AbstractPurposeThe purpose of this study was to examine the associations between ultrasonography (US) quality and clinical outcomes in patients undergoing surveillance for hepatocellular carcinoma.
MethodsBetween 2008 and 2013, 155 patients were diagnosed with liver cancer during regular surveillance by positive US results (US group, n=82) or by computed tomography (CT) or magnetic resonance image (MRI) scanning as alternative modalities (CT/MRI group, n=73). The quality of the echogenic window, macronodularity of the liver parenchyma, and occurrence of surveillance failure (initial tumor diagnosis beyond the Milan criteria or at Barcelona Clinic Liver Cancer stage B or C) were evaluated. Overall survival was compared according to whether surveillance failure occurred.
ResultsThe patients in the CT/MRI group with negative US results had a higher proportion of parenchymal macronodularity on US than those in the US group (79.5% vs. 63.4%, P=0.028). Surveillance failure tended to be more common in the US group than in the CT/MRI group (40.2% vs. 26.0% by the BCLC staging system [P=0.061]). In the US group, surveillance failure occurred more frequently when the echogenic window was inadequate (50.0% vs. 19.4% by the Milan criteria [P=0.046]). Significantly poorer 5-year overall survival was associated with surveillance failure (PŌēż0.001).
ConclusionParenchymal macronodularity hindered the detection of early-stage tumors during US surveillance. Using an alternative imaging modality may help prevent surveillance failure in patients with macronodular parenchyma on US. Supplemental surveillance strategies than US may also be necessary when the echogenic window is inadequate.
Liver cancer is the fourth leading cause of cancer-related death worldwide [1]. Surveillance is recommended for patients at high risk of developing hepatocellular carcinoma (HCC) to detect the tumor at an early stage and render curative treatment [2]. A general recommendation for HCC surveillance is biannual ultrasonography (US) with or without serum ╬▒-fetoprotein (AFP) testing [2,3]. Evidence suggests that surveillance for HCC is associated with significant improvements in patient survival [4,5].
However, concerns have been raised that the proportion of early-stage HCC diagnoses during surveillance is not sufficiently high. It has been reported that surveillance enables early-stage tumor diagnosis in only two-thirds of patients with cirrhosis [6,7]. In other words, surveillance failure, defined as late-stage HCC diagnosis despite surveillance, occurs in about one-third of patients under surveillance. Surveillance failure can occur due to a lack of regular periodic screening, the absence of a prompt diagnostic evaluation after obtaining a positive surveillance result, or even despite conforming to the recommended screening and diagnostic protocols [8].
Surveillance failure is meaningfully influenced by the performance of the imaging modality used for surveillance. It has been reported that US has suboptimal sensitivity for detecting early-stage HCC, which is likely a major cause of surveillance failure [9]. Therefore, it is crucial to elucidate the factors that contribute to the limited sensitivity of US. Although various clinical factors have been examined to identify possible associations with surveillance failure [6,10-12], few researchers have evaluated factors related to the US modality itself that may affect tumor detectability during surveillance [13]. At our institution, in addition to findings concerning nodules suspected to be malignant, the adequacy of the echogenic window and the status of the liver parenchyma are also routinely recorded in the surveillance US reporting form, as these factors may affect the detectability of small nodules. Our hypothesis was that the detectability of small nodules during surveillance US may be hindered by the inadequacy of the echogenic window and parenchymal macronodularity. Therefore, we aimed to investigate the associations between US quality, as assessed by adequacy of the echogenic window and the status of the liver parenchyma, and clinical outcomes in patients undergoing surveillance for HCC.
Approval from the local Institutional Review Board was obtained and the requirement for patient consent was waived due to the retrospective nature of this study. Our search of patients' electronic medical records revealed 2,166 patients at risk for HCC (due to hepatitis B virus surface antigen positivity, hepatitis C antibody positivity, or liver cirrhosis of any etiology) who underwent abdominal US for HCC surveillance from January 2008 to December 2013 at a single tertiary medical center. We excluded 1,783 patients who had been previously treated for liver cancer (n=465), who received no tumor diagnosis (n=1,220), or who underwent only a single screening US examination (n=98). Of the remaining 383 treatment-naïve at-risk patients who were diagnosed with liver cancer during surveillance, we excluded 206 patients who were diagnosed with liver cancer based on the initial surveillance US (n=91) or who did not have an interval of 4 to 8 months between the final screening round and the prior screening round (failure of screening, n=115) [10]. We also excluded 22 patients who did not undergo diagnostic computed tomography (CT) or magnetic resonance imaging (MRI) within 6 months after obtaining positive surveillance US (failure of follow-up, n=6), who received a histopathologic diagnosis of a non-HCC malignancy (n=11), or who were in Child-Pugh class C (n=5). Our final study cohort consisted of the remaining 155 patients (Fig. 1). The patients were either diagnosed with liver cancer by positive US surveillance results (US group, n=82) or by CT or MRI performed as an alternative modality after obtaining negative US surveillance results (CT/MRI group, n=73).
At our institution, surveillance for HCC is performed for atrisk patients by biannual abdominal US and serum AFP testing. Surveillance US is performed by hepatology residents or fellows with 1 to 4 years of experience in abdominal US. Our abdominal US reporting form includes (1) quality of the echogenic window (acceptable, poor, or unacceptable); (2) type of liver cirrhosis (macronodular, micronodular, or no gross evidence of cirrhosis); (3) degree of fatty liver (mild, moderate, severe, or no gross evidence of fatty liver); (4) number, size, and location of any space-occupying lesion; (5) presence or absence of splenomegaly; and (6) presence or absence of vascular thrombosis. The assessment of the echogenic window is based on the estimated proportion of scanned area to the entire liver; if some portions or only small portions of the liver are visualized on US, the echogenic window quality is rated as poor or unacceptable, respectively. Cirrhosis is classified as macronodular or micronodular depending on the presence of numerous cirrhosis-associated nodules Ōēź3 mm or <3 mm, respectively.
If any space-occupying lesion measuring >1 cm was newly detected by US, or the serum AFP level was above 20 ng/mL, the surveillance test was considered positive, and the patient underwent dynamic contrast-enhanced CT or MRI. In some cases, CT or MRI was performed to evaluate patientsŌĆÖ symptoms or as an alternative tool for surveillance under clinical suspicion of liver cancer. The diagnosis of HCC was based on the radiological findings of CT or MRI or on histological evidence, according to the guidelines of the American Association for the Study of Liver Disease [2].
A board-certified radiologist with 8 years of experience in abdominal imaging (C.A.) reviewed the electronic medical records, including the prospectively written radiology reports. To test our hypothesis, the quality of the echogenic window was dichotomized into adequate (acceptable) or inadequate (poor or unacceptable) based on the US reports. In addition, a board-certified radiologist with 4 years of experience in abdominal imaging (Y.Y.K.) retrospectively reviewed the sonograms, blinded to clinical information, in order to classify the patients as with or without parenchymal macronodularity. The presence of numerous cirrhosis-associated nodules Ōēź3 mm that made the hepatic echotexture markedly heterogeneous was considered indicative of macronodular liver cirrhosis. For patients in the US group, the US report and images from the prior screening round were reviewed; for those in the CT/MRI group, the US report and images from the final screening round were reviewed.
The clinical information that was examined included age, sex, body mass index (BMI), etiology of liver disease, Child-Pugh class, serum AFP level, serologic laboratory data, the presence or absence of diabetes mellitus (DM), final pathological diagnosis, and survival information. Based on radiology reports and clinical data, tumor stage at the time of the initial diagnosis was determined according to the Milan criteria or the Barcelona Clinic Liver Cancer (BCLC) staging system. Surveillance failure was defined as an initial tumor diagnosis beyond the Milan criteria or at BCLC stage B or C despite regular surveillance.
The patient characteristics and tumor stage were compared between the US group and the CT/MRI group using the chi-square test for categorical variables and the Student t test for continuous variables. The chi-square test or the Fisher exact test was used to compare tumor stage according to the quality of the echogenic window and the presence or absence of parenchymal macronodularity in the US group; this analysis was not performed in the CT/MRI group, as tumor stage can be affected by the intervention of CT and MRI scanning and by the quality of surveillance US. Inter-observer agreement in the evaluation of echogenic window quality between the final screening round and the prior screening round was compared using kappa statistics [14]. We used logistic regression to identify clinical factors associated with adequacy of the echogenic window or the presence of parenchymal macronodularity on surveillance US. Continuous variables were dichotomized according to the Child-Pugh classification or institutional reference values (aspartate transaminase >34 IU/L, alanine transaminase >46 IU/ L, and creatinine Ōēź0.91 mg/dL). Factors with a P-value less than 0.2 in the univariable analysis were included in the subsequent multivariable analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. The survival outcomes of the study subjects were analyzed according to whether surveillance failure occurred. Five-year overall survival (5YOS) was examined and compared using the Kaplan-Meier method with the log-rank test. For the survival analysis, patients were censored at the time of death or the last follow-up. All analyses were performed using statistical software (SPSS Statistics version 23.0, IBM Corp., Armonk, NY, USA), and a 2-sided P-value <0.05 was considered to indicate statistical significance.
The characteristics of the patients (113 men and 42 women) are summarized in Table 1. Hepatitis B virus infection was the predominant etiology of liver disease (81.9%), followed by hepatitis C virus infection (11.0%). Most patients (93.5%) were in Child-Pugh class A. The patients in the US group and those in the CT/MRI group did not differ significantly in terms of age, sex, BMI, etiology of liver disease, and Child-Pugh classification. The proportion of patients with DM or an AFP elevation Ōēź20 ng/mL tended to be higher in the CT/MRI group (50.7% vs. 35.4% for DM [P=0.054] and 50.7% vs. 36.6% for AFP elevation [P=0.077]), but this trend did not reach statistical significances.
With regard to the quality of surveillance US, 16.8% (26 of 155) and 71.0% (110 of 155) of patients had an inadequate echogenic window and parenchymal macronodularity, respectively. Patients in the CT/MRI group had higher proportions of an inadequate echogenic window (Fig. 2) and parenchymal macronodularity (Fig. 3) than those in the US group (19.2% vs. 14.6% for an inadequate echogenic window [P=0.450] and 79.5% vs. 63.4% for parenchymal macronodularity [P=0.028]). The inter-observer agreement for adequacy of the echogenic window was moderate (╬║=0.44).
In the entire study population, tumors were initially detected beyond the Milan criteria in 31 patients (20.0%) and at BCLC stage B or C in 52 patients (33.5%). The proportion of surveillance failure tended to be higher in the US group than in the CT/MRI group (23.2% vs. 16.4% by the Milan criteria [P=0.296] and 40.2% vs. 26.0% by the BCLC staging system [P=0.061]). The proportion of diagnoses at BCLC stage 0 tended to be lower in the US group than in the CT/MRI group (20.7% vs. 31.5%, P=0.126).
As summarized in Table 2, surveillance failure was more frequently observed in the US group in patients with an inadequate echogenic window (50.0% vs. 19.4% by the Milan criteria [P=0.046] and 60.0% vs. 37.5% by the BCLC staging system [P=0.191]). The proportion of surveillance failure did not differ between patients with parenchymal macronodularity and those without parenchymal macronodularity (23.1% vs. 23.3% by the Milan criteria [P=0.979] and 38.5% vs. 43.3% by the BCLC staging system [P=0.665]). The frequency of diagnosis at BCLC stage 0 was not significantly different according to adequacy of the echogenic window (10.0% vs. 22.2%, P=0.679) or parenchymal status (19.2% vs. 23.3%, P=0.659).
The results of the univariable and multivariable regression analyses performed to examine the relationship of various clinical factors with an inadequate echogenic window and parenchymal macronodularity are presented in Table 3. In the multivariable analyses, BMI Ōēź25 kg/m2 (OR, 2.536; 95% CI, 0.968 to 6.643; P=0.058) showed a tendency to be associated with an inadequate echogenic window; hepatitis B virus infection (OR, 10.370; 95% CI, 3.129 to 34.270; P<0.001) and a platelet count <150├Ś109/L (OR, 3.167; 95% CI, 1.360 to 7.375; P=0.008) were independently associated with parenchymal macronodularity.
The results of our study indicate that the quality of surveillance US assessed by adequacy of the echogenic window and the presence of parenchymal macronodularity was associated with failure of surveillance for HCC. The majority of patients (79.5%) with HCC who were not diagnosed by US, but instead diagnosed by CT or MRI, had macronodular liver cirrhosis; this proportion was significantly higher than that in patients with HCC detected by surveillance US. HCC was detected by US beyond the Milan criteria in approximately one-fourth of study subjects; this was observed significantly more frequently in patients having an inadequate echogenic window, with surveillance failure occurring in 50% to 60% of such patients. We also found that diagnosis of HCC at later stages due to surveillance failure led to poorer survival outcomes.
Although US, the mainstay of HCC surveillance, has suboptimal sensitivity for the detection of early-stage HCC [9], few studies have evaluated the association between the adequacy of surveillance US and surveillance failure. A previous study demonstrated that subcapsular location was more frequent among sonographically undetected nodules than among sonographically detected nodules [15]. The presence of an inadequate echogenic window, which was a variable assessed in our study, suggests that a non-negligible proportion of the liver is inappropriately visualized on US. Windows for subcapsular and dome locations would be particularly limited. Therefore, US may not be an appropriate modality for the detection of early-stage tumors when the echogenic window is not adequate.
We also hypothesized that the detection of early-stage tumors may be challenging when numerous regenerative or dysplastic nodules are present in the liver. However, parenchymal macronodularity did not show a significant relationship with surveillance failure in the US group. This can be attributed to the fact that study subjects with parenchymal macronodularity frequently underwent CT or MRI due to clinical suspicion of liver cancer despite negative results on surveillance US. Interestingly, patients diagnosed by these alternative modalities showed a tendency to be diagnosed at early stages. Thus, we can speculate that parenchymal macronodularity may hinder the detection of small tumors and that using an imaging modality other than US may help prevent surveillance failure. A previous study demonstrated that parenchymal macronodularity was a significant factor related to surveillance failure, especially in patients with hepatitis B virus infection [13]. Those results seem to be applicable to our study population, probably due to the high prevalence of hepatitis B virus infection in our study.
We found that a high BMI was marginally associated with an inadequate echogenic window, even though only a small proportion of our study subjects was classified as overweight based on BMI values. Although a previous analysis revealed no significant association between BMI and surveillance failure [10], another study that recruited a larger number of obese or morbidly obese patients demonstrated a significant association between high BMI and surveillance failure [11]. Our results indicate that a large patient body habitus can negatively affect the quality of the echogenic window and thereby interfere with tumor detection by US. However, BMI was not an independent contributor to a poor echogenic window. This may be explained by the fact that thin patients occasionally have an inadequate echogenic window depending on the configuration of the rib cage and intra-abdominal organs; liver parenchymal atrophy due to advanced liver cirrhosis can also make the echogenic window inadequate. Therefore, routine assessment of echogenic window quality in each patient during surveillance would be practically useful and provide critical information for planning the next surveillance modality.
It should be noted that patients with hepatitis B infection were more likely to exhibit parenchymal macronodularity than were patients with hepatitis C infection, with an OR of 10.370. This result, in accordance with prior knowledge [16], is significant in that the quality of surveillance US may depend on the etiology of liver disease. In patients with hepatitis B virus infection, US surveillance may show a limited ability to detect early-stage HCC. A low platelet count also showed a significant association with parenchymal macronodularity. A low platelet count, representing portal hypertension [17], may reflect the advanced status of the underlying liver disease itself, as is demonstrated by a macronodular appearance of the liver parenchyma on US.
The results of our study suggest that US surveillance has limitations in patients with an inadequate echogenic window or parenchymal macronodularity. Moreover, BCLC stage 0 tumors accounted for approximately 20% of the total tumors in the US group, regardless of the quality of the echogenic window or parenchymal status. This indicates that diagnosing tumors at a very early stage is challenging when US is used for surveillance, even with adequate US quality. Therefore, an alternative surveillance method, such as MRI, may be helpful to prevent surveillance failure and to facilitate tumor diagnosis at the earliest stage. Compared to US, MRI with a hepatobiliary contrast agent has shown superior detection sensitivity for early-stage HCC and a lower false-positive rate [15]. Further investigations are warranted to validate the utility of imaging modalities other than US, including unenhanced MRI, in patients prone to surveillance failure [18,19].
This study has some limitations. First, this is a single-center study that included noncirrhotic hepatitis C patients, which may limit the generalizability of our results. Second, as US examinations were performed by multiple operators, inter-observer variability in reporting echogenic window quality existed. However, we could not perform a retrospective analysis of this variable, as the degree to which echogenic window quality can be determined based on images alone is limited. Third, we only examined the adequacy of US examinations in terms of the echogenic window and parenchymal status. Other factors, including the degree of fatty liver, tumor size, tumor location, as well as elastography measurements of liver stiffness, may also be related to tumor detectability on US. Lastly, the major etiology of liver disease was hepatitis B virus infection in our study population, which limits the applicability of our results to the general high-risk population. However, our study demonstrated that parenchymal macronodularity significantly influenced the detection sensitivity of surveillance US in an area with endemic hepatitis B virus infections.
In conclusion, an inadequate echogenic window and parenchymal macronodularity may be associated with a higher risk of surveillance failure. Alternative surveillance strategies may be helpful for early tumor detection in patients with inadequate US quality.
NotesAuthor Contributions Conceptualization: An C, Kim DY. Data acquisition: Kim YY, An C, Aljoqiman KS. Data analysis or interpretation: Kim YY, An C. Drafting of the manuscript: Kim YY. Critical revision of the manuscript: An C, Kim DY, Choi JY, Kim MJ. Approval of the final version of the manuscript: all authors. Conflict of InterestConflict of interest No potential conflict of interest relevant to this article was reported. AcknowledgementsThe authors acknowledge Minsu Park from the Department of Biomedical Systems Informatics, Yonsei University College of Medicine, for providing statistical advice.
References1. Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683ŌĆō1691.
2. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358ŌĆō380.
3. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASLEORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908ŌĆō943.
4. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624.
5. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417ŌĆō422.
6. Del Poggio P, Olmi S, Ciccarese F, Di Marco M, Rapaccini GL, Benvegnu L, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1927ŌĆō1933.
7. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37ŌĆō47.
8. Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol 2013;108:425ŌĆō432.
9. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706ŌĆō1718.
10. Chon YE, Jung KS, Kim MJ, Choi JY, An C, Park JY, et al. Predictors of failure to detect early hepatocellular carcinoma in patients with chronic hepatitis B who received regular surveillance. Aliment Pharmacol Ther 2018;47:1201ŌĆō1212.
11. Wong LL, Reyes RJ, Kwee SA, Hernandez BY, Kalathil SC, Tsai NC. Pitfalls in surveillance for hepatocellular carcinoma: how successful is it in the real world? Clin Mol Hepatol 2017;23:239ŌĆō248.
12. Mancebo A, Varela M, Gonzalez-Dieguez ML, Navascues CA, Cadahia V, Mesa-Alvarez A, et al. Incidence and risk factors associated with hepatocellular carcinoma surveillance failure. J Gastroenterol Hepatol 2018;33:1524ŌĆō1529.
13. Sinn DH, Yi J, Choi MS, Choi D, Gwak GY, Paik YH, et al. Incidence and risk factors for surveillance failure in patients with regular hepatocellular carcinoma surveillance. Hepatol Int 2013;7:1010ŌĆō1018.
15. Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI with liverspecific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol 2017;3:456ŌĆō463.
16. Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis: definition, nomenclature, and classification. Bull World Health Organ 1977;55:521ŌĆō540.
17. Procopet B, Berzigotti A. Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol Rep (Oxf) 2017;5:79ŌĆō89.
Flowchart of the study participants.HCC, hepatocellular carcinoma; US, ultrasonography; CT, computed tomography; MRI, magnetic resonance imaging.
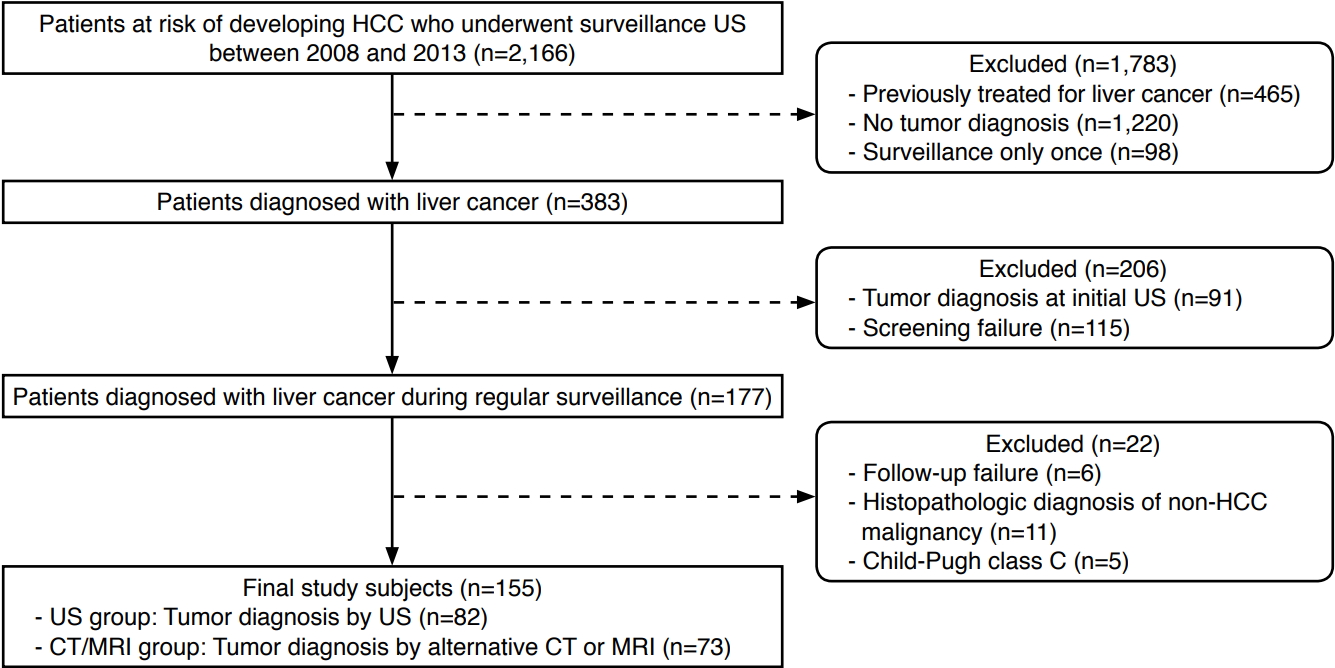 Fig.┬Ā1.Inadequate echogenic window of surveillance ultrasonography (US) obscuring hepatocellular carcinoma in a 65-year-old man with B-viral liver cirrhosis.A, B. On US obtained through a subcostal view, segment IV of the liver is poorly visualized due to posterior shadowing from (A) hepatic flexure of the colon (arrowheads) and (B) the costochondral junction (arrow). C, D. Liver dynamic computed tomography was performed after 3 months due to elevated serum ╬▒-fetoprotein levels and revealed a 6.3-cm mass in hepatic segment IV. The mass with hyperenhancement in the arterial phase (C) and washout in the portal venous phase (D) is located posterior to the hepatic flexure of the colon (arrowhead in C) and costochondral junction, which limited the echogenic window for this mass (arrow in C) on US.
 Fig.┬Ā2.Parenchymal macronodularity hindering detection of hepatocellular carcinoma in a 45-year-old man with B-viral liver cirrhosis.A. On the background of macronodular parenchyma, a 2.0-cm hypoechoic nodule (arrow) was undetected on surveillance ultrasonography (US). B. On the following US, performed after 6 months, the mass (arrow) increased in size but was still missed. C. On gadoxetate-enhanced liver dynamic magnetic resonance imaging (MRI) obtained after another 6 months, the mass in hepatic segment V/VI measured 5.4 cm and showed hyperenhancement in the arterial phase and washout in the portal venous phase (not shown). D. T2-weighted MRI depicts the mildly hyperintense mass and the macronodular liver parenchyma.
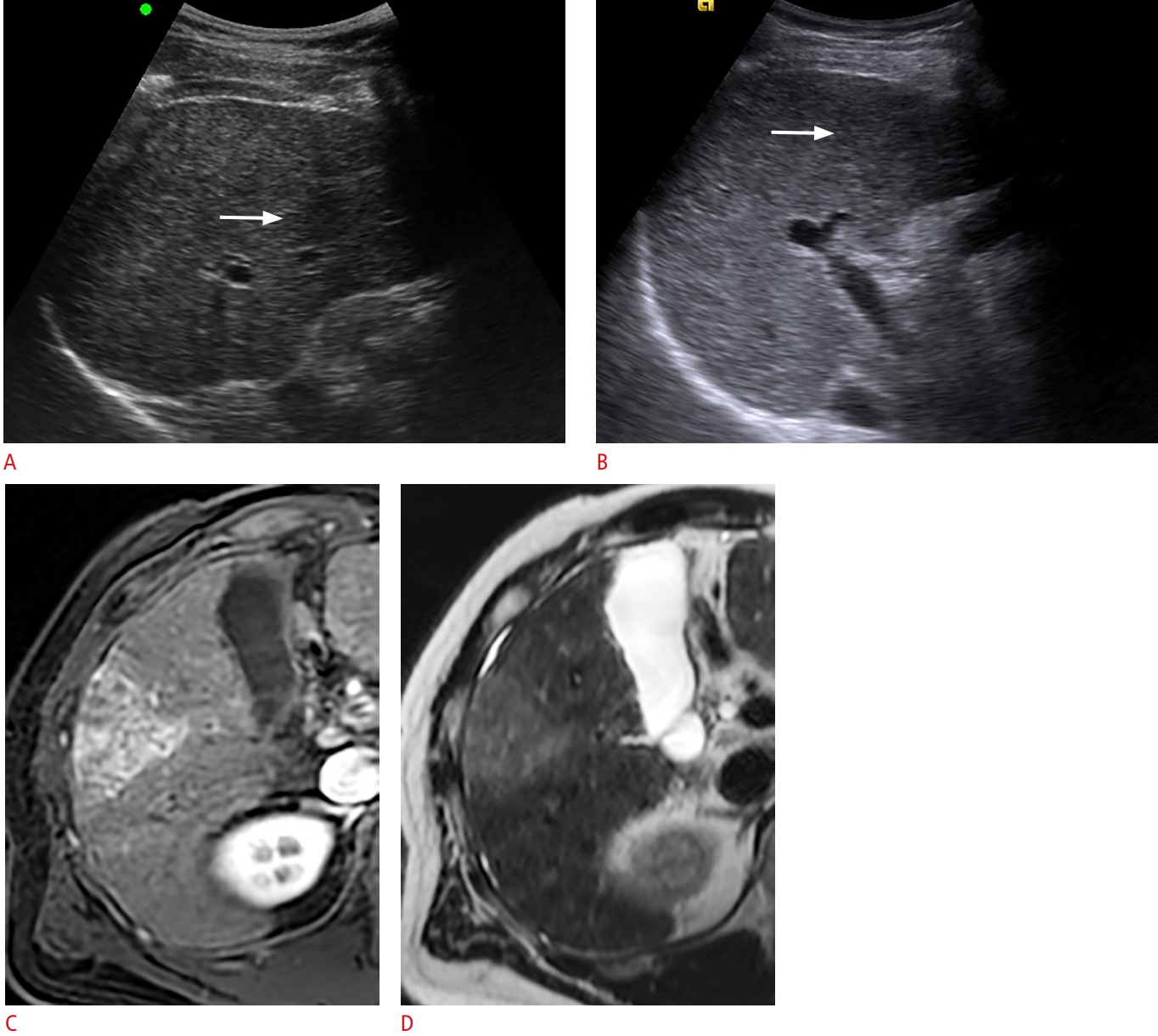 Fig.┬Ā3.Kaplan-Meier survival curves according to whether surveillance failure occurred.Survival outcomes were poorer in patients with surveillance failure (A, 5-year overall survival, 36.4% vs. 69.9%; P<0.001 by the Milan criteria; B, 46.8% vs. 71.3%; P=0.001 by the Barcelona Clinic Liver Cancer [BCLC] staging system).
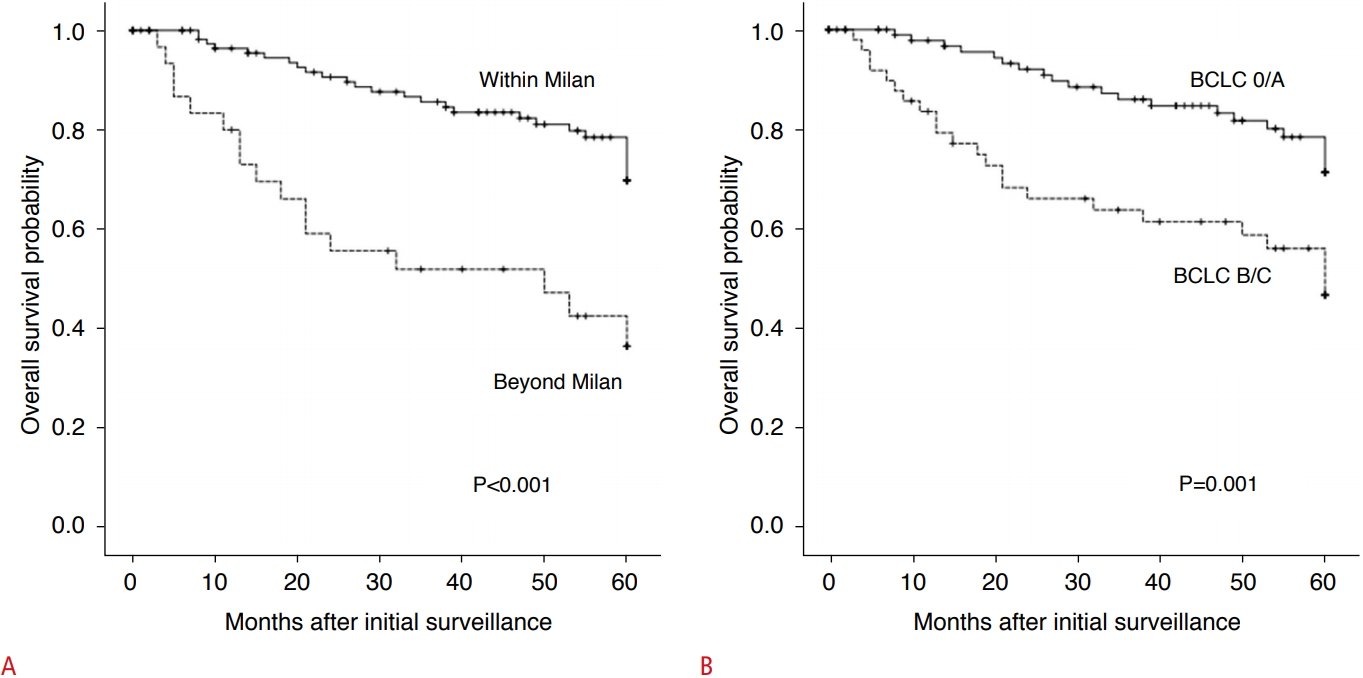 Fig.┬Ā4.Table┬Ā1.Patient characteristics and tumor stages at initial diagnosis Table┬Ā2.Tumor stages at initial diagnosis in the US group according to the quality of surveillance US
Table┬Ā3.Univariable and multivariable analyses of factors related to inadequate US quality |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




