AbstractPurposeThis study aimed to determine whether the normal parotid gland (PG) and submandibular gland (SMG) can be used as reference standards for normal thyroid echogenicity.
MethodsIn total, 1,302 consecutive patients with normal salivary glands were included in this study. The echogenicity of the SMG and PG was assessed during real-time ultrasound examinations, and the glands were categorized as hyperechogenic, isoechogenic, and hypoechogenic relative to the thyroid parenchyma in patients without diffuse thyroid disease (group 1, n=1,106) and with diffuse thyroid disease (group 2, n=196). The frequency of the echogenicity categories of the normal PG and SMG was assessed according to patientsŌĆÖ age.
ResultsIn group 1, the normal PG showed isoechogenicity in 94.0% and hypoechogenicity or hyperechogenicity in 6.0%, and the normal SMG showed isoechogenicity in 73.6% and hypoechogenicity in 26.4% of patients (P<0.001). There was no significant association of the frequency of isoechoic PG with age (P=0.834); however, there was a trend for an increasing frequency of isoechoic SMG with aging (22.9%-81.4%) (P<0.001). Similar findings were found in group 2 patients without decreased thyroid echogenicity.
ConclusionThe normal PG was mostly isoechoic to the normal thyroid parenchyma, whereas the normal SMG showed hypoechogenicity at various frequencies according to age. The echogenicity of the normal PG can be used as an alternative reference standard for normal thyroid echogenicity; however, the normal SMG is not suitable for a reference standard when assessing thyroid nodule echogenicity in patients who have diffuse thyroid disease with decreased parenchymal echogenicity.
The ultrasound (US) echogenicity of thyroid nodules has been used to stratify the risk of malignancy in various risk stratification systems and Thyroid Imaging Reporting and Data Systems (TI-RADSs) [1-6]. The echogenicity of a thyroid nodule is determined by the reference structures of the strap or anterior neck muscle and thyroid parenchyma in TI-RADSs [3-7]. The American College of Radiology (ACR) TI-RADS and Chinese (C)-TI-RADS recommend determining nodule echogenicity by comparing it with the adjacent thyroid parenchyma and describing the abnormal thyroid echogenicity in patients who have diffuse thyroid disease with decreased parenchymal echogenicity [3,5,7]. However, the true echogenicity of a nodule may not be accurately assessed by this recommendation, and a hypoechoic nodule can be misclassified as an isoechoic nodule when the thyroid parenchyma shows hypoechogenicity similar to the nodule echogenicity. The recently revised 2021 Korean (K)-TI-RADS adopted the presumed normal thyroid echogenicity for the reference structure instead of the adjacent thyroid parenchyma to avoid the possible misclassification in case of diffuse thyroid disease with decreased parenchymal echogenicity [6].
The European TI-RADS and the Italian US reporting system recommend using a normal submandibular gland (SMG) as an alternative reference standard for normal thyroid echogenicity to describe nodule echogenicity in patients with decreased thyroid parenchymal echogenicity [4,8], and a few studies [9,10] have used the echogenicity of the normal SMG as a reference for the determination of normal thyroid echogenicity in the evaluation of diffuse thyroid disease. The use of a normal SMG as the alternative reference structure for normal thyroid echogenicity is based on the assumption that the normal SMG has a homogeneous hyperechogenicity similar to that of the normal thyroid gland [11-13]; however, this assumption has not been validated.
The normal parotid gland (PG) and SMG have been generally considered to have homogeneous hyperechogenicity comparable to the echogenicity of the normal thyroid gland [12,14]. A recent study [15] demonstrated that 27.0% of normal SMGs showed hypoechogenicity compared with normal PGs in adults and reported that the normal SMG may exhibit physiologic hypoechogenicity. However, in the previous study [15], the echogenicity of the normal PG and SMG was not compared to the normal thyroid gland, and a limitation of that study was that it could not prove whether normal echogenicity of the PG was similar to that of the normal thyroid gland. To the authorsŌĆÖ knowledge, no previous study has investigated whether the normal PG or SMG has similar echogenicity to that of the normal thyroid gland. Therefore, the aim of this study was to determine whether the normal PG and SMG have a similar echogenicity to that of normal thyroid parenchyma and whether these glands can be used as an alternative reference standard for normal thyroid echogenicity when assessing the echogenicity of thyroid nodules in patients who have diffuse thyroid disease with decreased parenchymal echogenicity.
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by GangNeung Asan Hospital Institutional Review Board (2020-02-009) with a waiver of the requirement for informed consent.
Between November 2018 and July 2019, 1,844 consecutive patients underwent neck US for thyroid nodules (n=987), postoperative surveillance of thyroid cancer (n=439), cervical lymphadenopathy (n=177), suspected diffuse thyroid disease (n=87), neck mass (n=47), salivary gland mass (n=39), goiter (n=30), neck pain or discomfort (n=26), suspected parathyroid disease (n=8), and voice change (n=4). This study included only patients with normal salivary glands among those who underwent neck US during the study period.
Patients with normal salivary glands were defined as those who had no clinical history of salivary disease and no suspicion of focal or diffuse salivary gland disease on clinical and US assessments. Chronic sialadenitis, including Sj├Čgren syndrome or IgG4-related disease, was suspected on US upon the presence of any suspicious US feature (heterogeneous echotexture, nodular hypoechoic lesions, asymmetrical echogenicity, glandular enlargement, or atrophic change) [16-18]. Bilateral symmetrical homogeneous hypoechogenicity or hyperechogenicity compared to the normal thyroid parenchyma was considered indicative of a normal US feature of the PG or SMG, if the patient had no clinical history or symptoms of salivary gland disease and no suspicious US features for chronic sialadenitis. Patients without diffuse thyroid disease were defined as those who had no clinical history of diffuse thyroid disease and no suspicion of diffuse thyroid disease on clinical and US assessments. The thyroid parenchyma was determined to be normal on US when the echogenicity of the thyroid parenchyma showed typical homogeneous hyperechogenicity of the presumed normal thyroid gland. Patients with diffuse thyroid disease were defined as individuals who had a clinical history of diffuse thyroid disease or thyroid hormonal abnormality or any suspicious US feature of diffuse thyroid disease (decreased parenchymal echogenicity, coarse or nodular parenchymal echotexture, marginal nodularity, or increased or decreased parenchymal vascularity) [8,19,20].
We excluded 542 patients after reviewing their electronic medical records and assessing the US features of the thyroid and salivary glands. The exclusion criteria were as follows: (1) patients who underwent thyroid surgery (n=439), (2) patients who underwent salivary gland surgery or had salivary gland disease (focal or diffuse) (n=68), (3) patients who underwent radiation therapy on the neck or were not eligible for US evaluation (n=35) (Fig. 1). Following the exclusion of 542 patients, the study population comprised 1,106 patients without diffuse thyroid disease (group 1) and 196 patients with diffuse thyroid disease on clinical or US assessments (group 2), who had a clinical history of diffuse thyroid disease or thyroid hormonal abnormality (n=188) or suspected diffuse thyroid disease on US only (n=8). The patients in group 2 were classified into patients without decreased thyroid echogenicity (n=74) and patients with decreased thyroid echogenicity (n=122) upon US assessment. All patients in each group were categorized by age into four groups (age group 1, 0-20 years; age group 2, 21-40 years; age group 3, 41-60 years; age group 4, >60 years).
The interobserver agreement study included 273 patients for the thyroid gland and 163 patients for normal salivary glands among the consecutive 362 patients who underwent neck US between March and April 2020. We excluded 89 patients for the interobserver agreement study of the thyroid gland and 110 patients for the interobserver agreement study of normal salivary glands (Fig. 2).
All US examinations were performed with a 5-12 MHz linear-array transducer (EPIQ7, Philips Healthcare, Bothell, WA, USA). The US echogenicity of the thyroid gland, PG, and SMG was assessed during a real-time US examination by one experienced radiologist (D.G.N.) with 22 years of experience in performing neck US. Both the PG and SMG were examined with the patient in a supine position with the head turned to the contralateral side. We assessed the echogenicity of the thyroid gland, PG, and SMG in each patient with the same US scan parameters, including the level of gain, time gain compensation, and the application of compound imaging (SonoCT). The echogenicity of the PG was determined in the superficial region of the superficial lobe in consideration of the attenuation of US waves in deep glandular tissue. The observer determined the echogenicity of the thyroid parenchyma with the reference standard of presumed normal thyroid echogenicity (typical homogeneous hyperechogenicity). The patients with diffuse thyroid disease were classified as those with or without decreased thyroid echogenicity on US assessment regardless of the echotexture of the thyroid parenchyma. The observer compared the degree of echogenicity of the PG and SMG to that of the thyroid parenchyma; the assessed echogenicity of PG and SMG was categorized as hyperechogenicity, isoechogenicity, and hypoechogenicity compared to the echogenicity of the thyroid parenchyma. Isoechogenicity was determined when the echogenicity of PG and SMG were similar to the echogenicity of the thyroid parenchyma, and hypoechogenicity or hyperechogenicity of salivary glands was determined when the echogenicity was obviously different from the echogenicity of the thyroid parenchyma.
We performed the interobserver agreement study for the echogenicity of the thyroid and normal salivary glands between two operators (one experienced radiologist [D.G.N.] and a third-year resident [C.I.S.] with 1 year of experience in general US and 1 month of experience dedicated to neck US). The resident participated in the interobserver study after a month of training on assessing the echogenicity of the normal thyroid parenchyma, PG, and SMG. The interobserver agreement was compared between experienced and less experienced operators because the thyroid US examinations are widely used by less experienced primary care physicians. In the interobserver agreement study, two observers independently assessed the echogenicity of the thyroid parenchyma and salivary glands during real-time US examinations. The observers determined the echogenicity of the thyroid gland in comparison with the typical homogeneous hyperechogenicity of the presumed normal thyroid gland and classified the echogenicity as normal or abnormal thyroid echogenicity (decreased or heterogeneous echogenicity). The observers also independently assessed the echogenicity of the PG and SMG and categorized it into hyperechogenicity, isoechogenicity, and hypoechogenicity in comparison to the echogenicity of the normal thyroid parenchyma when the observer determined that the echogenicity of the thyroid gland was normal.
The ages of patients are presented using the median (interquartile range [IQR]) due to their non-parametric distribution and were compared between group 1 and group 2 by using the Mann-Whitney U test. Categorical variables are reported as frequencies and percentages for each category. The chi-square test or the Fisher exact test was used to compare sex and other categorical variables between the two study groups and subgroups. The McNemar test was used to compare the frequency of isoechogenicity of the salivary glands between the PG and SMG. The association between age groups and the frequency of isoechogenicity of PG and SMG was investigated by the chi-square test for trend.
Interobserver agreement was assessed using the Cohen ╬║ statistics [21]. The strength of the agreement was defined as follows: 0.81-1.00, almost perfect agreement; 0.61-0.80, substantial agreement; 0.41-0.60, moderate agreement; 0.21-0.40, fair agreement; and 0.00-0.20, slight agreement. The statistical analysis was performed using SPSS version 24.0 for Windows (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA); a significant difference was defined as a P-value <0.05.
The age of the 1,106 patients without diffuse thyroid disease (group 1) ranged from 22 days to 88 years (IQR, 47.0-65.0 years; median, 57.0 years old), and 813 (73.5%) female patients were included. The age of the 196 patients with diffuse thyroid disease (group 2) ranged from 50 days to 89 years (IQR, 39.0-60.0 years; median, 50.0 years), and 146 female patients (74.5%) were included. There was no significant difference in sex (P=0.773) between the two groups; however, the median age of the patients in group 2 was significantly younger compared to the patients in group 1 (P<0.001). Of the 1,106 patients in group 1, 48 patients (4.3%) were included in age group 1, 133 patients (12.0%) in age group 2, 501 patients (45.3%) in age group 3, and 424 patients (38.3%) in age group 4.
In the interobserver study for the thyroid gland, the age of the 273 patients ranged from 50 days to 91 years (IQR, 48.0-67.0 years; median, 59.0 years), and 203 female patients (74.4%) were included. In the interobserver study for the normal PG and SMG, the age of the 163 patients ranged from 1 to 91 years (IQR, 51.0-69.5 years; median, 61.0 years) and 118 (72.4%) female patients were included.
Relative to the echogenicity of the normal thyroid parenchyma, the US echogenicity of the normal PG was classified as isoechoic in 1,040 patients (94.0%), hypoechoic in 56 patients (5.1%), and hyperechoic in 10 patients (0.9%), and the US echogenicity of the normal SMG was isoechoic in 814 patients (73.6%) and hypoechoic in 292 of the 1,106 patients (26.4%) (Figs. 3-5). There was a significant difference in the frequency of isoechogenicity between the normal PG and SMG (P<0.001) (Table 1).
The number of patients with isoechoic PG was 44 (91.7%), 129 (97.0%), 466 (93.0%), and 401 (94.6%) patients in age groups 1, 2, 3, and 4, respectively, and the frequency of isoechoic PG was not significantly different among the age groups (P=0.919). The number of patients with isoechoic SMG was 11 (22.9%), 84 (63.2%), 374 (74.7%), and 345 (81.4%) in each age group, respectively. There was a trend for an increasing frequency of isoechoic SMG as age increased (P<0.001) (Table 2). Among the patients in age group 1 (0-20 years), an isoechoic PG was found in 100% of patients (5/5) <1 year of age, 91.7% of patients (22/24) 1-10 years of age, and 89.5% of patients (17/19) 11-20 years of age; however, an isoechoic SMG was found in 0% of patients (0/5) <1 year of age, 16.7% of patients (4/24) 1-10 years of age, and 36.8% of patients (7/19) 11-20 years of age (Fig. 3).
Slightly more female than male patients had an isoechoic PG (96.3% vs. 87.7%, P<0.001) and an isoechoic SMG (76.5% vs. 65.5%, P<0.001). There was a trend for an increasing frequency of isoechoic SMG as age increased in each subgroup of female and male patients (all, P<0.001), however, there was no significant trend for an increasing frequency of isoechoic PG as age increased in each subgroup of female and male patients (P=0.073 and P=0.261, respectively).
Relative to the echogenicity of the thyroid parenchyma, the US echogenicity of the normal PG was classified as hyperechoic in 119 patients (60.7%), isoechoic in 74 (37.8%) patients, and hypoechoic in three patients (1.5%), and the US echogenicity of the normal SMG was hyperechoic in 92 patients (46.9%), isoechoic in 67 patients (34.2%), and hypoechoic in 37 patients (18.9%). There was no significant difference in the frequency of isoechogenicity between the normal PG and SMG (P=0.450) (Table 3).
In the subgroup of 74 patients without decreased thyroid echogenicity, the normal PG and normal SMG were isoechoic in 71 (95.9%) patients and in 37 (50.0%), respectively (P<0.001). The frequency of isoechoic PG in this subgroup was similar to that in the patients without diffuse thyroid disease (group 1) (95.9% vs. 94.0%, P=0.533). The frequency of isoechoic SMG was significantly lower than in group 1 (50.0% vs. 73.6%, P<0.001). The number of patients with an isoechoic PG was 16 (100.0%), 16 (94.1%), 22 (95.7%), and 17 (94.4%) in age groups 1, 2, 3, and 4, respectively, and the frequency of isoechoic PG was not significantly different among the age groups (P=0.495). The number of patients with an isoechoic SMG was 1 (6.3%), 8 (47.1%), 16 (69.6%), and 12 (66.7%) in each age group, respectively. There was a trend for an increasing frequency of isoechoic SMG as age increased (P<0.001).
In the subgroup of 122 patients with decreased thyroid echogenicity, the normal PG and normal SMG were isoechoic to the thyroid parenchyma in three patients (2.5%) and in 30 patients (24.6%), respectively (P<0.001). The frequency of isoechoic PG was not significantly different among the age groups (P=0.859), but the frequency of isoechoic SMG tended to decrease as age increased (P<0.001) in contrast to the subgroup of patients without decreased thyroid echogenicity.
With regard to interobserver agreement on the echogenicity of the thyroid gland and normal salivary glands, substantial agreement was observed for the echogenicity of the thyroid gland (╬║=0.76; 95% confidence interval [CI], 0.66-0.86; agreement rate, 92.3%), moderate agreement was observed for the isoechogenicity of the PG (╬║=0.49; 95% CI, 0.06-0.92; agreement rate, 97.5%), and almost perfect agreement was observed for the isoechogenicity of the SMG (╬║=0.84; 95% CI, 0.74-0.93; agreement rate, 93.3%) (Table 4).
This study demonstrated that 94.0% of normal PGs exhibited isoechogenicity, whereas 73.6% of normal SMGs exhibited isoechogenicity compared to the normal thyroid parenchyma in patients without diffuse thyroid disease. The frequency of isoechoic SMG increased with age; however, the frequency of isoechoic PG did not correlate with age. In patients with diffuse thyroid disease, similar findings were consistently found in the subgroup of patients without decreased thyroid echogenicity; meanwhile, the normal PG showed isoechogenicity only in 2.5% of patients, in contrast to 24.6% of normal SMGs in the subgroup of patients with decreased thyroid echogenicity.
When nodule echogenicity is determined by comparison with the echogenicity of the adjacent thyroid parenchyma in patients who have diffuse thyroid disease with decreased parenchymal echogenicity, mildly hypoechoic nodules are classified as isoechoic or hyperechoic according to the degree of the hypoechogenicity of thyroid parenchyma, and this may be misleading in terms of the accuracy of the risk classification and appropriateness of management of nodules. The present study showed that approximately a quarter of normal SMGs were hypoechoic, while most normal PGs were isoechoic to the echogenicity of the normal thyroid parenchyma. Although some guidelines [4,8] recommend using the echogenicity of the normal SMG as a reference standard for normal thyroid echogenicity, these results suggest that the normal SMG is inadequate as a reference standard for evaluating nodule echogenicity in patients with diffuse thyroid disease showing decreased parenchymal echogenicity because SMG may show hypoechogenicity in the majority of children and in 18.6%-36.8% of adults, with the precise frequency depending on their age. However, the PG may be used as an alternative reference standard for normal thyroid echogenicity regardless of the patientsŌĆÖ age in patients with suspected diffuse thyroid disease showing decreased echogenicity of thyroid parenchyma. When there is suspicion of abnormal echogenicity of the PG, the presumed normal thyroid echogenicity may be used to evaluate the echogenicity of thyroid nodules.
A previous study [15] that investigated the echogenicity of normal PG and SMG included only adult patients with normal PGs showing typical homogeneous hyperechogenicity and did not compare the echogenicity of normal PG and SMG with thyroid gland. The present study included consecutive patients of all ages with normal salivary glands and compared the echogenicity between the normal salivary glands with thyroid parenchyma in patients with or without diffuse thyroid disease. This study showed a similar frequency (24.1%) of hypoechoic normal SMGs compared to the normal thyroid parenchyma in adults (Ōēź21 years old) among patients without diffuse thyroid disease, and validated that the echogenicity of the normal SMG is strongly associated with patientsŌĆÖ age, unlike the PG. It is notable that all the infants and the majority of group 1 patients (Ōēż20 years of age) had hypoechoic SMG, unlike the normal PG, which mostly showed isoechogenicity regardless of patientsŌĆÖ ages.
In patients with diffuse thyroid disease, the normal PG exhibited isoechogenicity compared to thyroid parenchyma in most patients without decreased thyroid echogenicity and exhibited hyperechogenicity in most patients with decreased thyroid echogenicity (Table 3). These results suggest that the normal PG can be reliably used as a reference standard for normal thyroid echogenicity in patients with diffuse thyroid disease.
Although the hyperechogenicity of the normal major salivary gland has been explained by the presence of a large amount of intraglandular fat tissue [22,23], the amount of fat tissue may not be the only factor that affects the echogenicity of the salivary gland. Fat tissue can show variable echogenicity depending on the heterogeneity of its histologic architecture [24]. The computed tomography attenuation of the normal SMG was substantially higher than that of the normal PG in patients who had similar echogenicity of the SMG and the PG [15]. PGs with complete fatty changes after radioactive iodine ablation for the treatment of thyroid cancer usually exhibit hypoechogenicity [25], and there was no significant relationship between diffuse fatty changes and parenchymal echogenicity upon histologic examinations of PGs with incidental diffuse parotid disease in patients who underwent parotid surgery [18].
Although the mechanism of physiologic hypoechogenicity of the normal SMG is uncertain and it has rarely been investigated, it may be related to changes in the histologic architecture of the SMG and the amount of intraglandular adipose tissue with aging. The acini of the SMG are generally compact and uniform in size and shape, and the proportion of intralobular ducts is low in young adults [26]. The SMG shows more loosely structured lobules with acini of disparate size and a high proportion of intralobular ducts in aged glands [26]. The proportion of intraglandular fat tissue increases, and that of parenchymal cells decreases with aging in SMG [27]. The compact and uniform histologic features and the relatively small amount of fat tissue in young SMGs may explain the hypoechogenicity of SMGs found in the majority of children. The heterogeneous histologic changes of the SMG with aging [26,27] may be a main factor contributing to the isoechogenicity of the SMG found in the majority of older patients because the heterogeneous glandular architecture contains numerous interfaces, resulting in increased echogenicity of glandular tissue. Unlike the SMG, most normal PGs did not show hypoechogenicity regardless of the patientsŌĆÖ age, including even neonates, in the present study. This different US feature of the normal PG may be related to differences in the histologic features of the PG compared to the SMG; in particular, severe fat infiltrations can be found and the glandular architecture is less compact in the young gland [26], and the proportional volume of adipose tissue was found to be similar between young and old adults in the under 70-year age group [28].
The interobserver agreement study showed high observed agreement rates of more than 90% and moderate to almost-perfect agreement for the echogenicity of the thyroid gland and normal PG and SMG. The relatively low ╬║ value (0.49, moderate agreement), despite the high agreement rate (97.5%) for the echogenicity of the normal PG, can be explained by the kappa paradox due to the imbalanced marginals of the data [29,30]. The findings of this study suggest that less experienced operators can reliably determine the echogenicity of the thyroid gland and salivary glands compared to experienced operators after a training period.
This study has several limitations. First, the number of pediatric patients and young adults included in the study was relatively small. Second, interobserver agreement was evaluated only between two observers. The number of observers participating in the study was limited because the US assessment of thyroid and salivary glands was conducted during real-time US examinations of patients. Further studies are needed on interobserver agreement for the echogenicity of the normal PG to establish the reliability of the normal PG as a reference standard for normal thyroid echogenicity.
Third, this study only used one type of high-resolution US machine from one institution. The echogenicity of the thyroid and salivary glands may depend on the parameters and type of US machines. A further investigation with various US machines from multiple institutions may be necessary to validate the results of the present study.
In conclusion, normal PGs were mostly isoechoic to the normal thyroid parenchyma, whereas normal SMGs showed hypoechogenicity at various frequencies that decreased as age increased. The echogenicity of normal PG can be used as an alternative reference standard for normal thyroid echogenicity; however, that of the normal SMG is not suitable for a reference standard when assessing the echogenicity of thyroid nodules in patients who have diffuse thyroid disease with decreased parenchymal echogenicity.
NotesAuthor Contributions Conceptualization: Na DG. Data acquisition: Choi I, Na DG. Data analysis or interpretation: Choi I, Na DG. Drafting of the manuscript: Choi I, Na DG. Critical revision of the manuscript: Choi I, Na DG. Approval of the final version of the manuscript: all authors. References1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1ŌĆō133.
2. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update appendix. Endocr Pract 2016;22(Suppl 1):S1ŌĆōS60.
3. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587ŌĆō595.
4. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TI-RADS. Eur Thyroid J 2017;6:225ŌĆō237.
5. Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TI-RADS. Endocrine 2020;70:256ŌĆō279.
6. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean Thyroid Imaging Reporting and Data System and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol 2021;22:2094ŌĆō2123.
7. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting lexicon: white paper of the ACR Thyroid Imaging, Reporting and Data System (TI-RADS) Committee. J Am Coll Radiol 2015;12:1272ŌĆō1279.
8. Rago T, Cantisani V, Ianni F, Chiovato L, Garberoglio R, Durante C, et al. Thyroid ultrasonography reporting: consensus of Italian Thyroid Association (AIT), Italian Society of Endocrinology (SIE), Italian Society of Ultrasonography in Medicine and Biology (SIUMB) and Ultrasound Chapter of Italian Society of Medical Radiology (SIRM). J Endocrinol Invest 2018;41:1435ŌĆō1443.
9. Kim DW, Eun CK, In HS, Kim MH, Jung SJ, Bae SK. Sonographic differentiation of asymptomatic diffuse thyroid disease from normal thyroid: a prospective study. AJNR Am J Neuroradiol 2010;31:1956ŌĆō1960.
10. Jeong SH, Hong HS, Lee JY. The association between thyroid echogenicity and thyroid function in pediatric and adolescent Hashimoto's thyroiditis. Medicine (Baltimore) 2019;98:e15055.
11. Raber W, Gessl A, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid 2002;12:725ŌĆō731.
12. Zajkowski P, Ochal-Choinska A. Standards for the assessment of salivary glands: an update. J Ultrason 2016;16:175ŌĆō190.
13. Halenka M, Frysak Z. Atlas of thyroid ultrasonography. Berlin: Springer, 2017:
14. Som PM, Curtin HD. Atlas of thyroid ultrasonography. Berlin: Springer, 2017:
15. Choi I, Na DG, Paik W. Ultrasonographic echogenicity of normal salivary glands in adults: comparison of submandibular and parotid glands. Ultrasonography 2021;40:342ŌĆō348.
16. Baldini C, Luciano N, Tarantini G, Pascale R, Sernissi F, Mosca M, et al. Salivary gland ultrasonography: a highly specific tool for the early diagnosis of primary Sjogren's syndrome. Arthritis Res Ther 2015;17:146.
17. Narayan AK, Baer A, Fradin J. Sonographic findings of IgG4-related disease of the salivary glands: case report and review of the literature. J Clin Ultrasound 2018;46:73ŌĆō77.
18. Kim DH, Kim DW, Park JY, Lee YJ, Choo HJ, Ha TK, et al. Ultrasound detection of incidental diffuse parotid disease: a single-center study. PLoS One 2019;14:e0219308.
19. Ahn HS, Kim DW, Lee YJ, Baek HJ, Ryu JH. Diagnostic accuracy of real-time sonography in differentiating diffuse thyroid disease from normal thyroid parenchyma: a multicenter study. AJR Am J Roentgenol 2018;211:649ŌĆō654.
20. Baek HJ, Kim DW, Lee YJ, Ahn HS, Ryu JH. Comparison of real-time and static ultrasonography diagnoses for detecting incidental diffuse thyroid disease: a multicenter study. Ultrasound Q 2019;35:233ŌĆō239.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159ŌĆō174.
22. Garcia CJ, Flores PA, Arce JD, Chuaqui B, Schwartz DS. Ultrasonography in the study of salivary gland lesions in children. Pediatr Radiol 1998;28:418ŌĆō425.
23. Bialek EJ, Jakubowski W, Zajkowski P, Szopinski KT, Osmolski A. US of the major salivary glands: anatomy and spatial relationships, pathologic conditions, and pitfalls. Radiographics 2006;26:745ŌĆō763.
24. Spencer GM, Rubens DJ, Roach DJ. Hypoechoic fat: a sonographic pitfall. AJR Am J Roentgenol 1995;164:1277ŌĆō1280.
25. Kim DW. Ultrasonographic features of the major salivary glands after radioactive iodine ablation in patients with papillary thyroid carcinoma. Ultrasound Med Biol 2015;41:2640ŌĆō2645.
27. Waterhouse JP, Chisholm DM, Winter RB, Patel M, Yale RS. Replacement of functional parenchymal cells by fat and connective tissue in human submandibular salivary glands: an age-related change. J Oral Pathol 1973;2:16ŌĆō27.
28. Scott J, Flower EA, Burns J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J Oral Pathol 1987;16:505ŌĆō510.
A 5-month-old infant with a hypoechoic submandibular gland and isoechoic parotid gland.The left submandibular gland shows homogeneous hypoechogenicity compared to the thyroid gland. The left parotid gland (anterior part of superficial lobe) shows homogeneous isoechogenicity similar to the thyroid gland: normal thyroid gland (A), normal submandibular gland (B), and normal parotid gland (C).
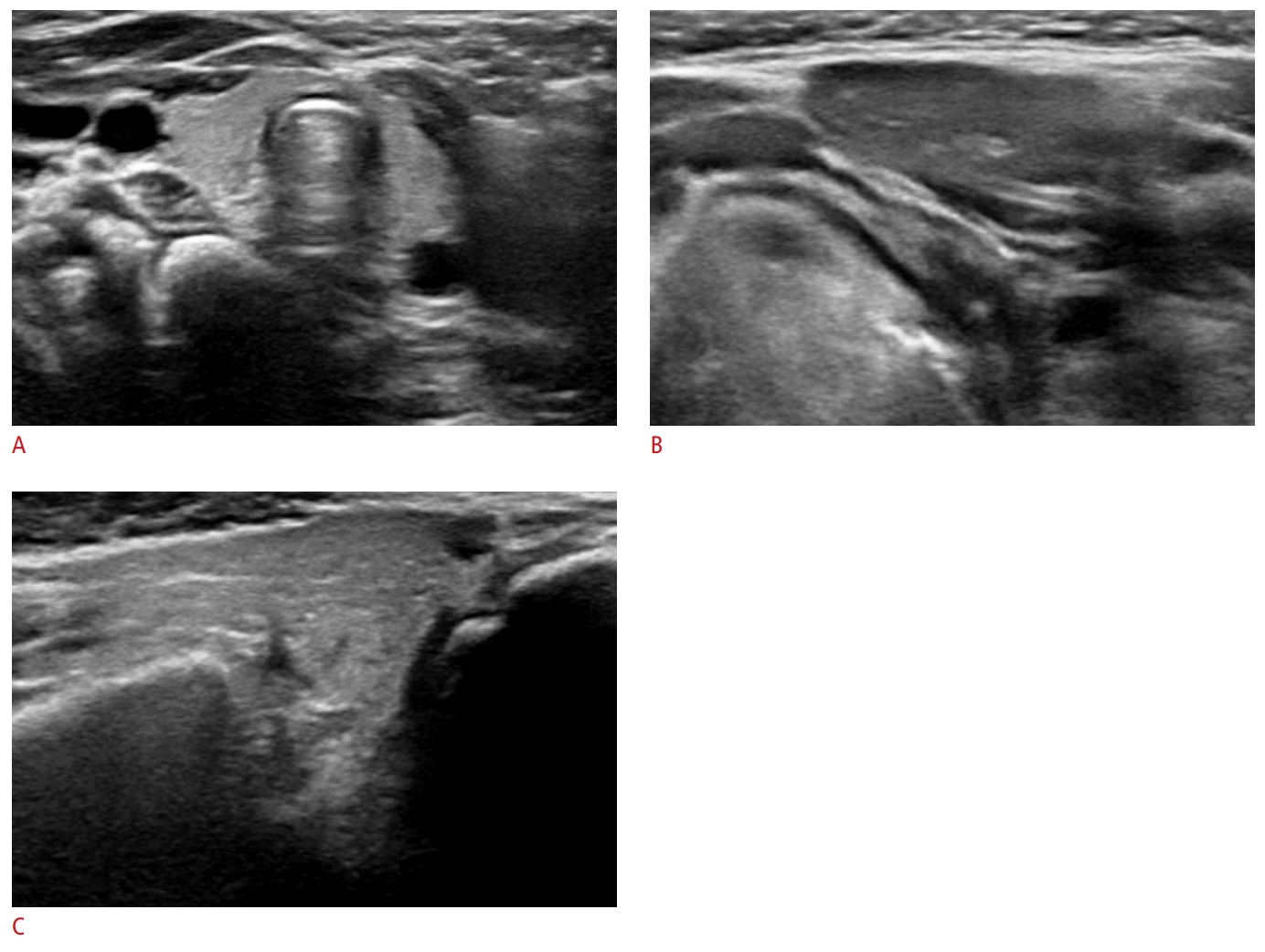 Fig.┬Ā3.A 25-year-old woman with a hypoechoic submandibular gland and isoechoic parotid gland.The right submandibular gland shows homogeneous hypoechogenicity compared to the thyroid gland. The right parotid gland (superficial lobe) shows homogeneous isoechogenicity similar to the thyroid gland: normal thyroid gland (A), normal submandibular gland (B), and normal parotid gland (C).
 Fig.┬Ā4.A 70-year-old woman with isoechoic submandibular and parotid glands.The left submandibular and parotid glands (superficial lobe) show homogeneous isoechogenicity similar to the thyroid gland: normal thyroid gland (A), normal submandibular gland (B), and normal parotid gland (C).
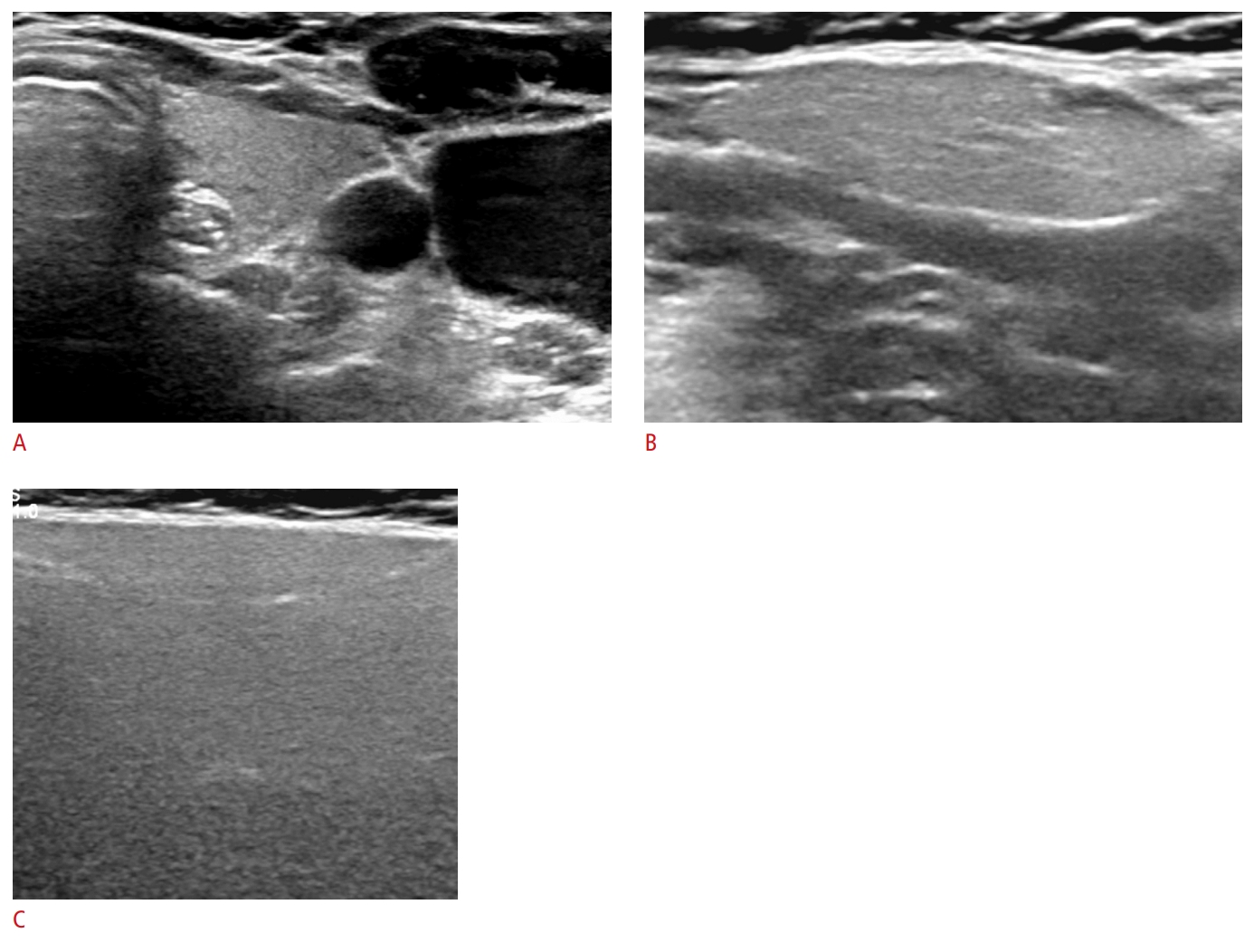 Fig.┬Ā5.Table┬Ā1.Echogenicity of normal parotid and submandibular glands compared to thyroid glands in patients without diffuse thyroid disease (n=1,106)
Table┬Ā2.Echogenicity of normal PG and SMG according to age groups in patients without diffuse thyroid disease (n=1,106)
Table┬Ā3.Echogenicity of normal parotid and submandibular glands compared to thyroid glands in patients with diffuse thyroid disease (n=196)
Table┬Ā4.Interobserver agreement for the echogenicity of thyroid glands (n=273) and the echogenicity of normal parotid and submandibular glands compared to the normal thyroid parenchyma (n=163)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




