AbstractPurposeThe purpose of this study was to elucidate whether contrast-enhanced ultrasonography (CEUS) can visualize orally administered Sonazoid leaking into the peritoneal cavity in a postoperative stomach leakage mouse model.
MethodsAdult female mice (n=33, 9–10 weeks old) were used. Preoperative CEUS was performed after delivering Sonazoid via intraperitoneal injection and the per oral route. A gastric leakage model was then generated by making a surgical incision of about 0.5 cm at the stomach wall, and CEUS with per oral Sonazoid administration was performed. A region of interest was drawn on the CEUS images and the signal intensity was quantitatively measured. Statistical analysis was performed using a mixed model to compare the signal intensity sampled from the pre-contrast images with those of the post-contrast images obtained at different time points.
ResultsCEUS after Sonazoid intraperitoneal injection in normal mice and after oral administration in mice with gastric perforation visualized the contrast medium spreading within the liver interlobar fissures continuous to the peritoneal cavity. A quantitative analysis showed that in the mice with gastric perforation, the orally delivered Sonazoid leaking into the peritoneal cavity induced a statistically significant (P<0.05) increase in signal intensity in all CEUS images obtained 10 seconds or longer after contrast delivery. However, enhancement was not observed before gastric perforation surgery (P=0.167).
Contrast-enhanced ultrasonography (CEUS) with oral Sonazoid administration in normal mice did not show a contrast enhancement effect in the vessels, liver, or peritoneal cavity. CEUS with intraperitoneal Sonazoid injection visualized the contrast medium spreading within the peritoneal cavity, but contrast enhancement was not observed in the vessels or liver. CEUS with oral Sonazoid administration in a stomach leakage mouse model visualized the leaked contrast medium spreading within the peritoneal cavity.
Ultrasonography enables a prompt assessment at the bedside and therefore has been increasingly applied to the assessment of critically ill patients [1,2]. Ultrasonography has proven its usefulness not only in the emergency department and the intensive care unit, but also in the operating room and postoperative care unit because of the consistent demand for prompt bedside evaluation of acutely ill patients [1–4].
Gastrointestinal tract (GIT) perforation can occur due to various underlying causes, including but not limited to peptic ulcers, inflammatory diseases, trauma, foreign bodies, neoplasms, or anastomosis site leakage [5,6]. These are emergency conditions demanding early recognition and usually timely surgical treatment [5]. Computed tomography (CT) is considered the modality of choice for the assessment of patients with suspected GIT perforation, as it can accurately detect small amounts of free intraperitoneal air, as well as fluid collections, inflammation, and thickened gastric or duodenal walls [6–9].
Meanwhile, ultrasonography does not typically play a role in the first-line workup of GIT perforation. However, in various situations during daily clinical practice, ultrasonography sometimes has to replace the role of CT. Several studies have reported that ultrasonography can effectively detect intraperitoneal free air and therefore may be used to diagnose GIT perforation [5,7,10].
However, it is well recognized that the detection of free air by ultrasonography is often challenging because intraperitoneal free air can be easily mistaken for air within the bowel [5,11]. Moreover, the detection of free air alone is insufficient to establish the diagnosis of GIT perforation in patients who recently underwent surgery [6,12]. The hypothesis of this study was that ultrasonography contrast medium could be applied to diagnose GIT perforation by contrast-enhanced ultrasonography (CEUS).
This study was designed as a proof-of-concept to elucidate whether CEUS can directly visualize an orally administered ultrasonography contrast agent leaking into the peritoneal cavity, thereby improving the diagnostic confidence of GIT perforation, using a postoperative stomach leakage mouse model.
This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Samsung Biomedical Research Institute (SBRI) (approval number: 20210831001). SBRI is an Association for Assessment and Accreditation of Laboratory Animal Care international (AAALAC international) accredited facility and abides by the Institute of Laboratory Animal Resources (ILAR) guide.
We used adult female (9–10 weeks old) BALB/c nude mice (ORIENT BIO Inc., Seongnam, Korea) that were maintained in a pathogen-free environment and fed ad libitum.
A stomach leakage mouse model was surgically generated by making an incision in the stomach. In brief, the mice were fasted overnight by removal of feedstuff, but not water. The mice were put under general anesthesia using a mixture of isoflurane and oxygen. Anesthesia was initiated in an induction chamber using a mixture of 3% isoflurane and 97% oxygen at a flow rate of 1.5 L/min. Then the mice were positioned supine on a heating pad while anesthesia was maintained with an animal nose/mouth mask supplying a mixture of 2% isoflurane and 98% oxygen at a flow rate of 1.5 L/min.
A midline incision of approximately 2 cm in length was made to the skin, rectus muscle fascia and underlying peritoneum slightly below the xiphoid and the anterior stomach wall was exposed. A focal incision of approximately 0.5 cm was made at the anterior wall of the middle body of the stomach. The abdominal wall incision wound was repaired using layer-by-layer 4-0 black silk sutures (Ailee Co., Ltd., Busan, Korea).
Ultrasonography was performed with a small animal high-frequency ultrasound imaging system (VEVO2100, FUJIFILM VisualSonics, Toronto, Canada). Each mouse underwent three CEUS sessions throughout Sonazoid (GE Healthcare, Chicago, IL, USA) administration at the following time points: after intraperitoneal administration, after oral administration but before surgery (preoperative CEUS), and after gastric leakage had been surgically established (postoperative CEUS) (Supplementary Fig. 1). Each CEUS session was conducted at an interval of at least 1 week to ensure complete removal of residual Sonazoid.
Each mouse was maintained under anesthesia by connecting a nozzle supplying a mixture of 2% isoflurane and 98% oxygen at a flow rate of 1.5 L/min. The mouse was placed on an ultrasound machine platform with foot pad electrodes to monitor the respiratory cycle.
A 20 MHz high-frequency linear probe was used and an ultrasound coupling gel was applied between the mouse skin and transducer. Ultrasonography was performed with 4% transmit power, 39 dB contrast gain, and an acquisition rate of 100 frames per second. The probe was placed to image the liver in the subcostal orientation with the liver hilum at the center and the position of the probe was fixed by a dedicated stereotactic device. CEUS was performed using Sonazoid, which was prepared according to the manufacturer’s instructions. Prior to Sonazoid administration, an ultrasound burst was performed by transmitting the ultrasound pulse at the maximum setting for 1 second to remove unknown microbubbles in the scanned field and background data were acquired for 20 seconds. Afterward, Sonazoid was infused either by intraperitoneal injection or oral administration. For intraperitoneal injection, 100 μL of Sonazoid suspension was infused into the abdominal cavity via a 31G insulin syringe. The volume of intraperitoneal injection was set as 100 μL to match that of oral infusion. Prior to the day of CEUS with oral Sonazoid administration, the mice were fasted overnight by removing feedstuff, but not water. For oral Sonazoid administration, a polyethylene tube (BD, East Rutherford, NJ, USA) was inserted into the stomach through the oral cavity to serve as an orogastric tube. We first inserted an oral Zonde needle (JEUNGDO Bio & Plant, Seoul, Korea) which served as an outer sheath to guide the polyethylene tube. The polyethylene tube was pushed through the lumen of the zoned needle, which was then removed to leave the tip of the polyethylene tube inserted in the stomach. This polyethylene tube served as an orogastric tube to orally administer 100 μL of Sonazoid suspension for CEUS. During a pilot test, oral regurgitation was sometimes observed when larger volumes were infused, so the infusion volume was set as 100 μL in all experiments.
The CEUS images were visually assessed to detect any contrast medium leakage and to determine whether enhancement had occurred. For quantitative assessment the CEUS images were stored as every frame for at least 3 minutes after administration of contrast medium. To quantitatively measure the degree of enhancement, a CEUS image obtained between 60 to 120 seconds after contrast medium administration was picked to select the region with the most prominent enhancement on visual assessment. A round region of interest (ROI) was then placed within the liver boundaries to include the pixels showing prominent enhancement at the interlobar fissure, and non-enhancing pixels were avoided as much as possible during ROI placement. If enhancement could not be perceived, the ROI was randomly placed at the central part within the liver boundary while avoiding large vessels as much as possible. Once an ROI was placed on one image obtained at a specific time point, then the software automatically placed an ROI at the same location on all the other images obtained at different time points and the signal intensity (AU) was automatically measured. We used the VevoCQ software to quantify the signal intensity of all pixels included in the ROI every 10 seconds. Prior to quantification, the Correct Motion function built into the VevoCQ software was activated to minimize motion artifacts.
After CEUS was complete, oral Evans blue solution was infused to confirm the state of gastric leakage. In brief, under anesthesia, the mice were put in supine position and a gastric tube was placed, as previously described. The tied sutures of the mice’s abdominal wall were removed and the incision was extended to expose the stomach leakage site. We then infused 100 μL of Evans blue solution through the gastric tube and visually assessed the presence or absence of color dye leakage.
The mice were sacrificed, and specimens of the stomach and peritoneum were obtained and fixed in 10% formalin solution; these specimens were later used to produce hematoxylin and eosin–stained slides for microscopic examinations.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The signal intensity (AU) measured from the ROI drawn on the CEUS pre-contrast images was compared with that of post-contrast images obtained at different time points to elucidate whether a significant enhancement effect existed. A mixed-effects model with a random effect for the subject and a fixed effect for time was applied to the CEUS data obtained with intraperitoneal Sonazoid injection, oral Sonazoid administration in the preoperative and postoperative periods. P-values were corrected by the Bonferroni method due to multiple testing. For the CEUS dataset obtained from oral Sonazoid administered in the preoperative period, a mixed model was used after double natural logarithm transformation due to the highly skewed distribution. Continuous data were summarized as the mean and standard deviation. A two-tailed P-value <0.05 was considered as statistically significant.
CEUS was performed after intraperitoneally injecting 100 μL of Sonazoid mixture to elucidate whether Sonazoid retained in the peritoneal cavity can enter the systemic circulation and induce detectable intra-vascular enhancement. The spread of Sonazoid within the liver interlobar fissures was observed (Fig. 1A), but there was no perceivable enhancement in the large vessels or liver parenchyma.
A time-intensity curve sampled from a ROI placed at the interlobar fissure showed that the CEUS signal rapidly increased shortly after the intraperitoneal injection of Sonazoid (Fig. 1B). When the signal intensity measured at each time point was individually compared with that of the pre-contrast image, all CEUS images obtained at 10 seconds or more after contrast medium injection showed significantly higher intensity than the pre-contrast images (P<0.05) (Fig. 1B).
Next, baseline CEUS was performed with oral Sonazoid administration to elucidate whether the contrast agent would be absorbed from the bowel and induce systemic enhancement. No enhancement effect was detected within the peritoneal cavity, large vessels, or liver parenchyma (Fig. 2A). An analysis of the measured mean signal intensity of each ROI drawn on CEUS images obtained at different time points did not show statistically significant difference (P=0.167) (Fig. 2B).
A sudden gush of Sonazoid entering the stomach and small bowel was sometimes observed, but was easily differentiated from the spread of Sonazoid into the peritoneal cavity by the fact that the enhancement remained in a tubular shape within the boundaries of the bowel and peristalsis movement was present.
Finally, we performed CEUS with oral Sonazoid administration using the stomach leakage mouse model. In the majority of cases (90.9%, n=30/33), obvious spread of the contrast agent within the liver interlobar fissure was observed shortly after oral Sonazoid infusion (Fig. 3A). The overall pattern of contrast medium spread was similar to that observed during CEUS with intraperitoneal Sonazoid injection (Fig. 1A). When the signal intensity measured at each time point was compared with that of the pre-contrast images (Table 1), the CEUS images obtained at 10 seconds or more following contrast medium administration were significantly higher in intensity than the pre-contrast image (P<0.05) (Fig. 3B). After CEUS, oral Evans blue solution was infused through the orogastric tube and color dye leakage was obtained from the perforation site (Fig. 4).
However, in three mice (9.1%, n=3/33), contrast leakage was not detected on CEUS after oral Sonazoid administration. Under anesthesia, the surgical ties of the abdominal wall incision site were removed to expose the stomach. In all three mice, the stomach perforation site was plugged with foodstuff. Subsequent Evans blue solution infusion through an orogastric tube failed to demonstrate color dye leakage as well.
Microscopic examination of the hematoxylin and eosin–stained slides of the specimen of stomach revealed acute suppurative necrotizing exudates and submucosal edema at the perforation site (Supplementary Fig. 2).
In this proof-of-concept study, CEUS was performed with oral Sonazoid using a postoperative stomach leakage mouse model to demonstrate that the oral contrast agent leaking into the peritoneal cavity can be directly visualized. Although ultrasonography is already applied for detecting intraperitoneal free air to diagnose GIT perforation [5,7,10,13], the strategy presented herein will have added value for not only improving confidence in detecting GIT perforation, but also for diagnosing postoperative leakage in patients for whom free air already exists. Along similar lines to the detection of extraluminal oral contrast by CT or fluoroscopy being considered as a specific sign of GIT perforation and postoperative leakage [6,8,14–17], this approach could enable the diagnosis of postoperative gastric leakage even under conditions in which the detection of free air alone would have little if any clinical significance.
Ultrasonography is a versatile modality that offers irreplaceable advantages such as rapid availability in an emergency setting and easy application to patients who are bedridden, critically ill, or difficult to transport to the radiology department for a CT scan [3,18,19]. Moreover, CEUS has advantages in terms of safety issues. Orally administered iodine contrast medium is frequently used to diagnose bowel perforation on CT or fluoroscopy [6,8,14–17]. Although oral administration of iodine contrast medium is generally considered to be safe, anaphylactoid-like reactions do occur in an unpredictable manner and can be severe and life-threatening [20–24]. Ultrasound contrast agents are generally considered to be safe, with a low incidence of hypersensitivity or allergic events [25]. Although the possibility of shock and anaphylactoid reactions cannot be excluded, there have been no reports of such severe adverse events associated with Sonazoid [26]. Therefore, the authors suggest that CEUS with oral contrast medium administration may be considered a reasonable method for screening upper GIT perforation, especially in patients with a history of severe allergy to iodine contrast medium.
During daily practice, the authors have frequently performed CEUS with intravenous Sonazoid injection for critically ill patients who received liver transplantation, which led to the hypothesis that CEUS may be applied to screen for upper GIT leakage at low additional expense, as commercial ultrasonography contrast media such as Sonazoid or Sonovue are packaged in redundant amounts for a single-session study. It was speculated that after completing CEUS with intravenous contrast medium injection, it would be reasonable to perform a subsequent CEUS study with oral administration using the excess contrast medium to screen for GIT perforation.
Several studies have performed CEUS after orally administrating various ultrasound contrast agents for the evaluation of gastric focal lesions, inflammatory bowel disease, and esophageal hiatal hernia [27–32]. However, to the best of the authors’ knowledge, CEUS after oral ultrasound contrast administration to evaluate bowel perforation has not been described. CEUS was first performed in normal mice before surgery with oral delivery of Sonazoid (serving as a negative control) and intraperitoneal injection (serving as a positive control as Sonazoid enters the peritoneal cavity) to elucidate whether significant absorption into the systemic circulation would occur to induce perceivable vascular enhancement. CEUS after the oral administration of Sonazoid did not demonstrate any detectable enhancement in the peritoneal cavity, liver, or large vessels (Fig. 2). Meanwhile, CEUS after the intraperitoneal injection of Sonazoid demonstrated robust linear enhancement within the liver boundaries (Fig. 1). Unlike in humans, the liver of mice consists of four main lobes, and each lobe has its own pedicle forming prominent interlobar fissures, which are continuous with the peritoneal cavity [33,34]. It is suggested that this slender shape of contrast enhancement within the liver outline represented the spread of the contrast medium through the interlobar fissures. Meanwhile, enhancement could not be detected in the large vessels or the liver parenchyma.
When we performed CEUS with oral Sonazoid using the postoperative stomach leakage mouse model, contrast leakage was not detected in a minor portion of the mice (9.1%, n=3/33). In these three mice, surgical exploration performed shortly after CEUS revealed foodstuff plugging the perforation although overnight fasting was performed. Subsequent Evans blue infusion through the orogastric tube also failed to demonstrate color dye leakage. In the majority of cases (90.9%, n=30/33), obvious contrast agent spread was observed shortly after oral Sonazoid infusion (Fig. 3). However, the authors would like to make it clear that the intention was not to insist that CEUS using oral Sonazoid can replace the role of CT assessment in patients with suspected GIT perforation. As the limitations of ultrasonography during bowel assessment are evident, there is no doubt that CT should remain the modality of choice for the assessment of intra-abdominal emergencies. However, as patients with bowel perforation are often critically ill (and may not be movable to the CT unit) and may show a non-specific clinical presentation, or may have a history of severe allergic reaction to iodine-based contrast agents, the decision to perform a CT scan frequently may not be straightforward. Therefore, the authors do believe that our results support the suggestion that CEUS can be applied to diagnose GIT perforation in patients in whom a CT scan is not feasible or for the screening of patients who undergo ultrasonography for an ambiguous clinical presentation.
This study has a few limitations. First, a mouse model was used, and mice are much smaller than human patients and considerably different in terms of the available sonic window. Since mice are small, it could have been easier to avoid bowel gas, which frequently is a major interference with ultrasonographic assessments of the bowel. Anatomical differences, such as prominent liver interlobar fissures, would have made the detection of leaked contrast medium easier in mice. Therefore, the authors recognize the need for a subsequent clinical study or a preclinical study using a larger animal model. However, as this was designed as a proof-of-concept study, the conclusions of this study are still thought to be meaningful. Second, this study used a high-frequency linear probe equipped with superior resolution compared with that of average convex probes used for the assessment of adult patients. Therefore, a subsequent study with a low-frequency convex probe is necessary to assess the feasibility of detecting leaked contrast medium in adult patients. However, these results still could have significant implications in the evaluation of high-risk neonates, who are usually assessed with high-frequency linear probes.
In conclusion, CEUS efficiently detected the extraluminal spread of orally administered Sonazoid in a stomach leakage mouse model, suggesting that CEUS can be used to diagnose gastric perforation.
AcknowledgmentsThis work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2020R1F1A1075053).
NotesAuthor Contributions Conceptualization: Kim H. Data acquisition: Jung K, Kim AR, Kim H. Data analysis or interpretation: Jung K, Woo SY. Drafting of the manuscript: Jung K, Woo SY, Kim AR, Kim H. Critical revision of the manuscript: Kim H. Approval of the final version of the manuscript: all authors. Supplementary MaterialSupplementary Fig. 1.Schema of experimental design. All mice received contrast enhanced ultrasonography (CEUS) three times. First, CEUS with intraperitoneal Sonazoid injection was performed followed by preoperative CEUS with oral Sonazoid administration (preoperative CEUS) and finally CEUS with oral Sonazoid administration after gastric perforation has been surgically generated (post-operative CEUS). Each CEUS was performed with an interval of at least 1 week (https://doi.org/10.14366/usg.22192).
Supplementary Fig. 2.Microscopic image of stomach specimen demonstrating the perforation site (arrows) at the gastric wall with acute suppurative necrotizing exudates (arrowheads) and submucosal edema (hematoxylin and eosin staining; A, ×40; B, ×100) (https://doi.org/10.14366/usg.22192).
References1. Laursen CB, Sloth E, Lambrechtsen J, Lassen AT, Madsen PH, Henriksen DP, et al. Focused sonography of the heart, lungs, and deep veins identifies missed life-threatening conditions in admitted patients with acute respiratory symptoms. Chest 2013;144:1868–1875.
2. Zieleskiewicz L, Muller L, Lakhal K, Meresse Z, Arbelot C, Bertrand PM, et al. Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med 2015;41:1638–1647.
3. Nicola R, Dogra V. Ultrasound: the triage tool in the emergency department: using ultrasound first. Br J Radiol 2016;89:20150790.
4. Hoffmann B, Nurnberg D, Westergaard MC. Focus on abnormal air: diagnostic ultrasonography for the acute abdomen. Eur J Emerg Med 2012;19:284–291.
5. Coppolino F, Gatta G, Di Grezia G, Reginelli A, Iacobellis F, Vallone G, et al. Gastrointestinal perforation: ultrasonographic diagnosis. Crit Ultrasound J 2013;5(Suppl 1):S4.
6. Kim YE, Lim JS, Hyung WJ, Lee SK, Choi JY, Noh SH, et al. Clinical implication of positive oral contrast computed tomography for the evaluation of postoperative leakage after gastrectomy for gastric cancer. J Comput Assist Tomogr 2010;34:537–542.
7. Kuzmich S, Harvey CJ, Fascia DT, Kuzmich T, Neriman D, Basit R, et al. Perforated pyloroduodenal peptic ulcer and sonography. AJR Am J Roentgenol 2012;199:W587–W594.
8. Hainaux B, Agneessens E, Bertinotti R, De Maertelaer V, Rubesova E, Capelluto E, et al. Accuracy of MDCT in predicting site of gastrointestinal tract perforation. AJR Am J Roentgenol 2006;187:1179–1183.
9. Maniatis V, Chryssikopoulos H, Roussakis A, Kalamara C, Kavadias S, Papadopoulos A, et al. Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging 2000;25:373–379.
10. Chen SC, Wang HP, Chen WJ, Lin FY, Hsu CY, Chang KJ, et al. Selective use of ultrasonography for the detection of pneumoperitoneum. Acad Emerg Med 2002;9:643–645.
11. Seitz K, Reising KD. Ultrasound detection of free air in the abdominal cavity. Ultraschall Med 1982;3:4–6.
12. Yoo SY, Kim KW, Han JK, Kim AY, Lee HJ, Choi BI. Helical CT of postoperative patients with gastric carcinoma: value in evaluating surgical complications and tumor recurrence. Abdom Imaging 2003;28:617–623.
13. Chadha D, Kedar RP, Malde HM. Sonographic detection of pneumoperitoneum: an experimental and clinical study. Australas Radiol 1993;37:182–185.
14. Zissin R, Osadchy A, Gayer G. Abdominal CT findings in small bowel perforation. Br J Radiol 2009;82:162–171.
15. Tonouchi H, Mohri Y, Tanaka K, Ohi M, Kobayashi M, Yamakado K, et al. Diagnostic sensitivity of contrast swallow for leakage after gastric resection. World J Surg 2007;31:128–131.
16. Bruce J, Krukowski ZH, Al-Khairy G, Russell EM, Park KG. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157–1168.
17. Tirnaksiz MB, Deschamps C, Allen MS, Johnson DC, Pairolero PC. Effectiveness of screening aqueous contrast swallow in detecting clinically significant anastomotic leaks after esophagectomy. Eur Surg Res 2005;37:123–128.
18. Abushamat F, Dietrich CF, Clevert DA, Piscaglia F, Fetzer DT, Meloni MF, et al. Contrast-enhanced ultrasound (CEUS) in the evaluation of hemoperitoneum in patients with cirrhosis. J Ultrasound Med 2023;42:247–253.
19. Lv F, Tang J, Luo Y, Li Z, Meng X, Zhu Z, et al. Contrast-enhanced ultrasound imaging of active bleeding associated with hepatic and splenic trauma. Radiol Med 2011;116:1076–1082.
20. Davis PL. Anaphylactoid reactions to the nonvascular administration of water-soluble iodinated contrast media. AJR Am J Roentgenol 2015;204:1140–1145.
21. Miller SH. Anaphylactoid reaction after oral administration of diatrizoate meglumine and diatrizoate sodium solution. AJR Am J Roentgenol 1997;168:959–961.
22. Marik PE, Patel SY. Anaphylactoid reaction to oral contrast agent. AJR Am J Roentgenol 1997;168:1623–1624.
23. Schmidt BJ, Foley WD, Bohorfoush AG. Toxic epidermal necrolysis related to oral administration of diluted diatrizoate meglumine and diatrizoate sodium. AJR Am J Roentgenol 1998;171:1215–1216.
24. Ridley LJ. Allergic reactions to oral iodinated contrast agents: reactions to oral contrast. Australas Radiol 1998;42:114–117.
25. Rettenbacher T. Focal liver lesions: role of contrast-enhanced ultrasound. Eur J Radiol 2007;64:173–182.
26. Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al. The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using Sonazoid. Ultrasonography 2020;39:191–220.
27. Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease: a prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut 2004;53:1652–1657.
28. Badea R, Neciu C, Iancu C, Al Hajar N, Pojoga C, Botan E. The role of i.v. and oral contrast enhanced ultrasonography in the characterization of gastric tumors: a preliminary study. Med Ultrason 2012;14:197–203.
29. Shiyan L, Pintong H, Zongmin W, Fuguang H, Zhiqiang Z, Yan Y, et al. The relationship between enhanced intensity and microvessel density of gastric carcinoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol 2009;35:1086–1091.
30. Yu T, Wang X, Zhao Z, Liu F, Liu X, Zhao Y, et al. Prediction of T stage in gastric carcinoma by enhanced CT and oral contrastenhanced ultrasonography. World J Surg Oncol 2015;13:184.
31. Zheng XZ, Zhang LJ, Wu XP, Lu WM, Wu J, Tan XY. Oral Contrast-enhanced gastric ultrasonography in the assessment of gastric lesions: a large-scale multicenter study. J Ultrasound Med 2017;36:37–47.
32. Wang JY, Luo Y, Wang WY, Zheng SC, He L, Xie CY, et al. Contrast-enhanced ultrasound using SonoVue mixed with oral gastrointestinal contrast agent to evaluate esophageal hiatal hernia: Report of three cases and a literature review. World J Clin Cases 2021;9:2679–2687.
Intraperitoneal Sonazoid injection model.
A. Dual-mode display of contrast-enhanced ultrasonography (CEUS) images before and after intraperitoneal injection of Sonazoid are shown. Interlobar spread of contrast medium (arrows) was observed starting 10 seconds after contrast medium injection. The dotted circle represents the region of interest used for signal intensity measurement. B. Box-whisker plot of the time-intensity curve of the signal intensity sampled from all mice (n=33) during CEUS is shown. The value of each time point was compared with that of the pre-contrast image (○, outliers; *P<0.05).
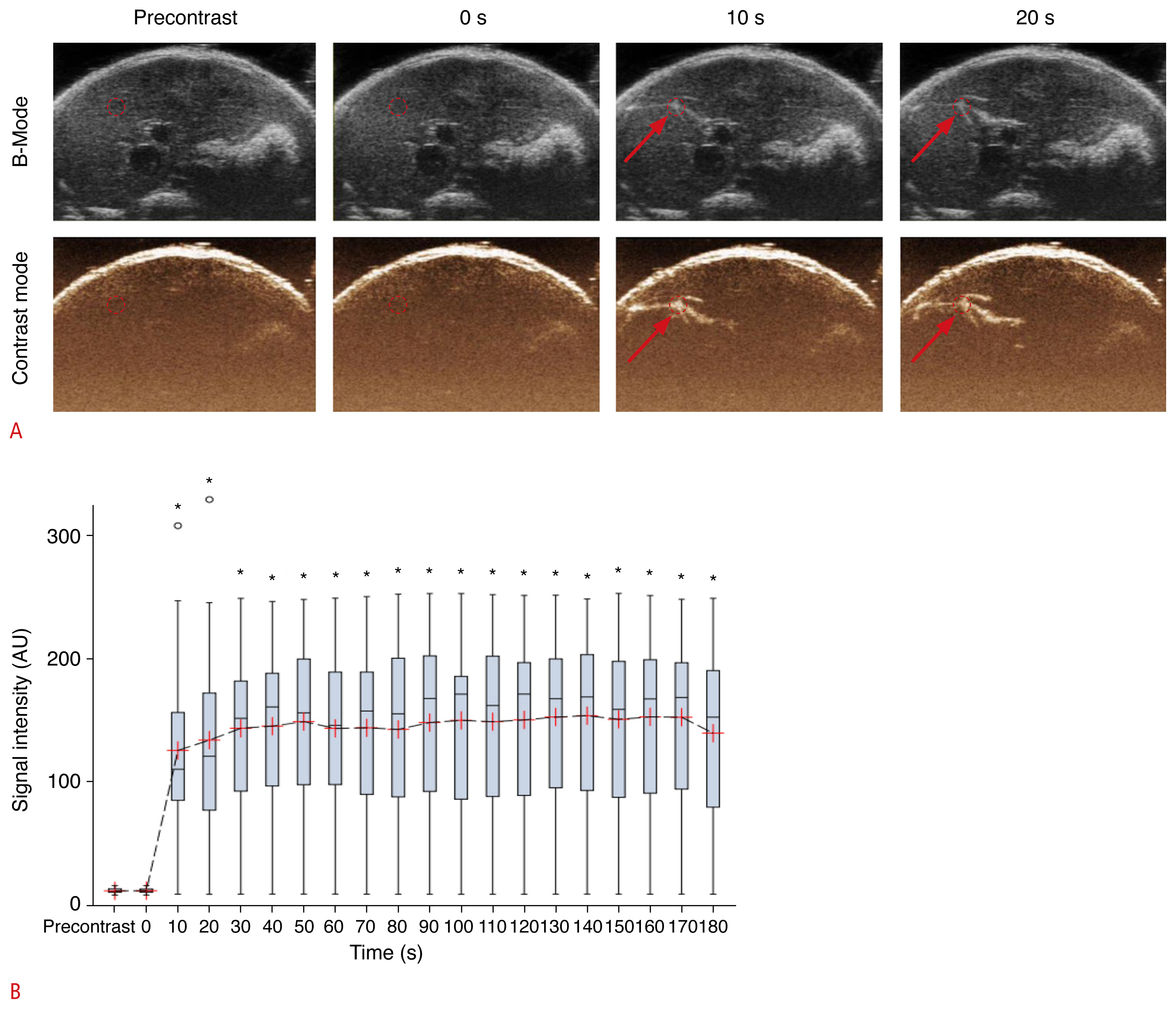 Fig. 1Preoperative model with per oral Sonazoid infusion.
A. Dual-mode display of contrast-enhanced ultrasonography (CEUS) images before and after oral Sonazoid administration in the baseline (preoperative) study are shown. No significant contrast enhancement was detected. The dotted circle represents the region of interest used for signal intensity measurement. B. Box-whisker plot of the time-intensity curve of the signal intensity sampled from all mice (n=33) during CEUS is shown. The value of each time point was compared with that of the pre-contrast image, but none of them showed statistically significant differences (○, outliers).
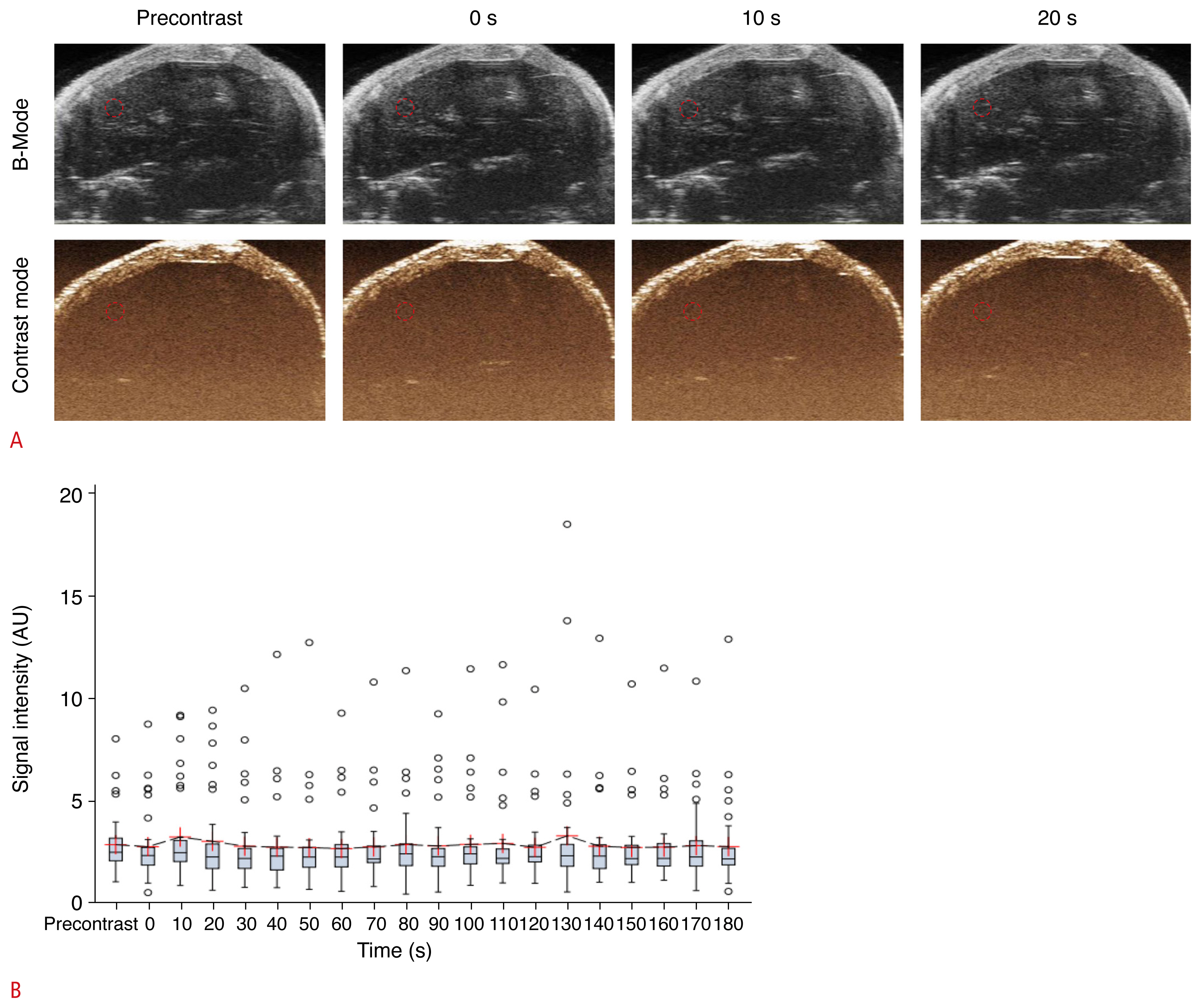 Fig. 2Post-operative gastric leakage model with Sonazoid per oral infusion.
A. Dual-mode display of contrast-enhanced ultrasonography (CEUS) images before and after oral Sonazoid administration in the stomach leakage model are shown. Interlobar spread of contrast medium (arrows) was observed starting 10 seconds after contrast medium injection. The dotted circle represents the region of interest used for signal intensity measurement. B. Box-whisker plot of the time-intensity curve of the signal intensity sampled from all mice (n=33) during CEUS is shown. The value of each time point was compared with that of the pre-contrast image (○, outliers; #P<0.05).
 Fig. 3Color dye (Evans blue) leakage study in a postoperative gastric leakage mouse model.
A. The stomach was surgically exposed, and a tweezer tip was inserted into the perforation site (arrow). B. Evans blue solution was infused via an orogastric tube, which leaked into the peritoneal cavity (arrows).
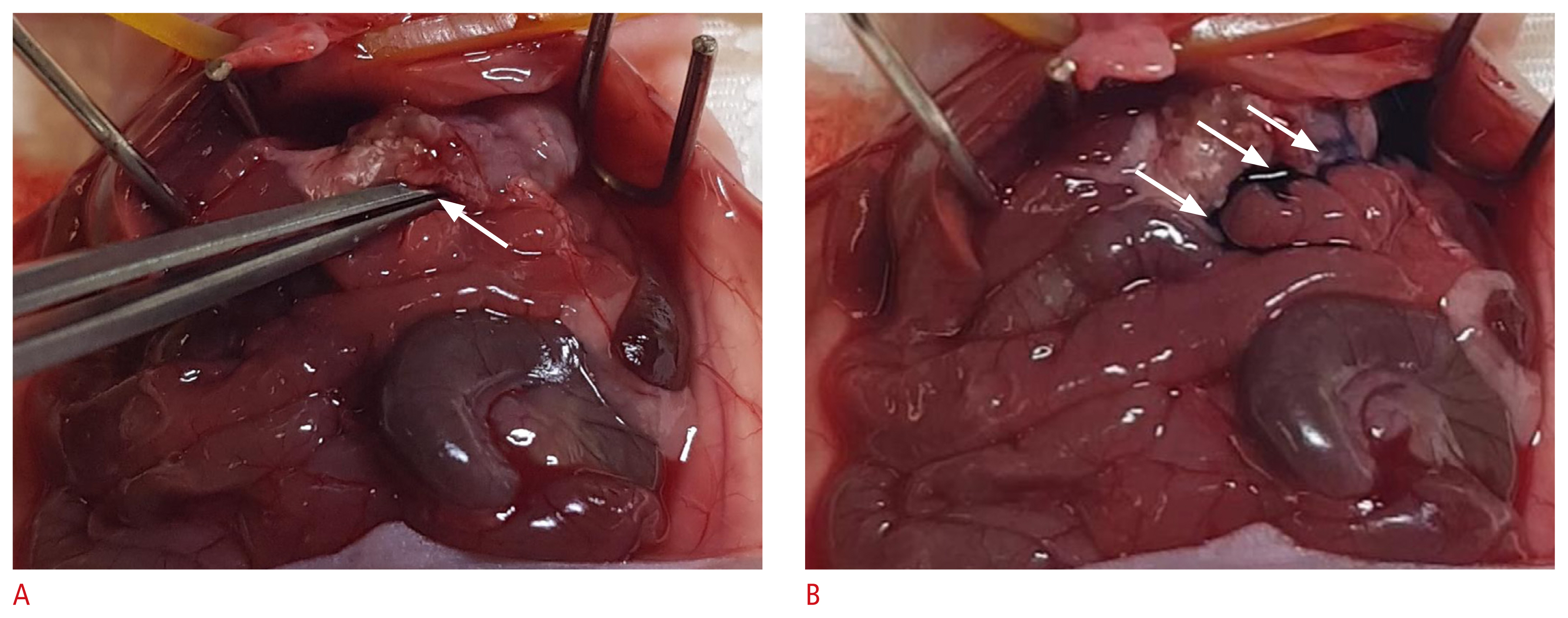 Fig. 4Table 1Signal intensity (AU) measured from a fixed region of interest on contrast-enhanced ultrasonography images obtained at different time points (time) before and after Sonazoid administration |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI




