AbstractPurpose:The goal of this study was to determine the risk of malignancy of thyroid nodules with minimal cystic changes.
Methods:A total of consecutive 1,000 thyroid nodules (Ōēź1 cm) with final diagnoses from twoinstitutions were included in this study. The risk of malignancy of thyroid nodules was analyzed according to the internal content, which was categorized as purely solid, minimally cystic (cystic changes Ōēż10%), and partially cystic (cystic changes >10%). We also assessed the risk of malignancy of nodules with minimal cystic changes depending on echogenicity and presence of any suspicious ultrasonografic (US) features.
Results:The overall frequency of purely solid, minimally cystic, and partially cystic noduleswas 730/1,000 (73%), 61/1,000 (6.1%), and 209/1,000 (20.9%), respectively, with risks ofmalignancy of 14.8% (108/730), 3.3% (2/61), and 3.3% (7/209), respectively. The risk ofmalignancy of nodules with minimal cystic changes was significantly lower than that of purelysolid nodules (P=0.013). The risk of malignancy of nodules with minimal cystic changes was also lower than that of purely solid nodules in the group of hypoechoic nodules (P=0.063) and in the group of nodules with suspicious US features (P=0.028), but was not significantly different from that of partially cystic nodules regardless of echogenicity or the presence of suspicious US features (PŌēź0.652).
Conclusion:Thyroid nodules with minimal cystic changes have a low risk of malignancy, similar to that of partially cystic nodules regardless of echogenicity or the presence of suspicious US features. The US lexicon could define solid nodules as nodules with purely solid internal content in order to enhance the accuracy of estimated risks of malignancy.
Ultrasonography (US) is the primary tool used to estimate the risk of malignancy of thyroid nodules. Moreover, US plays an essential role in decisions regarding fine-needle aspiration (FNA) and a complementary role in decisions regarding medical management after FNA is performed in thyroid nodules [1-5]. Recent meta-analyses [6-8] have consistently demonstrated that the gray-scale US features of microcalcification, microlobulated/spiculated (irregular) margins, and a nonparallel orientation (taller-than-wide shape) are strongly predictive of malignancy with a very high specificity, while the US features of solid internal content and hypoechogenicity are also independently predictive of malignancy with an intermediate specificity for thyroid malignancy.
The definition of solid internal content has not been standardized, and the lexicon of solid internal content is defined as purely solid internal content [9,10], nodules composed entirely or nearly entirely of soft tissue with only a few tiny cystic spaces [11], and nodules with a cystic portion comprising less than 10% of their volume [12,13]. Considering the role of the US lexicon in estimating the risk of malignancy of thyroid nodules, the US lexicon should include definitions that optimize the predictability of malignancy or benignity. However, to the best of our knowledge, the prevalence and risk of malignancy of nodules with minimal cystic changes have not been investigated.
The aim of this study was to determine the risk of malignancy of thyroid nodules with minimal cystic changes.
The Institutional Review Board (Human Medical Imaging and Intervention Center, Seoul National University Hospital) approved this retrospective study, and the requirement to obtain informed consent was waived.
A total of consecutive 1,000 thyroid nodules from 842 patients (635 women, 207 men; mean age, 52┬▒11.7 years) were included in this study. A total of 1,000 nodules with final diagnoses were selected from 1,943 thyroid nodules (Ōēź1 cm) of patients who underwent FNA or core needle biopsy (CNB) between January 2010 and May 2011 at two institutions. We excluded 943 nodules because the final diagnosis was not obtained in 937 nodules and the US characteristics could not be analyzed in six isolated macrocalcifications (entirely calcified nodules).
The final diagnosis of malignant tumors was made surgically. The final diagnosis of benign nodules was determined by (1) the pathological results of surgical resection, (2) benign cytology results from FNA or CNB that had been repeated at least twice, or (3) an initial benign result from FNA or CNB and a decreased or stable nodule size at a US follow-up performed more than 12 months after the original procedure.
High-resolution US scanning using a 10-12 MHz or 5-14 MHz linear-array transducer (AplioXG, Toshiba, Otawarashi, Japan; iU22, Philips Medical Systems, Bothell, WA, USA) was employed. The sonograms were retrospectively reviewed at two sessions with an interval of six months by one experienced thyroid radiologist (D.G.N., who had 19 years of experience in performing thyroid US and interventional procedures, respectively, at the time of the analysis). The reviewer, who had no previous knowledge of the FNA results or the final diagnoses, assessed the US features of internal content, echogenicity, and suspicious US features including microcalcification, nonparallel orientation, and spiculated/microlobulated margins [12]. At the first review session, the internal content of each thyroid nodule was categorized as solid (cystic portion Ōēż10%) and partially cystic (cystic portion >10%). At the second review session, the solid nodules (cystic portion Ōēż10%) identified in the previous review session were reclassified into purely solid nodules and nodules with minimal cystic changes in a blind fashion (Figs. 1, 2). Nodules were classified as having minimal cystic changes when an anechoic cystic portion was clearly found within a nodule. Echogenicity was categorized as hypoechogenicity (marked or mild hypoechogenicity) and isohyperechogenicity (isoechogenicity or hyperechogenicity) according to the predominant pattern of echogenicity in comparison to the normal thyroid gland and anterior neck muscle.
US-guided FNA was routinely performed for thyroid nodules greater than 1 cm, with the exception of pure cystic nodules, partially cystic nodules with comet-tail artifacts, and spongiform nodules [12]. FNA was performed using the conventional method and at least two samples were obtained from each nodule [14]. CNB was performed using a disposable 18-gauge single or double-action spring-activated needle (approximately 1 or 2 cm excursion; TSK Acecut or Stericut, Create Medic, Yokohama, Japan), as described elsewhere [15]. The interpretation of FNA was based on the Bethesda System for Reporting Thyroid Cytopathology [1] and the interpretation of CNB was based on a CNB pathology reporting system [16].
We assessed the risk of malignancy of nodules with minimal cystic changes in the thyroid nodules overall and according to echogenicity and the presence of any suspicious US features (microcalcification, nonparallel orientation, and spiculated/microlobulated margins). The chi-square test or Fisher exact test was used to identify associations between the US features of internal content and malignancy, as well as to compare the risk of malignancy of nodules according to the internal content of each group as categorized by echogenicity and the presence of any suspicious US features. The statistical analysis was performed using SPSS ver. 19.0 for Windows (IBM Co., Armonk, NY, USA), and P-values <0.05 were considered to indicate statistical significance.
The maximal size of nodules ranged from 10 to 87 mm (mean size, 18.3┬▒9.5 mm). The final diagnoses of the 1,000 nodules included in this study were 883 benign nodules (88.3%) (859 benign non-neoplastic nodules, 24 benign tumors) and 117 malignant nodules (11.7%). Final diagnoses were determined by surgical resection in 123 of 883 benign nodules (13.8%), including 89 nodular hyperplasias, 21 follicular adenomas, 10 cases of thyroiditis, two hyalinizing trabecular tumors, and one schwannoma. The final diagnosis of malignant tumors was made by surgical resection in all cases, including 105 papillary carcinomas, 11 follicular carcinomas, and one medullar carcinoma.
Table 1 presents the frequency and risk of malignancy of thyroid nodules according to their internal content. The frequency of purely solid, minimally cystic, and partially cystic nodules was 730 (73%), 61 (6.1%), and 209 (20.9%), respectively, with risks of malignancy of 14.8% (108/730), 3.3% (2/61), and 3.3% (7/209), respectively.
Thyroid nodules with minimal cystic changes were found in 6.1% of all nodules, and the frequency of minimal cystic changes was significantly higher in benign nodules than in malignant nodules (6.7% vs. 1.7%, P=0.035). The risk of malignancy of nodules with minimal cystic changes was 3.3%, which was the same as the risk of malignancy of partially cystic nodules and significantly lower than the risk of malignancy (14.8%) of solid nodules (P=0.013).
Table 2 presents the frequency and risk of malignancy of thyroid nodules according to the echogenicity of the nodule. Nodules with minimal cystic changes were found in nine of the 257 hypoechoic nodules (3.5%) and in 52 of the 743 isohyperechoic nodules (7%). The frequency of minimal cystic changes was slightly higher in benign nodules in both hypoechoic and isohyperechoic nodules, but this trend was statistically nonsignificant (P=0.118 and P=0.568, respectively). In hypoechoic nodules, a malignant tumor was not found in nine nodules with minimal cystic changes, and the risk of malignancy of nodules with minimal cystic changes was marginally lower than that of purely solid nodules (0% vs. 29.7%, P=0.063). In isohyperechoic nodules, the risk of malignancy of nodules with minimal cystic changes was similar to that of partially cystic nodules (3.8% vs. 2.7%, P=0.652), and it was lower than the risk of malignancy of purely solid nodules (8.3%), but to a statistically nonsignificant extent (P=0.414).
Table 3 presents the frequency and risk of malignancy of thyroid nodules according to the presence of suspicious US features. Nodules with minimal cystic changes comprised six of the 144 nodules (4.2%) with at least one suspicious US feature and 55 of the 856 nodules (6.4%) without suspicious US features. The frequency of minimal cystic changes was higher in the benign nodules in both the group of nodules with suspicious US features and the group without suspicious US features (P=0.087 and P=0.011, respectively). In nodules with suspicious US features, malignant tumors were not found in the six nodules with minimal cystic changes, and the risk of malignancy of nodules with minimal cystic changes was significantly lower than that of purely solid nodules (0% vs. 50.5%, P=0.028). In the nodules without suspicious US features, the risk of malignancy of nodules with minimal cystic changes was similar to that of partially cystic nodules (3.6% vs. 2.9%, P=0.68), and lower than the risk of malignancy of purely solid nodules (9.2%), but to a statistically nonsignificant extent (P=0.215).
Our data demonstrated that thyroid nodules with minimal cystic changes had a low prevalence (6.1%) and a low risk of malignancy (3.3%). The risk of malignancy of nodules with minimal cystic changes was similar to that of partially cystic nodules, and exhibited a tendency to be lower than the risk of malignancy of purely solid nodules regardless of nodule echogenicity or the presence of suspicious US features.
The US lexicon for thyroid nodules should be clinically useful for risk stratification of malignancy because one of the essential clinical roles of US imaging is to estimate the risk of malignancy of thyroid nodules in order to support decision-making in the management of patients with thyroid nodules. Recently, the US-based risk stratification system has been used for the management of thyroid nodules in clinical practice, and most risk stratification systems categorize thyroid nodules according to the risk of malignancy based on combined US patterns including solidity, echogenicity, and various US features [17-21]. The US feature of solid internal content is an independent US predictor for malignancy and has been used for the risk stratification of thyroid nodules. However, it has not been determined whether the risk of malignancy of nodules with minimal cystic changes is similar to that of purely solid nodules or partially cystic nodules. If the risk of malignancy of thyroid nodules with minimal cystic changes is similar to that of purely solid nodules, the US lexicon definition of solid internal content should include minimal cystic changes; however, if it is similar to the risk of malignancy of partially cystic nodules, solid internal content should be defined as purely solid content.
Accurately determining the presence of minimal cystic changes can be difficult in some nodules because the cystic content may not manifest as a typically anechoic lesion depending on the characteristics of the cyst content, and markedly hypoechoic solid components such as fibrosis may show cyst-like hypoechogenicity [22]. Minimal cystic changes were more commonly found in isohyperechoic nodules, which may be associated with higher tendency for cystic changes in benign nodules and a tendency for minimal cystic changes to be more clearly identified by higher contrast in isohyperechoic nodules than in with hypoechoic nodules.
We analyzed the risk of malignancy of nodules with minimal cystic changes depending on nodule echogenicity and the presence of suspicious US features because those parameters are strongly associated with the risk of malignancy of thyroid nodules [23]. Our data demonstrated that thyroid nodules with minimal cystic changes had a low risk of malignancy, similar to the risk of malignancy of partially cystic nodules. This result suggests that thyroid nodules with minimal cystic changes should be categorized as partially cystic nodules and that the US lexicon definition of solid nodules could be defined as referring to nodules with purely solid internal content. This categorization for internal content may prove helpful in increasing the accuracy of US findings for predicting the risk of malignancy of thyroid nodules.
In our study, hypoechogenicity and suspicious US features showed a tendency to increase the risk of malignancy of purely solid or partially cystic nodules. However, no malignant tumor was found in a minimally cystic nodule with hypoechogenicity or suspicious US features. The most likely explanation for this is the low frequency of minimally cystic nodules with hypoechogenicity or suspicious US features, which comprise less than 1% of overall nodules. Therefore, although the risk of malignancy of minimally cystic nodules with hypoechogenicity or suspicious US features was lower than that of purely solid nodules, the accurate risk of malignancy of these nodules may not have been determined by the present study due to the small number of nodules that were studied.
This study has several limitations. First, the retrospective assessment of static sonograms places an inevitable limitation on the accuracy of the US interpretation of minimal cystic changes. Second, the sonograms were analyzed by one interpreter, meaning that interobserver reliability was not assessed. Third, the accurate risk of malignancy of minimally cystic thyroid nodules with hypoechogenicity or suspicious US features should be determined by a subsequent study incorporating a larger sample.
In conclusion, thyroid nodules with minimal cystic changes have a low risk of malignancy (3.3%), which is similar to the risk of malignancy of partially cystic nodules, regardless of echogenicity or the presence of suspicious US features. In the US lexicon, solid nodules could be defined as nodules with purely solid internal content in order to enhance the accuracy of US in predicting the risk of malignancy of thyroid nodules.
AcknowledgementsThis study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.
References1. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19:1159ŌĆō1165.
2. Lee SW, Lee HJ, Kim HJ, Lee J, Park JY, Kim SH, et al. Combined categorical reporting systems of US and cytology findings for thyroid nodules: guidance on repeat fine-needle aspiration cytology. Radiology 2013;266:956ŌĆō963.
3. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid 2013;23:703ŌĆō708.
4. Moon HJ, Kim EK, Kwak JY. Malignancy risk stratification in thyroid nodules with benign results on cytology: combination of thyroid imaging reporting and data system and Bethesda system. Ann Surg Oncol 2014;21:1898ŌĆō1903.
5. Rosario PW. Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda Category III): importance of ultrasonography and cytological subcategory. Thyroid 2014;24:1115ŌĆō1120.
6. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab 2014;99:1253ŌĆō1263.
7. Remonti LR, Kramer CK, Leitao CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 2015;25:538ŌĆō550.
8. Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol 2014;170:R203ŌĆōR211.
9. Henrichsen TL, Reading CC, Charboneau JW, Donovan DJ, Sebo TJ, Hay ID. Cystic change in thyroid carcinoma: prevalence and estimated volume in 360 carcinomas. J Clin Ultrasound 2010;38:361ŌĆō366.
10. Su HK, Dos Reis LL, Lupo MA, Milas M, Orloff LA, Langer JE, et al. Striving toward standardization of reporting of ultrasound features of thyroid nodules and lymph nodes: a multidisciplinary consensus statement. Thyroid 2014;24:1341ŌĆō1349.
11. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J Am Coll Radiol 2015;12:1272ŌĆō1279.
12. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011;12:1ŌĆō14.
13. Andrioli M, Carzaniga C, Persani L. Standardized ultrasound report for thyroid nodules: the endocrinologist's viewpoint. Eur Thyroid J 2013;2:37ŌĆō48.
14. Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the korean society of thyroid radiology. Korean J Radiol 2015;16:391ŌĆō401.
15. Na DG, Min HS, Lee H, Won JK, Seo HB, Kim JH. Role of core needle biopsy in the management of atypia/follicular lesion of undetermined significance thyroid nodules: comparison with repeat fine-needle Aspiration in subcategory nodules. Eur Thyroid J 2015;4:189ŌĆō196.
16. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med 2015;49:288ŌĆō299.
17. Haugen BR, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2015 Oct 14 [Epub]. http://dx.doi.org/10.1089/thy.2015.0020.
18. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract 2010;16 Suppl 1:1ŌĆō43.
19. Wemeau JL, Sadoul JL, d'Herbomez M, Monpeyssen H, Tramalloni J, Leteurtre E, et al. Guidelines of the French society of endocrinology for the management of thyroid nodules. Ann Endocrinol (Paris) 2011;72:251ŌĆō281.
20. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81 Suppl 1:1ŌĆō122.
21. National Comprehensive Cancer Network. 2014 Practice Guidelines in Oncology: Thyroid Carcinoma v.2 [Internet] Fort Washington: National Comprehensive Cancer Network, 2014. [cited 2015 May 18]. Available from: http://www.nccn.org/.
An isoechoic nodule with minimal cystic changes in a 47-year-old woman.The isoechoic nodule (40 mm) has a smooth margin, ovoid shape, parallel orientation, and focal minimal cystic change (arrow). The final diagnosis was benign follicular nodule.
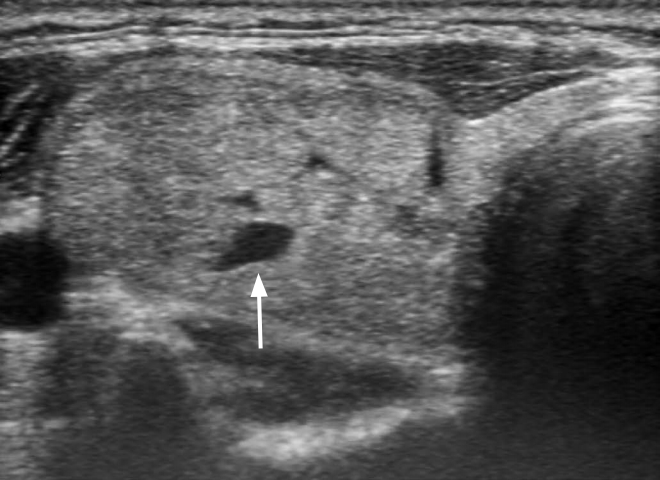 Fig.┬Ā1.A hypoechoic nodule with minimal cystic changes in a 50-year-old woman.The mildly hypoechoic nodule (30 mm) has a smooth margin, ovoid shape, parallel orientation, and focal minimal cystic change (arrow). The final diagnosis was benign follicular nodule.
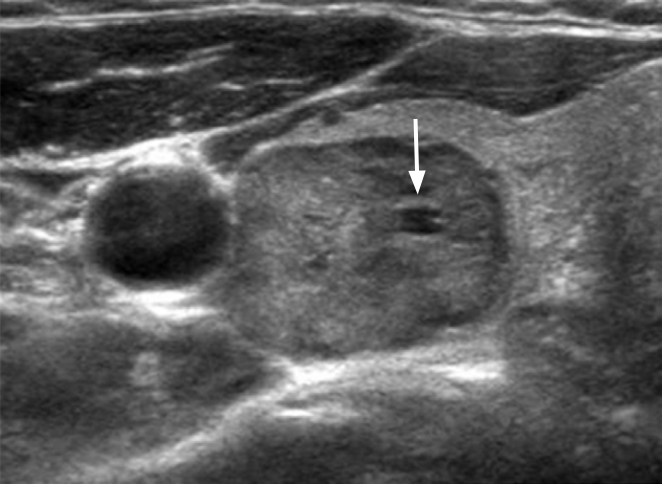 Fig.┬Ā2.Table┬Ā1.The frequency and risk of malignancy of thyroid nodules according to internal content
Table┬Ā2. The frequency and risk of malignancy of thyroid nodules according to internal content and echogenicity
Table┬Ā3.The frequency and risk of malignancy of thyroid nodules according to internal content and the presence of suspicious ultrasound (US) featuresa)
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




