AbstractPurposeThis study aimed to assess the diagnostic performance of supersonic impulse (SSI) elastography in differentiating malignant and benign cervical lymph nodes.
MethodsThe Medline, Embase, and Cochrane Central databases were searched until December 1, 2020. Two different reviewers checked the studies and extracted the data. The diagnostic yields were quantitatively synthesized using a Bayesian bivariate model with an integrated nested Laplace approximation in R.
ResultsIn total, 590 patients with 892 cervical lymph nodes who underwent SSI elastography were included. The total prevalence of malignancy was 33.7% (301/892), and the four elastic modulus values (mean, maximum, minimum, and standard deviation) were significantly different between malignant and benign lymph nodes. For the mean elastic modulus, the summary estimates for sensitivity and specificity were 0.720 (95% credible interval [CrI], 0.592 to 0.824) and 0.877 (95% CrI, 0.727 to 0.969), respectively. The estimated area under the curve (AUC) was 0.845 (95% CrI, 0.672 to 0.914). For the maximum elastic modulus, the sensitivity and specificity were estimated to be 0.809 (95% CrI, 0.698 to 0.899) and 0.816 (95% CrI, 0.643 to 0.924), respectively. The estimated AUC was 0.834 (95% CrI, 0.579 to 0.938). The minimum and standard deviation of the elastic modulus and the outcomes of the positive and negative likelihood ratio, diagnostic odds ratio, and risk difference were also calculated.
The assessment of the status of cervical lymph nodes is critical in clinical decision-making, especially in terms of whether they are malignant or benign. Cervical lymph node assessment can predict the prognosis and contribute to the management plans of patients with primary tumors in other places [1]. Conventional ultrasonography (US) is the first choice of imaging modality for diagnosing malignant cervical lymph nodes preoperatively. It can characterize the distribution and morphology of superficial lymph nodes, including their shape, border, internal structure, and vascularity [2]. However, there are no standardized criteria for US in the diagnosis of malignant lymph nodes with satisfactory sensitivity and specificity [3,4].
In recent years, US elastography has been introduced to measure the elasticity of lymph nodes, which is a parameter based on the strain or deformation in response to a physical force related to the degree of malignancy, as most malignancies reliably exhibit reduced elasticity and increased stiffness due to the nature of neoplastic growth [5]. Elastography can be divided into quasi-static elastography and shear wave elastography, including supersonic shear impulse (SSI) elastography and acoustic radiation force impulse (ARFI) elastography. Quasi-static elastography involves collecting numerical data with a quasi-static method, such as the elasticity score, which is a qualitative classification, and the strain index or the muscle-to-lymph node strain ratio, which are often considered as semiquantitative; this technique is widespread and has been extensively studied. A meta-analysis of quasi-static elastography demonstrated that the summary sensitivity and specificity for diagnosing superficial malignant lymph nodes were 0.74 and 0.90 for the elasticity score and 0.88 and 0.81 for the strain ratio, respectively [1]. SSI elastography is an objective and quantitative modality, which is less operator-dependent. SSI elastography analyzes tissue stiffness using the elastic modulus (kPa), which provides absolute quantification of lymph node elasticity [6], while ARFI uses the shear wave speed (m/s) for the quantitative analysis. In addition, SSI elastography does not require freehand compression, making it independent of the compression technique [6].
A previous systematic review reported that the sensitivity and specificity of shear wave elastography in the diagnosis of cervical lymph nodes were 0.81 and 0.85, respectively [3]. However, that study used the maximum elastic modulus for SSI and shear wave velocity (m/s) for ARFI as a cutoff value, and did not systematically provide results for the maximum, mean, minimum, and standard deviation (SD) values of the elastic modulus. Furthermore, studies of SSI elastography for evaluating cervical lymph nodes have been carried out in small and selected patient samples, and have had observational designs. Therefore, this study was conducted to provide new clinical evidence regarding differences in elastic modulus values using SSI elastography between malignant and benign cervical lymph nodes and the diagnostic performance of SSI elastography for diagnosing malignant cervical lymph nodes.
This systematic review and meta-analysis of diagnostic test accuracy was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) extension for diagnostic test accuracy statement [7]. This systematic review and meta-analysis was registered with PROSPERO (https://www.crd.york.ac.uk/prospero/) with an ID of CRD42020165574.
A systematic search was conducted in PubMed, Embase, and Cochrane Library Central Register of Controlled Trials databases up to December 1, 2020. The search strategy employed Boolean logic to merge discrepant concepts and synonyms. The search terms were the following: (cervical lymph node OR neck lymph node OR cervical lymphadenopathy OR neck node OR cervical node) AND (elastography OR sonoelastography OR sonoelastography OR supersonic imaging OR supersonic shear imaging OR supersonic shear wave elastography OR supersonic shear-wave elastography). Two independent investigators first screened the title and abstract of each article. The references of the eligible articles and reviews were then screened.
First, English studies or their subsets that reported SSI elastography data as a predictor of malignant cervical lymph nodes, were eligible for inclusion. Then, the final diagnoses of cervical lymph nodes were determined based on histopathology reports after surgery or fine-needle aspiration biopsy. The exclusion criteria were as follows: (1) articles that did not fall within the scope of this review; (2) review articles, editorials or letters, comments, and conference proceedings; (3) case reports or case series.
Descriptive data were extracted by one investigator and then confirmed by another researcher. The extracted descriptive data included the study characteristics (authors, year of publication, region, study design, duration of patient recruitment, and sample size), clinical characteristics (the number of lymph nodes, both benign and malignant, patient age, type of primary tumor, elastic modulus [kPa] and cutoff values), and diagnostic accuracy test characteristics. Two reviewers independently extracted the numerical data. Inconsistent extractions were resolved by consensus. When the data were not extractable, an attempt was made to contact the corresponding authors for additional data.
Two reviewers independently assessed the risk of bias and concerns about applicability based on the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [8]. Discrepancies were resolved by consensus.
For all mean, maximum, minimum, and SD values of the elastic modulus, the mean difference (MD) with a 95% confidence interval (CI) was analyzed. Heterogeneity was examined using the Q-test and the I2 statistic. When studies were heterogeneous (P<0.1 or I2>50%), a random effects model was used; otherwise, a fixed effects model was applied [9].
For diagnostic characteristics, a Bayesian bivariate model was implemented using integrated nested Laplace approximation (INLA) [10]. Accurate posterior marginal distributions for sensitivity and specificity, as well as all hyperparameters and covariates, were directly obtained by a bivariate model with no need for conventional Markov-chain Monte Carlo sampling [11,12]. Further, univariate estimates of sensitivity and specificity with 95% credible intervals (CrIs), as well as the summary receiver operating characteristic (SROC) curve, were directly available for interpretation. Moreover, the area under the receiver operating characteristic curve (AUC) values with 95% CrIs were calculated. The summary positive and negative likelihood ratios (LR+ and LR-, respectively) were calculated from the summary sensitivity and specificity estimates, and the same procedure was carried out for diagnostic odds ratios (ORs) and risk difference (RD). Alternative models were compared using the deviance information criterion (DIC), with a lower DIC considered to be better. In model 1, the sensitivity and specificity were modeled in the bivariate model. Models 2, 3, and 4 indicate that the sensitivity and false-negative rate (1-specificity), false-positive rate (1-sensitivity) and specificity, false-positive rate (1-sensitivity) and false-negative rate (1-specificity) were modeled in the bivariate model, respectively. The threshold effect was tested using Spearman correlations, and a P-value less than 0.05 was considered to indicate a significant threshold effect. Funnel plot asymmetry was also examined to enable a valid assessment of the extent and impact of publication and selective reporting bias in studies of diagnostic accuracy. Subgroup analyses were performed according to the study design (prospective or retrospective), age of included patients (over or less than the median age), histopathology (papillary thyroid cancer or other carcinoma), and prevalence of malignancy (over or less than the median rate).
All analyses were conducted using R software version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org) with the R package meta (4.18-2), meta4diag (2.0.8), and INLA (21.02.23) with their required packages.
After excluding duplicate results and abstract screening, the full texts of potentially eligible publications were reviewed. After applying the exclusion criteria, 10 studies were included in this systematic review. The study selection process is described in Fig. 1.
Ten eligible articles with 590 patients (333 women and 257 men) were included in the study [13-22]. Seven of them were designed prospectively, and three had retrospective designs. Of 892 cervical lymph nodes, 301 (33.7%) were malignant. The prevalence of malignancy ranged from 19.5% to 60.7%, with a median of 29.7%. SSI elastography data included mean, maximum, minimum, and SD values of the elastic modulus, which were provided in six, nine, four, and three articles, respectively. The detailed characteristics of the 10 included studies are summarized in Table 1.
Two reviewers independently assessed the risk of bias and concerns about applicability based on the QUADAS-2 [8]. The results of the quality assessment using the QUADAS-2 tool are shown in Fig. 2. Overall, the applicability of the included studies was moderate to high, and all the studies satisfied at least four of the seven items.
Comparisons of the mean, maximum, minimum, and SD values of the elastic modulus between malignant and benign lymph nodes are displayed in Fig. 3. The pooled differences of all elastic modulus values were statistically significant (P<0.05). However, the heterogenicity between studies could not be ignored.
For the mean elastic modulus, the summary estimates for sensitivity and specificity were 0.720 (95% CrI, 0.592 to 0.824) and 0.877 (95% CrI, 0.727 to 0.969), respectively. The summary estimates for LR+ and LR- were 5.79 (95% CrI, 4.02 to 12.49) and 0.31 (95% CrI, 0.23 to 0.47), respectively. The summary estimates for the diagnostic OR and RD were 16.87 (95% CrI, 11.17 to 55.39) and 0.58 (95% CrI, 0.47 to 0.72), respectively. The estimated AUC was 0.845 (95% CrI, 0.672 to 0.914). There was no threshold effect according to Spearman correlation analysis (P=0.064). For the maximum elastic modulus, the summary estimates for sensitivity and specificity were 0.809 (95% CrI, 0.698 to 0.899) and 0.816 (95% CrI, 0.643 to 0.924), respectively. The summary estimates for LR+ and LR- were 5.78 (95% CrI, 3.15 to 11.82) and 0.24 (95% CrI, 0.13 to 0.34), respectively. The summary estimates for OR and RD were 27.67 (95% CrI, 11.15 to 65.75) and 0.67 (95% CrI, 0.54 to 0.76), respectively. The estimated AUC was 0.834 (95% CrI, 0.579 to 0.938). No threshold effect was found (P=0.699). The details regarding the minimum and SD values of the elastic modulus are shown in Table 2. The corresponding forest and SROC plots are displayed in Figs. 4 and 5, respectively.
Data for the mean and maximum values of elastic modulus were divided into subgroups for further analysis. For thyroid cancer, the summary estimates for sensitivity and specificity were 0.741 (0.616-0.834) and 0.870 (0.583-0.979), respectively. The summary estimates for LR+ and LR- were 11.75 (95% CrI, 7.55 to 28.19) and 0.30 (95% CrI, 0.28 to 0.40), respectively. The summary estimates for the OR and RD were 41.68 (95% CrI, 19.22 to 94.96) and 0.67 (95% CrI, 0.55 to 0.69), respectively. The estimated AUC was 0.768 (95% CrI, 0.381 to 0.859). A threshold effect was found (P<0.001). For the maximum elastic modulus value, the summary estimates for sensitivity and specificity were 0.808 (0.711-0.878) and 0.814 (0.372-0.975), respectively. The summary estimates for LR+ and LR- were 6.14 (95% CrI, 5.59 to 12.65) and 0.16 (95% CrI, 0.14 to 0.25), respectively. The summary estimates for the OR and RD were 39.04 (95% CrI, 22.35 to 87.87) and 0.72 (95% CrI, 0.64 to 0.80), respectively. The estimated AUC was 0.913 (95% CrI, 0.889 to 0.959). No threshold effect was found (P=0.800). Additional subgroup analyses regarding study design, other cancers, age, and malignancy prevalence are shown in Tables 3 and 4.
The rising incidence of head and neck cancers requires better operative imaging assessments of the neck region to facilitate surgical decision-making. As an emerging technology, SSI is a shear wave-based elastography technique that is based on the use of an acoustic radiation force to generate a shear wave [23]. ARFI is another technique in this category; however, these techniques are different in terms of the frame rate and the strength of the acoustic radiation force [23]. SSI generates superior impulses that induce and amplify shear waves, which are then tracked throughout the entire window using ultrafast US tracking impulses, while ARFI generates an impulse with a single pushing beam [5,6]. As mentioned in a previous meta-analysis, shear wave elastography (including SSI and ARFI) is an acceptable imaging method for diagnosing malignant cervical lymph nodes, with a sensitivity of 0.81 (95% CI, 0.72 to 0.88) and a specificity of 85% (95% CI, 0.70 to 0.93) [3]. However, the results of SSI elastography from the subgroup analysis showed a sensitivity of 0.80 (95% CI, 0.69 to 0.91) and a specificity of 0.84 (95% CI, 0.67 to 1.00), although only four original articles were included. In addition, the previous meta-analysis only considered the diagnostic performance of the maximum elastic modulus value.
The current systematic review and meta-analysis first summarized the differences of elastic modulus values (mean, maximum, minimum, and SD) between malignant and benign cervical lymph nodes. Although all elastic modulus values were significantly different, heterogeneity was also obvious. No difference was found between malignant and benign cervical lymph nodes in 28.6% (2/7) of the articles for the mean elastic modulus, 11.1% (1/9) for the maximum elastic modulus, 60% (3/5) for the minimum elastic modulus, and 0% (0/3) for the SD of the elastic modulus. These findings indicate that not all parts of malignant lymph nodes were always significantly stiffer than benign lymph nodes, especially for the softest parts. Of course, according to these results, the fact that the maximum elastic modulus of malignant and benign lymph nodes was significantly different should be undisputed, and this finding aligns with the hypothesis that the heterogeneous histology of lymph node metastasis is more accurately reflected by the maximum value for detecting focal cortical metastasis or metastasis where the lymph node is entirely replaced by tumor cells [19,21]. It can also be concluded that the variation in the elastic modulus inside the malignant lymph nodes was significantly larger than that in the benign lymph nodes. Bhatia et al. also found that malignant lymph nodes were more likely to have heterogeneous elasticity, even when the nodes appeared uniform on US [22]. Nonetheless, the present results for the MD between malignant and benign cervical lymph nodes regarding each elastic modulus value could be helpful for building a future consensus about cutoff values.
According to the present meta-analysis, the mean elastic modulus showed potential superiority relative to the maximum elastic modulus regarding AUC (0.845 vs. 0.834), but the mean elastic modulus had lower sensitivity (0.720 vs. 0.809) and higher specificity (0.877 vs. 0.816). However, this difference might have resulted from a different number of included articles and different tumors, and more importantly, no direct evidence was provided. Many previous articles reported comparisons between the mean and maximum elastic modulus values. Bhatia et al. [22] reported the same AUC 0.77 for both mean and maximum elastic modulus values; however, they had distinct advantages regarding sensitivity (0.484 vs. 0.419) and specificity (0.918 vs. 1.000). Chen et al. [18] found the same trend as in the present study; that is, the AUC of the mean elastic modulus was higher than that of the maximum value (0.879 vs. 0.819), but the sensitivity and specificity were possibly lower (0.846 vs. 1.000) and higher (0.830 vs. 0.500), respectively. Another article by Jung et al. [20] also reported data supporting the findings of the present study that the mean elastic modulus had a higher AUC (0.748 vs. 0.738), lower sensitivity (0.7647 vs. 0.8431), and higher specificity (0.6667 vs. 0.3030). Therefore, the choice between different elastic modulus should depend on the situation and actual primary carcinoma. According to the present results, the mean elastic modulus might be more suitable for usual screening while the maximum elastic modulus might be more useful in preoperative evaluations. Meanwhile, the European Federation for Ultrasound in Medicine and Biology guidelines and recommendations suggest that elastography is unlikely to be suitable for the differential diagnosis, but is more likely to be useful for targeting malignant lymph nodes for fine-needle aspiration if multiple lymph nodes are present [24,25]. The heterogeneity inside metastatic lymph nodes, represented as the SD of the elastic modulus, was also a good parameter for distinguishing malignant and benign lymph nodes; however, further assessments should be conducted in the future, since only three articles reported this value.
A subgroup analysis showed that the diagnostic performance of SSI for lymph node metastasis from thyroid cancer was not significantly different from its performance for other cancers. The intrinsic curvature of the neck and the superficial location of cervical lymph nodes can cause an inhomogeneous stress distribution in the field of observation, although primary thyroid cancer would have advantages in SSI due to its hardness [14,22]. In addition, the older subgroups showed a trend for lower sensitivity, but higher specificity for both mean and maximum elastic modulus values. Older age is usually a risk factor for early metastasis or recurrence, especially for head and neck cancers [26], and elderly patients were more likely to have been selected for fine-needle biopsy at an early stage, which could have contributed to a higher false-negative rate [26,27]. Differences in trends in diagnostic performance according to age could be further investigated by direct comparisons.
Considering that the available studies were all studies with small samples and potential selection bias, a summarized meta-analysis is necessary to elicit results close to the truth. The use of Bayesian theory rather than the I2 method to assess heterogeneity is more precise according to the PRISMA extension for diagnostic test accuracy statement [7]. However, the present meta-analysis has the following limitations. First, only 10 articles were included, and not all articles provided data for all four elastic modulus values, which made it impossible to compare the outcomes directly. Secondly, mixed primary carcinomas still contributed to the bias in the present analysis, and it is hoped that further studies will focus on a single tumor type to improve the better validity.
SSI is an acceptable imaging technology for diagnosing malignant cervical lymph nodes and can play a complementary role currently. Both maximum and mean elastic modulus values should be taken into consideration to make a clinical judgment.
NotesAuthor Contributions Conceptualization: Qiu Y, Luo Y. Data acquisition: Qiu Y, Xing Z, Yang Q. Data analysis or interpretation: Qiu Y, Xing Z. Drafting of the manuscript: Qiu Y, Xing Z. Critical revision of the manuscript: Yang Q, Luo Y. Approval of the final version of the manuscript: all authors. References1. Ying L, Hou Y, Zheng HM, Lin X, Xie ZL, Hu YP. Real-time elastography for the differentiation of benign and malignant superficial lymph nodes: a meta-analysis. Eur J Radiol 2012;81:2576–2584.
3. Suh CH, Choi YJ, Baek JH, Lee JH. The diagnostic performance of shear wave elastography for malignant cervical lymph nodes: a systematic review and meta-analysis. Eur Radiol 2017;27:222–230.
4. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab 2007;92:3590–3594.
5. Saadi R, LaRusso S, Vijay K, Goldenberg D. Elastography as a potential modality for screening cervical lymph nodes in patients with papillary thyroid cancer: a review of literature. Ear Nose Throat J 2018;97:31–39.
6. Choi YJ, Lee JH, Baek JH. Ultrasound elastography for evaluation of cervical lymph nodes. Ultrasonography 2015;34:157–164.
7. McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM; PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018;319:388–396.
8. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536.
9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560.
10. Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Series B Stat Methodol 2009;71:319–392.
11. Guo J, Riebler A, Rue H. Bayesian bivariate meta-analysis of diagnostic test studies with interpretable priors. Stat Med 2017;36:3039–3058.
12. Simpson D, Rue H, Riebler A, Martins TG, Sorbye S. Penalising model component complexity: a principled, practical approach to constructing priors. Stat Sci 2017;32:1–28.
13. Lo WC, Hsu WL, Wang CT, Cheng PW, Liao LJ. Incorporation of shear wave elastography into a prediction model in the assessment of cervical lymph nodes. PLoS One 2019;14:e0221062.
14. Kang HJ, Seo M, Sohn YM, Yun SJ, Min SY, You MW, et al. Comparison of diagnostic performance of B-mode ultrasonography and shear wave elastography in cervical lymph nodes. Ultrasound Q 2019;35:290–296.
15. You J, Chen J, Xiang F, Song Y, Khamis S, Lu C, et al. The value of quantitative shear wave elastography in differentiating the cervical lymph nodes in patients with thyroid nodules. J Med Ultrason (2001) 2018;45:251–259.
16. Kim HJ, Choi IH, Jin SY, Park HK, Byun DW, Suh K, et al. Efficacy of shear-wave elastography for detecting postoperative cervical lymph node metastasis in papillary thyroid carcinoma. Int J Endocrinol 2018;2018:9382649.
17. Chang W, Tang L, Lu C, Wu M, Chen M. Shear wave elastography in the evaluation of level VI lymph nodes in papillary thyroid carcinoma: combined with gray-scale ultrasound ex vivo. BMC Cancer 2018;18:1001.
18. Chen BB, Li J, Guan Y, Xiao WW, Zhao C, Lu TX, et al. The value of shear wave elastography in predicting for undiagnosed small cervical lymph node metastasis in nasopharyngeal carcinoma: a preliminary study. Eur J Radiol 2018;103:19–24.
19. Desmots F, Fakhry N, Mancini J, Reyre A, Vidal V, Jacquier A, et al. Shear wave elastography in head and neck lymph node assessment: image quality and diagnostic impact compared with B-mode and Doppler ultrasonography. Ultrasound Med Biol 2016;42:387–398.
20. Jung WS, Kim JA, Son EJ, Youk JH, Park CS. Shear wave elastography in evaluation of cervical lymph node metastasis of papillary thyroid carcinoma: elasticity index as a prognostic implication. Ann Surg Oncol 2015;22:111–116.
21. Choi YJ, Lee JH, Lim HK, Kim SY, Han MW, Cho KJ, et al. Quantitative shear wave elastography in the evaluation of metastatic cervical lymph nodes. Ultrasound Med Biol 2013;39:935–940.
22. Bhatia KS, Cho CC, Tong CS, Yuen EH, Ahuja AT. Shear wave elasticity imaging of cervical lymph nodes. Ultrasound Med Biol 2012;38:195–201.
23. Suh CH, Kim SY, Kim KW, Lim YS, Lee SJ, Lee MG, et al. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology 2014;271:895–900.
24. Saftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, Amy D, et al. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: update 2018. Ultraschall Med 2019;40:425–453.
25. Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy 2007;39:952–957.
26. Dong W, Horiuchi K, Tokumitsu H, Sakamoto A, Noguchi E, Ueda Y, et al. Time-varying pattern of mortality and recurrence from papillary thyroid cancer: lessons from a long-term follow-up. Thyroid 2019;29:802–808.
27. Sengul D, Sengul I, Van Slycke S. Risk stratification of the thyroid nodule with Bethesda indeterminate cytology, category III, IV, V on the one surgeon-performed US-guided fine-needle aspiration with 27-gauge needle, verified by histopathology of thyroidectomy: the additional value of one surgeon-performed elastography. Acta Chir Belg 2019;119:38–46.
Quality assessment of the included studies according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria.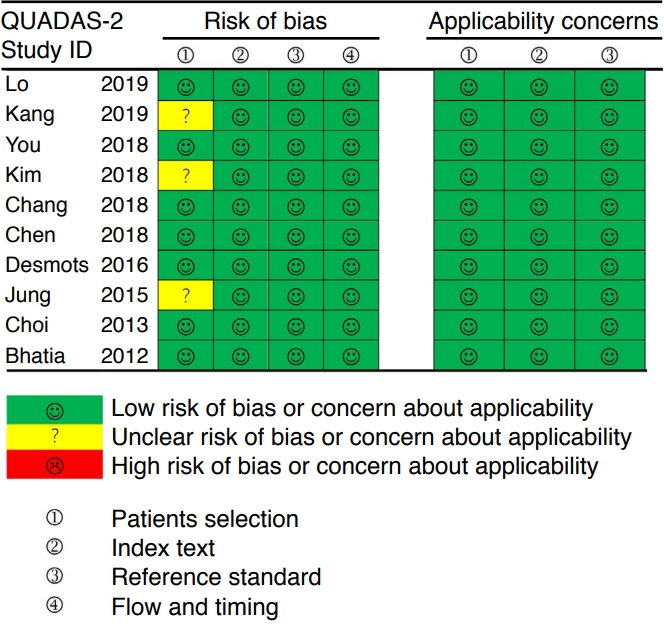 Fig. 2.Mean differences between malignant and benign cervical lymph nodes according to the mean (A), maximum (B), minimum (C), and standard deviation (SD) (D) of the elastic modulus.CI, confidence interval.
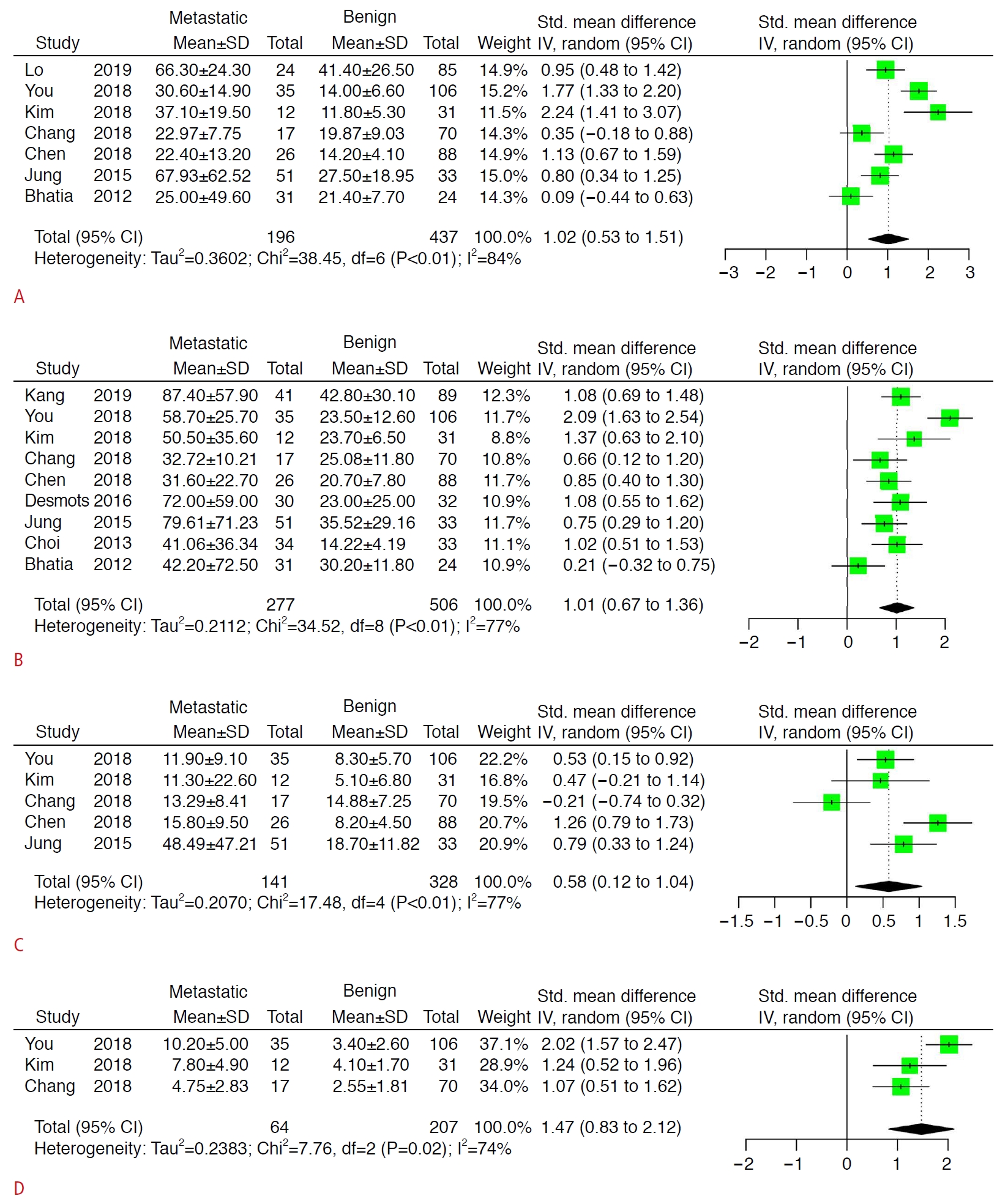 Fig. 3.Estimates of sensitivity and specificity for the mean (A), maximum (B), minimum (C), and standard deviation (D) of the elastic modulus.CrI, credible interval.
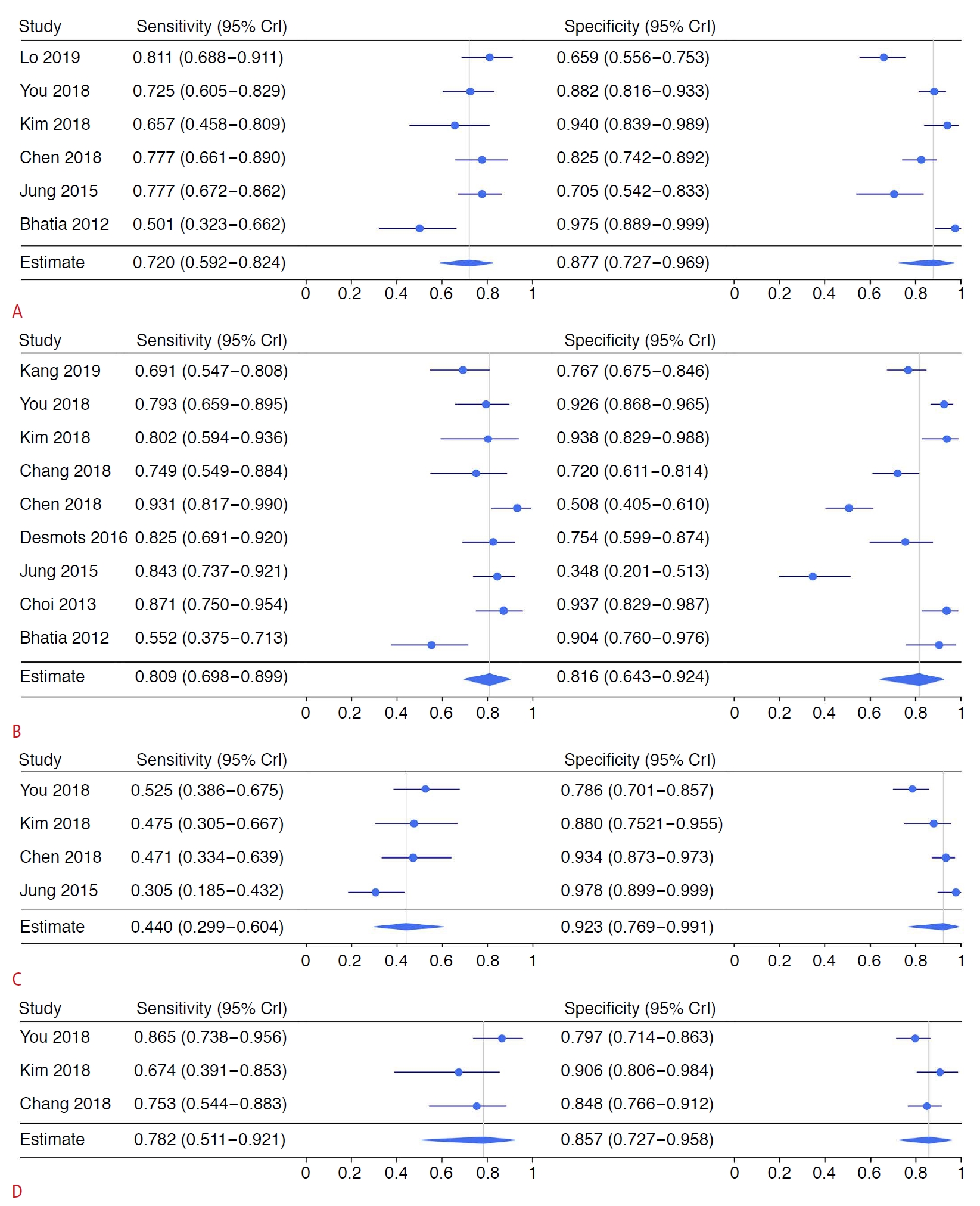 Fig. 4.Summary receiver operating characteristic (SROC) curves for the mean (A), maximum (B), minimum (C), and standard deviation (D) of the elastic modulus.The SROC curve shows individual study posterior-point estimates (the size of each circle is proportional to the sample size for each study). The dashed elliptical boundary represents the 95% credible region for the summary estimates (closed diamond). The standard (black) and latent-class model analyses based on the conditional dependence model (blue) and the conditional independence model (red) are presented.
 Fig. 5.Table 1.Characteristics of the included studies
Table 2.Estimates of diagnostic accuracy from the included studies
SSI, supersonic shear imaging; AUC, area under the summary receiver operating characteristic curve; CrI, credible interval; LR+, positive likelihood ratio; LR-, negative likelihood ratio; OR, diagnostic odds ratio; RD, risk difference; Max, maximum; Min, minimum; SD, standard deviation. a) Due to the absence of data, Chan et al’s study [17] was omitted from SSI index of Mean and Min. Table 3.Subgroup analysis of the differences in SSI index values between malignant and benign lymph nodes Table 4.Subgroup analysis of the differences in SSI index values between the diagnostic accuracy (sensitivity and specificity) |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




