging in patients with breast cancer [4-6]. After the ACOSOG Z0011 trial findings were reported [3], the usefulness of axillary US and US-guided FNAB in patients with early-stage breast cancer needed to be revisited. Several studies suggested that the combined use of axillary US and US-guided FNAB can accurately diagnose a heavy nodal burden (three or more metastatic LNs) and, therefore, may reduce the unnecessary use of sentinel lymph node biopsy (SLNB) in cases with proven metastasis [7,8]. However, some patients with fewer than three LN metastases may undergo ALND directly because of a positive FNAB result. Therefore, the necessity of axillary US and US-guided FNAB has been discussed, especially when only a few abnormal axillary LNs are present [9-11]. In addition, no reports have discussed the correlation between US findings and FNAB results for identifying early-stage breast cancer patients with a heavy nodal burden. Therefore, one purpose of this study was to investigate the accuracy of axillary US and US-guided FNAB in diagnosing axillary metastasis and predicting a heavy nodal burden in patients with early-stage breast cancer. Another purpose was to identify the clinicopathological factors and US findings affecting the prediction of a heavy nodal burden.
In this retrospective study, we reviewed the medical records of patients with breast cancer from our institution from January 2006 to December 2015. The institutional review board approved this study at Seoul National University Bundang Hospital in Korea (B-1504-296-106) and waived the requirement for informed patient consent.
Initially, we included 565 pathological T1 or T2 stage breast cancers, including one bilateral cancer, in 564 women who underwent preoperative US-guided FNAB for axillary LN and breast surgery during the study period. However, 161 cancers in 161 patients were excluded because of undisclosed SLNB or ALND (n=89) and administration of preoperative systemic therapy (n=72). We included elderly patients (older than 65 years) and patients with luminal A type cancers or other coexistent malignancies who did not undergo preoperative systemic therapy, although they had positive LN results in axillary FNAB. Finally, 404 cancers in 403 women were included in this study.
Patients with newly diagnosed breast cancer routinely underwent axillary US at our institution. Ultrasonographic assessment of axillary LNs was performed in real time by one of three radiologists (with 20, 14, and 7 years of experience in breast imaging, respectively) using high-resolution US equipment with 12-5 MHz, focused, linear-array transducers (HDI 5000 or IU 22, Philips Ultrasound, Bothell, WA, USA).
US-guided FNAB was performed by the same radiologist who performed the patient’s US examination. US-guided FNAB was considered when LNs showed a cortical thickening of 2.5 mm or greater, or abnormal morphology [12]. If multiple LNs showed suspicious features, the largest one was selected. US-guided FNAB was performed with a 21-gauge needle attached to a 10-mL syringe with moderate (1-3 mL) negative pressure. Numerous multi-directional passes were made through the thickened cortex to increase the probability of successful sampling. Specimens were smeared on two slides and fixed with 95% ethanol. Using a longitudinal freehand approach, FNAB was performed two or three times in all patients.
Two blinded radiologists (with 20 and 5 years of experience in breast US interpretation, respectively) retrospectively reviewed the US images and reached a consensus in all cases. Reviewers evaluated the number of suspicious LNs and the cortical thickness (mm), and reported the following suspicious features: shape of the fatty hilum (compression or loss), shape (oval or round), margin (circumscribed or non-circumscribed), and extranodal extension (none or present). Compression or loss of the hilum, a round shape, a non-circumscribed margin, and the presence of extranodal extension were regarded as abnormal findings. The definitions of individual findings were as follows; hilar compression: partial effacement of fatty hilum, hilar loss: complete effacement of fatty hilum, non-circumscribed margin: partially or completely indistinct margin, and extranodal extension: LN matting and perinodal edema.
The LN tissue obtained by ALND and SLNB was sectioned. After preparing five slides and staining them with hematoxylin and eosin, a pathologist with 15 years of experience in breast pathology examined them. We described the tumor and nodal stage according to the TNM staging system from the seventh edition of the American Joint Committee on Cancer staging manual [13]. A nodal stage of N0(i+) was considered a negative final pathology [13]. In the patient with bilateral cancer, surgery and histopathological review were performed separately for each side. Tumor molecular subtypes were divided into estrogen receptor (ER)/progesterone receptor (PR)-positive, human epidermal growth factor receptor 2 (HER2)-positive, or triple-negative subtypes [14], as described in the results and tables.
The clinical, radiological, and pathological data of the 404 cancers were collected for statistical analysis. The final surgical histopathologic findings of either ALND or SLNB were used as the reference standard (presence of metastasis, presence of heavy nodal burden [three or more metastatic axillary LNs]). Indeterminate FNAB results (a few atypical cells) were considered as indicating metastasis, while insufficient FNAB results were considered as indicating no metastasis.
Sensitivity and specificity for the diagnostic performance of US and US-guided FNAB were calculated and compared using the McNemar test. Positive predictive value (PPV) and negative predictive value (NPV), calculated using the generalized estimating equation, were also compared using the McNemar test.
Categorical variables from clinicopathological characteristics and US findings were compared between the <3 and ≥3 metastatic LN groups using the chi-square test or Fisher exact test. Continuous variables were compared between the two groups using the Student t-test. The Firth correction was used for rare US findings including a round shape (n=7) and the presence of extranodal extension (n=5) of the LN. A multivariate logistic regression model was used to analyze significant findings with at least one marginal predictive value (P<0.05) in the univariate analysis.
Stata (version 13 IC, StataCorp., College Station, TX, USA) was used for all statistical analyses. A P-value of <0.05 was considered to indicate statistical significance.
The mean age of patients was 50.8 years (range, 20 to 89 years). The study included 369 invasive ductal carcinomas, two ductal carcinomas in situ with microinvasion, and 33 other malignancies including invasive lobular (n=9), metaplastic (n=7), papillary (n=7), mucinous (n=5), mixed ductal and lobular (n=3), and tubular carcinomas (n= 2). Among these, 25 patients showed palpable axillary LNs on physical examination, including one patient with clinical stage N2a. Axillary nodal staging was determined by SLNB (n=226, 55.9%) and ALND (n=178, 44.1%), and the mean number of excised LNs was 4.7 in SLNB (range, 1 to 15) and 27.2 in ALND (range, 7 to 66). Patient and tumor characteristics are summarized in Table 1.
Among the 404 cancers, axillary US assessment revealed that 29.2% of the LNs were positive and 70.8% were negative. Among the US-determined positive LNs, final pathology revealed metastasis in 67.8%, and among the US-determined negative LNs, final pathology revealed metastasis in 36.0% (P<0.001). The sensitivity, specificity, PPV, and NPV of axillary US were 43.7%, 82.8%, 67.8%, and 64.0%, respectively (Table 2).
US-guided FNAB showed that 28% of LNs were positive and 72% were negative. Among the FNAB-determined positive LNs, 96.5% contained metastases, and among the FNAB-determined negative LNs, 25.4% involved metastases (P<0.001). The sensitivity, specificity, PPV, and NPV of US-guided FNAB were 59.6%, 98.2%, 96.5%, and 74.6%, respectively (Table 2). US-guided FNAB showed significantly higher diagnostic values for axillary LN metastasis than did US (all P<0.001).
The final pathology of the US-determined positive LNs showed three or more LN metastases in 43.2% of cases, while that of the US-determined negative LNs showed three or more LN metastases in 10.5% of cases (P<0.001). The sensitivity, specificity, PPV, and NPV of US were 63.0%, 79.3%, 43.2%, and 89.5%, respectively (Table 2).
The final pathology of the FNAB-determined positive LNs showed three or more LN metastases in 56.6% of cases, and that of the FNAB-determined negative LNs showed three or more LN metastases in 5.8% of cases (P<0.001). The sensitivity, specificity, PPV, and NPV of US-guided FNAB were 79.0%, 84.8 %, 56.6%, and 94.2%, respectively (Table 2). US-guided FNAB showed significantly higher diagnostic values for a heavy nodal burden than did axillary US (all P<0.05).
The clinicopathological characteristics and US findings of axillary LNs are shown in Table 3. A high tumor stage (T2, P<0.001), less cortical thickening (P<0.001), large number of suspicious LNs (P<0.001), hilar compression or loss (P<0.001), round shape (P=0.012), a non-circumscribed margin (P=0.001), extranodal extension (P=0.018), a positive FNAB result (P<0.001), ER/PR-positivity (P=0.006), and HER2 positivity (P=0.042) were more frequently found in the heavy nodal burden group.
Multivariate analysis of the significant findings showed that the presence of two or more suspicious LNs (P<0.001) and positive FNAB results (P<0.001) were significantly correlated with a heavy nodal burden (Table 4). The odds ratio for two suspicious LNs was 4.20 (95% CI, 1.90 to 9.39), while that for three or more suspicious LNs was 9.40 (95% CI, 2.99 to 29.54). The odds ratio for positive FNAB results was 14.22 (95% CI, 6.78 to 29.82). For the prediction of a heavy nodal burden, the sensitivity decreased gradually as the number of suspicious LNs on US increased, as follows: one suspicious LN, 45.7% (37 of 81); two suspicious LNs, 29.6% (24 of 81); and three or more suspicious LNs, 24.7% (20 of 81). However, the specificity, PPV, NPV, and accuracy were higher when there were three or more suspicious LNs, and there was a trend toward an increasing number of suspicious LNs on US as these diagnostic values increased (Supplementary Table 1). A subgroup analysis of the FNAB-positive group (n=64) showed that the presence of two or more suspicious LNs was significantly correlated with a heavy nodal burden on multivariate analysis (Supplementary Table 2). The odds ratio for two suspicious LNs was 4.34 (95% CI, 1.39 to 13.53) and that for three or more suspicious LNs was 36.15 (95% CI, 2.68 to 488.37) (Figs. 1-3). Multivariate analysis for the FNAB-negative group (n=17) showed that clinicopathological and US findings, except margin type and extranodal extension, were significantly correlated with a heavy nodal burden (Supplementary Table 3). Among these findings, the odds ratio for two suspicious LNs was 3.77 (95% CI, 3.69 to 3.84), while that for three or more suspicious LNs was 2.78 (95% CI, 2.67 to 2.90).
The present study showed that a larger number of suspicious LNs detected on US and positive FNAB results were significantly correlated with a heavy nodal burden in multivariate analysis. The odds ratios for a heavy nodal burden were 4.20 in patients with two suspicious LNs, 9.40 in patients with three or more suspicious LNs, and 14.22 in patients with a positive FNAB result on axillary US. Regardless of the preoperative FNAB result, the presence of a larger number of suspicious LNs on axillary US was significantly correlated with a heavy nodal burden. These findings can help clinicians decide whether ALND and SLNB must be performed on patients with early-stage breast cancer.
In the post-ACOSOG Z0011 trial era, the role of preoperative axillary US and US-guided FNAB in early-stage breast cancer needs to be reconsidered and used to identify patients with three or more axillary metastases. Van Wely et al. [8] first reported that patients with axillary LN metastases identified using US-guided FNAB had more positive LNs and were more likely to have three or more LNs involved than those with SLNB-identified nodal metastases. In a recent meta-analysis involving 532 patients with breast cancer whose axillary involvement was diagnosed by US-guided FNAB, 56% had three or more positive LNs in the final pathologic examination [15], which is similar to our results. According to another study by Lloyd et al. [16], more than 40% of patients who had positive LNs, as determined by axillary US-guided biopsy, were reported to have only one or two LNs with macrometastases; in such cases, direct implementation of ALND could result in overtreatment.
Our data suggested that the detection of a larger number of suspicious LNs on axillary US was an independent predictive factor; several previous studies have reported similar results [17-19]. Hieken et al. [17] reported that the rate of detection of three or more pathologically metastatic axillary LNs was 13.5% in patients with a solitary suspicious axillary LN observed on axillary US images and 30.8% in those with multiple abnormal LNs observed on axillary US images for clinically LN-negative breast cancer (P<0.001). Caudle et al. [18] found that 45% of patients with two or more abnormal LNs identified by US had three or more positive LNs identified using their axillary LN dissection specimens, whereas 19% of patients who were identified by SLNB after negative US findings had three or more positive LNs (P<0.001). In another study by Zhu et al. [19], 84.8% of patients with a solitary suspicious LN detected by axillary US had limited positive LNs; however, for patients with multiple suspicious LNs detected by axillary US, 38.6% of patients had limited positive LNs (P<0.001). These data and ours suggest that thorough axillary US in patients with early-stage breast cancer with suspicious LNs can help predict a heavy nodal burden of the axillae. Therefore, physicians must consider the number of suspicious axillary LNs on preoperative US in order to predict a heavy nodal burden.
In this study, 17 of 291 patients (5.8%) with FNAB-determined negative LNs showed three or more LN metastases. Because of this low rate of a heavy nodal burden in the FNAB-negative group, Liang et al. [20] suggested that such patients can be treated similarly to those who test negative using axillary US. In their study, Liang et al. [20] observed a similar heavy nodal burden in the FNAB-determined and US-determined negative groups (3.3% vs. 2.4%, P=0.405). Unlike our study, they did not analyze the number of suspicious LNs using US. In this study, the patients showed a larger number of suspicious LNs on US, even in the FNAB-determined negative LN group (one suspicious LN, 10 patients [58.8%, 10 of 17]; two suspicious LNs, five patients [29.4%, 5 of 17]; three or more suspicious LNs, two patients [11.7%, 2 of 17]) (Table 3). Therefore, regardless of FNAB results, we observed a larger number of suspicious LNs on US in the heavy nodal burden group than in patients who did not have a heavy nodal burden. We performed subgroup and multivariate analyses on the FNAB-negative group to discover predictors of a heavy nodal burden; the presence of two or more suspicious LNs on US was significantly correlated with a heavy nodal burden (Supplementary Table 3). These results suggest that the presence of a large number of US-determined suspicious LNs is a reliable predictor of a heavy nodal burden, regardless of preoperative FNAB results. The combined use of the number of suspicious LNs using axillary US and preoperative FNAB results may improve the accuracy of prediction of the nodal burden.
Among the clinicopathological findings in our study, a high tumor stage and ER-/PR-positivity and HER2-positivity of tumors were more common, and the triple-negative subtype was less common, in the heavy nodal burden group than in the group without a heavy nodal burden. Kim et al. [21] reported that a higher T stage (≥2) was independently associated with a higher nodal burden on both breast US and magnetic resonance imaging. Furthermore, Zhu et al. [19] suggested that false-positive axillary US results were more frequent in patients with early-stage breast cancer who had a T1 stage tumor. They also described that ER-/PR-negative tumors had a lower risk of nodal metastases than ER-/PR-positive tumors in patients with early-stage breast cancer. In addition, Vane et al. [22] reported the highest NPV of axillary US in the triple-negative subtype (90.3%), and Caudle et al. [18] found that triple-negative tumors were associated with less extensive nodal involvement. Our results in this regard are consistent with those of previous studies.
This study has several limitations. First, this was a retrospective study performed over a long study period (about 10 years); therefore, the possibility of selection bias should be considered. In addition, variability in management methods used by different physicians is also possible; for instance, variation may be present among radiologists who perform US and US-guided FNAB. However, this variation could not be assessed because of the retrospective study design. Second, we did not correlate the findings for suspicious LNs, as determined by US, with their final pathological results obtained using a node-by-node analysis. Third, we included only patients who underwent FNAB; therefore, patients who had normal axillary LNs were not included in the study. However, we applied not only cortical thickening (≥2.5 mm), but also other US features such as hilar change, round shape, indistinct margin, and extranodal extension as abnormal US findings. Therefore, the diagnostic performance of axillary US obtained in this study was similar to that obtained in a previous systematic review [4]. Fourth, the small number of cases showing a heavy nodal burden in the FNAB-negative group may influence the generalizability of our subgroup results. Additional larger studies are needed in the future.
It is well known that SLNB could reduce the morbidity and complications associated with ALND, and the major reason for performing axillary US to predict heavy nodal burden should be to guide appropriate treatments for early-stage breast cancer. However, ALND is preferred in FNAB-positive patients, even in those with only one or two metastatic LNs. According to our results, physicians may decide to perform ALND in patients with axillary LNs showing FNAB-determined positivity and two or more suspicious LNs on axillary US. Because this study was performed over a long period with a retrospective study design, further prospective studies should be performed to determine the precise US features of axillary LNs that predict LN metastasis and a heavy nodal burden.
In conclusion, the number of suspicious LNs detected on axillary US and preoperative FNAB results can help predict a heavy axillary nodal burden in patients with early-stage breast cancer. These findings may help clinicians determine the requirement for ALND after performing US-guided FNAB in patients with early-stage breast cancer showing suspicious findings on axillary US.
NotesAuthor Contributions Conceptualization: Kim SM. Data acquisition: Kim SM, Jang M, Yun BL, Kang E, Kim EK, Park SY, Kim B. Data analysis or interpretation: Ahn HS, Kim SM. Drafting of the manuscript: Ahn HS, Kim SM. Critical revision of the manuscript: Kim SM, Jang M, Yun BL, Kang E, Kim EK, Park SY, Kim B. Approval of the final version of the manuscript: all authors. Supplementary MaterialSupplementary Table 1.Diagnostic performance of the number of suspicious LNs on axillary US in predicting a heavy nodal burden (https://doi.org/10.14366/usg.20143).
Supplementary Table 2.Results of multivariate analysis: significant findings in the FNAB-positive group (n=64) (https://doi.org/10.14366/usg.20143).
Supplementary Table 3.Results of multivariate analysis: significant findings in the FNAB-negative group (n=17) (https://doi.org/10.14366/usg.20143).
References1. Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer 1983;52:1551–1557.
2. Montemurro F, Rossi V, Cossu Rocca M, Martinello R, Verri E, Redana S, et al. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer 2012;118:17–26.
3. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569–575.
4. Alvarez S, Anorbe E, Alcorta P, Lopez F, Alonso I, Cortes J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 2006;186:1342–1348.
5. Podkrajsek M, Music MM, Kadivec M, Zgajnar J, Besic N, Pogacnik A, et al. Role of ultrasound in the preoperative staging of patients with breast cancer. Eur Radiol 2005;15:1044–1050.
6. Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer 2002;95:982–988.
7. Hu X, Zhou X, Yang H, Wei W, Jiang Y, Liu J. Axillary ultrasound and fine needle aspiration biopsy in the preoperative diagnosis of axillary metastases in early-stage breast cancer. Oncol Lett 2018;15:8477–8483.
8. van Wely BJ, de Wilt JH, Schout PJ, Kooistra B, Wauters CA, Venderinck D, et al. Ultrasound-guided fine-needle aspiration of suspicious nodes in breast cancer patients: selecting patients with extensive nodal involvement. Breast Cancer Res Treat 2013;140:113–118.
9. Leenders MW, Broeders M, Croese C, Richir MC, Go HL, Langenhorst BL, et al. Ultrasound and fine needle aspiration cytology of axillary lymph nodes in breast cancer: to do or not to do? Breast 2012;21:578–583.
10. Verheuvel NC, van den Hoven I, Ooms HW, Voogd AC, Roumen RM. The role of ultrasound-guided lymph node biopsy in axillary staging of invasive breast cancer in the post-ACOSOG Z0011 trial era. Ann Surg Oncol 2015;22:409–415.
11. Zhu Y, Zhou W, Zhou JQ, Fei XC, Ye TJ, Huang O, et al. Axillary staging of early-stage invasive breast cancer by ultrasound-guided fine-needle aspiration cytology: which ultrasound criteria for classifying abnormal lymph nodes should be adopted in the post-ACOSOG Z0011 trial era? J Ultrasound Med 2016;35:885–893.
12. Cho N, Moon WK, Han W, Park IA, Cho J, Noh DY. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol 2009;193:1731–1737.
13. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–1474.
14. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies: improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546.
15. van Wely BJ, de Wilt JH, Francissen C, Teerenstra S, Strobbe LJ. Meta-analysis of ultrasound-guided biopsy of suspicious axillary lymph nodes in the selection of patients with extensive axillary tumour burden in breast cancer. Br J Surg 2015;102:159–168.
16. Lloyd P, Theophilidou E, Newcombe RG, Pugh L, Goyal A. Axillary tumour burden in women with a fine-needle aspiration/core biopsy-proven positive node on ultrasonography compared to women with a positive sentinel node. Br J Surg 2017;104:1811–1815.
17. Hieken TJ, Trull BC, Boughey JC, Jones KN, Reynolds CA, Shah SS, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery 2013;154:831–838.
18. Caudle AS, Kuerer HM, Le-Petross HT, Yang W, Yi M, Bedrosian I, et al. Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol 2014;21:3440–3447.
19. Zhu Y, Zhou W, Jia XH, Huang O, Zhan WW. Preoperative axillary ultrasound in the selection of patients with a heavy axillary tumor burden in early-stage breast cancer: what leads to false-positive results? J Ultrasound Med 2018;37:1357–1365.
20. Liang Y, Chen X, Zhan W, Garfield DH, Wu J, Huang O, et al. Can clinically node-negative breast cancer patients with suspicious axillary lymph nodes at ultrasound but negative fine-needle aspiration be approached as having node-negative disease? Ann Surg Oncol 2017;24:1874–1880.
Invasive carcinoma of the left breast in a 40-year-old woman.A. Transverse axillary ultrasonography shows a lymph node with 3 mm even cortical thickness (arrows). Ultrasound-guided fine-needle aspiration biopsy confirmed metastasis. B. Transverse breast ultrasonography shows an indistinct, irregular, hypoechoic mass with internal microcalcifications (arrows). On pathological examination following mastectomy and axillary lymph node dissection, a 1.4 cm invasive ductal carcinoma-associated ductal carcinoma in situ [ER (+), PR (-), HER2 (-), and Ki-67 10%], with two out of 20 axillary lymph node metastases (T1c, N1a), was confirmed. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2.
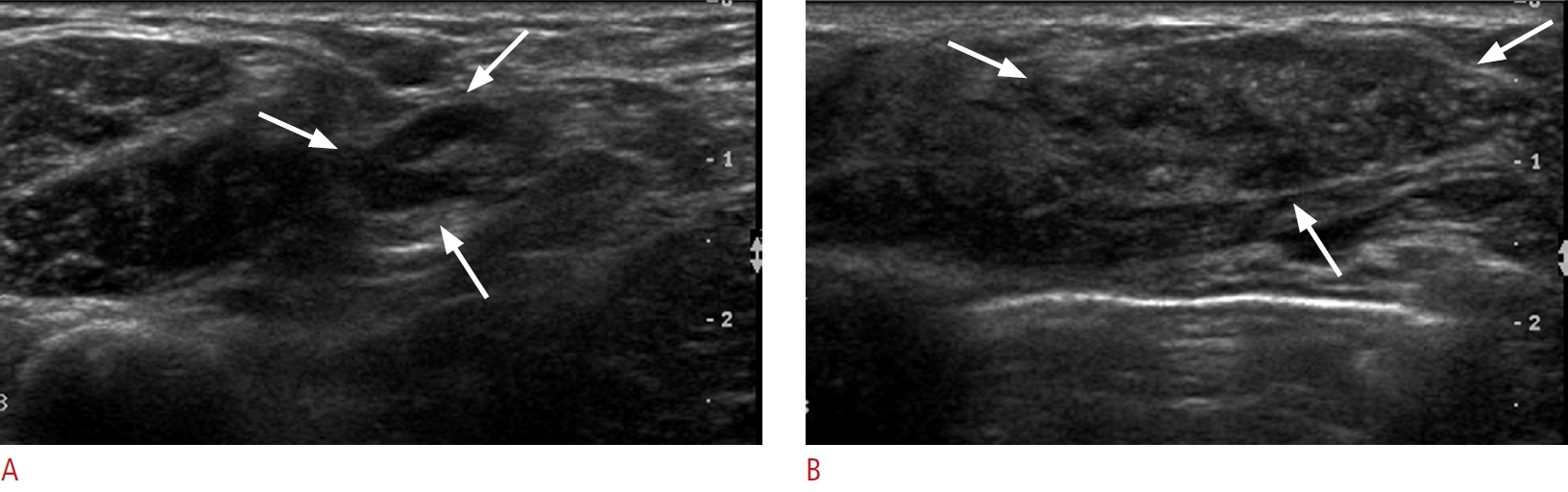 Fig. 1.Invasive carcinoma of the right breast in a 50-year-old woman.A. Longitudinal axillary ultrasonography shows two lymph nodes with uneven cortical thickness (arrows). Ultrasound-guided fine-needle aspiration biopsy confirmed metastasis. B. Transverse breast ultrasonography shows an indistinct, irregular, hypoechoic mass with internal microcalcification (arrows). On pathological examination following lumpectomy and axillary lymph node dissection, a 3.5 cm invasive ductal carcinoma [ER (+), PR (+), HER2 (-), and Ki-67 5%] with three out of 29 axillary lymph node metastases (T2, N1) was confirmed. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2.
 Fig. 2.Invasive carcinoma of the left breast in a 75-year-old woman.A, B. Transverse axillary ultrasonography shows three round or oval hypoechoic lymph nodes with hilar changes (medial [arrowhead] and lateral [arrow]/inferior [arrow]). Ultrasound-guided fineneedle aspiration biopsy proved metastasis. C. Transverse breast ultrasonography shows an angular, irregular, hypoechoic mass. On a pathological examination following lumpectomy and axillary lymph node dissection, a 1.5 cm invasive ductal carcinoma [ER (-), PR (-), HER2 (+), and Ki-67 20%] with 18 out of 43 axillary lymph node metastases (T1, N3) was confirmed. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2.
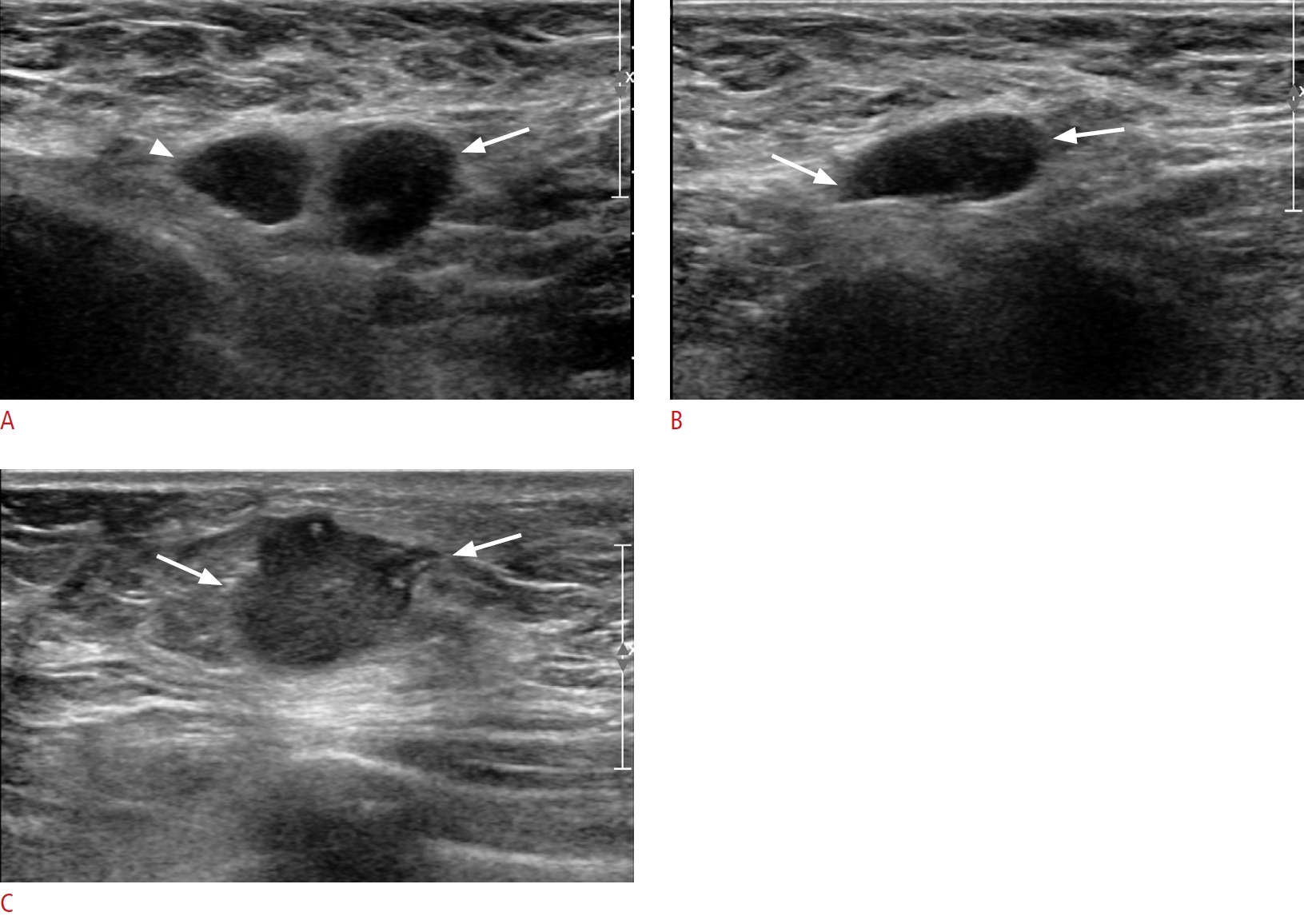 Fig. 3.Table 1.Patient and tumor characteristics Table 2.Diagnostic performance of US and US-guided FNAB
Table 3.Results of univariate analysis: clinicopathological characteristics and US findings
Values are presented as number (%) unless otherwise indicated. US, ultrasonography; LN, lymph node; CI, confidence interval; FNAB, fine-needle aspiration biopsy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2. Table 4.Results of multivariate analysis: significant clinicopathological characteristics and US findings
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




