Introduction
Nipple discharge accounts for 2%-10% of the symptoms that women report when they seek care at breast clinics [1,2] and is alarming for both patients and clinicians since it is a presenting sign of breast cancer. Nipple discharge can result from various physiologic or pathologic causes, and accurately identifying the causative condition is critical for patient management. Despite the anxiety it causes, most underlying causes of nipple discharge are benign, as the cancer rate in patients with nipple discharge has been reported to be 5%-21.3% [1,3-5]. A thorough investigation of the patient is warranted, with a physical examination and breast imaging to accurately distinguish patients who require surgical treatment for breast cancer from those who may be managed conservatively. Herein, we review the causes of nipple discharge, the ultrasonographic (US) imaging techniques used to evaluate the cause of nipple discharge, and the management of patients with nipple discharge.
Pathogenesis of Nipple Discharge
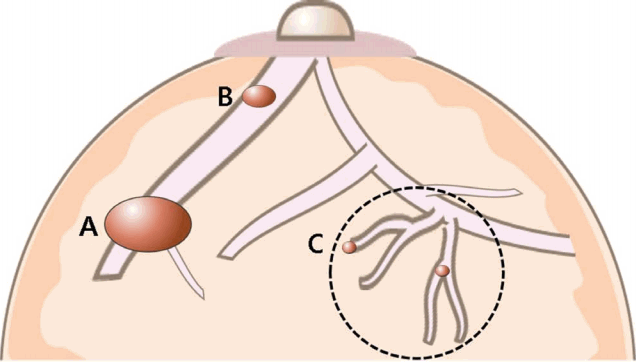
Nipple discharge is defined as a true, direct drainage from the mammary ducts that grossly appears at the surface of the nipple [2]. Grossly apparent nipple discharge is due to (1) excess secretions due to physiologic or hormonal causes, or (2) obstructive breast masses or lesions located within the ductal structures that either block the ductal drainage system or independently cause excess secretions within the duct (Fig. 1). Based on its origin, nipple discharge can be categorized as physiologic or pathologic discharge. Physiologic nipple discharge includes galactorrhea following normal hormonal stimulation during pregnancy or lactation. This condition can persist for more than 1 year after discontinuing breast feeding. Other than pregnancy or lactation, elevated levels of prolactin or thyroid-stimulating hormone can induce galactorrhea, and the clinician should determine whether underlying conditions causing these hormonal abnormalities are present.
Pathologic nipple discharge is defined as spontaneous, unilateral, bloody, or serous discharge, often arising from a single duct [1]. Common causes for pathologic nipple discharge are intraductal papilloma, duct ectasia, inflammation, and breast cancer, among which intraductal papilloma is most common, accounting for approximately 57% of cases [1]. Table 1 summarizes the characteristics of physiologic and pathologic nipple discharge.
Evaluation of Patients with Nipple Discharge
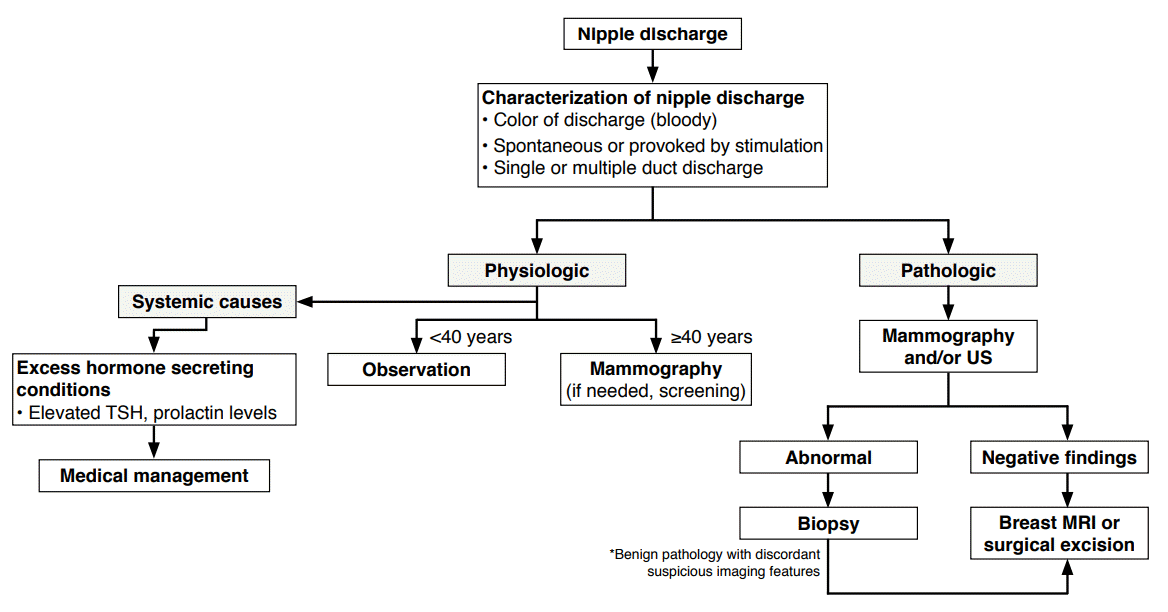
With the exception of physiologic galactorrhea seen in pregnant or lactating women, a thorough investigation of the cause of nipple discharge must be performed. A detailed history including the patient’s medical history and a physical examination evaluating the characteristics of the nipple discharge should be carried out as the first step; this is important, because these steps provide information useful for characterizing the nipple discharge and deciding upon the next step in patient management. A physical examination is needed to locate breast masses associated with the nipple discharge. If the discharge is considered to be physiologic based on the clinical information and characteristics of the nipple discharge, no further imaging studies of the breast are warranted if the patient is under the age of 40 years or over 40 years with up-to-date routine screening mammography. If the discharge is considered to be pathologic, all women should have breast imaging examinations, regardless of age (Fig. 2).
Imaging Workup
Approximately 80%-90% of patients with pathologic nipple discharge have been reported to have benign conditions [1,3-6], but since the risk of breast cancer cannot be completely excluded, surgical duct excision is commonly considered. Breast imaging is used to evaluate women with pathologic nipple discharge for two common purposes: first, to localize the lesion that can explain the origin of the pathologic nipple discharge, and second, to determine whether the pathologic nipple discharge is caused by a cancerous lesion. Several reports have proven that mammography and breast US are useful for detecting the pathologic causes of nipple discharge [3,7], and especially for detecting findings indicative of breast cancer. Still, controversy remains regarding whether breast imaging helps in triaging patients who need immediate surgical intervention from those who can be managed conservatively, but in general the role of breast imaging is being increasingly emphasized for the following reasons. First, breast imaging enables the localization of the breast abnormalities causing the pathologic nipple discharge, which helps to minimize the number of operations and/or the extent of surgery. Second, localizing the origin of pathologic nipple discharge enables percutaneous biopsy under imaging guidance, which allows clinicians to be more confident when deciding upon management, and in particular when choosing whether to perform minimally invasive percutaneous vacuum-assisted excision in these patients.
Imaging Modalities Used for Women with Nipple Discharge
The imaging findings associated with pathologic nipple discharge may vary according to its origin and the imaging modality applied. Among the common imaging modalities currently available for breast imaging, mammography is commonly recommended for women presenting with nipple discharge. Mammography can reveal masses, microcalcifications, and architectural distortions that may be associated with the underlying pathologic cause of nipple discharge. However, the sensitivity of mammography in detecting the lesion causing the pathologic nipple discharge is very low [6,7], since intraductal masses or masses in the subareolar region tend to be small and lack microcalcifications, in addition to the fact that the subareolar region normally shows increased density that can easily [7]. Ductography and galactography have been used in the past to visualize the number, location, and extent of the involved milk ducts in women with pathologic nipple discharge [5,8,9], but ductography is an invasive imaging method that requires iodinated contrast media injection, and also has low diagnostic accuracy since the differential diagnosis of the causative lesion cannot be completed based on ductographic findings alone [10,11]. Several studies have proposed applying breast magnetic resonance imaging (MRI) to evaluate patients who have pathologic nipple discharge, especially in patients who have negative findings on mammography or US [7,12-14], but the usefulness of MRI is limited by the fact that it is very expensive, not readily available in all areas, and requires contrast media injection.
Breast US in Women with Nipple Discharge
Breast US is used as an adjunct to mammography for breast imaging, enabling the further characterization of abnormalities detected on mammography and providing biopsy guidance. With recent improvements in technology, US is particularly useful in women with pathologic nipple discharge, since it enables the visualization of ductal pathologies that are smaller than a centimeter, as well as associated ductal changes that cannot be detected on mammography, especially in women with dense breasts [1,5,9,13]. For women with pathologic nipple discharge, US enables detection of the causative lesion(s), along with orientation of the surrounding ductal structures involved, which is helpful in planning the method of biopsy or the extent of surgery. Although mammography is recommended for women over 40 years old who exhibit pathologic nipple discharge, a recent study showed that adding US to diagnostic mammography can help detect additional cancers in women with pathologic nipple discharge [4]. Also, the addition of US in patients with pathologic nipple discharge who had negative findings on mammography led to the detection of malignancies in 15.1% of these patients by US-guided core needle biopsy, without additional diagnostic surgery [15]. Applying subareolar US in patients with pathologic nipple discharge has also eliminated ductography from diagnostic evaluations [6,16], since US is a less invasive imaging method that is more comfortable for the patient and involves no radiation exposure, while showing similar diagnostic performance to ductography [7]. The findings in the literature regarding the diagnostic performance of mammography, US, and breast MRI in patients with pathologic nipple discharge are summarized in Table 2.
Interpretation of Causative Lesions Detected on Breast US
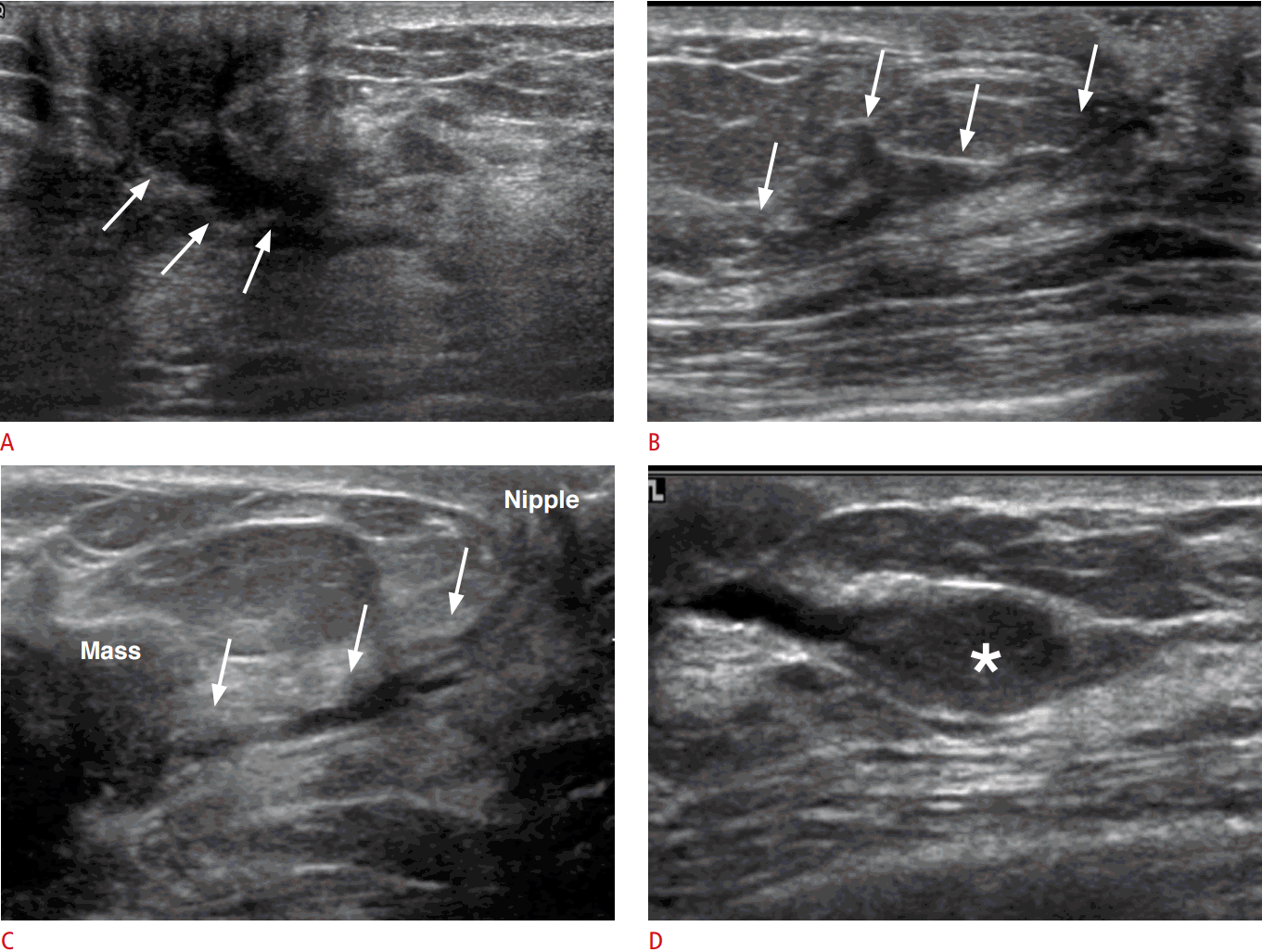
In patients with grossly apparent pathologic nipple discharge, localizing the breast mass causing the nipple discharge and evaluating the ductal structures involved with, surrounding, or connected to the causative mass is important for planning the extent of surgery or the further management of the patient. According to the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS) [17], various US features are used for describing and differentiating benign and malignant breast lesions. The ACR BI-RADS categorizes duct changes as “associated features,” and abnormal duct changes are defined as (1) cystic dilatation of duct/ducts containing irregular calibers or branching; (2) extension of dilated ducts from a malignant mass; or (3) the presence of an intraductal mass, thrombus, or debris (Fig. 3) [17]. Among the duct changes, intraductal masses are at present recommended to be assessed as category 4a, indicating a need for biopsy because these intraductal masses have an 8% risk of malignancy [17-19]. Still, it is not clear whether all intraductal masses should be biopsied, and considerable overlap is seen between benign and malignant intraductal masses, as demonstrated in a recent study [18]; although none of the intraductal masses that only partially filled the duct were proven to be cancers, approximately 92% of the intraductal masses that completely filled the duct were likewise shown to be benign. In addition, in cases when irregular masses show intraductal extension, a precise description of the extent of the surrounding ductal structures in addition to the irregular mass is required, because these ductal extensions often represent ductal carcinoma in situ components surrounding an invasive carcinoma. For intraductal masses, a description of the length of the duct segment containing the mass or debris, the size of the intraductal mass, and the distance from the nipple are important factors that are required in US reports.
Tips on Identifying Causes of Nipple Discharge Using Breast US
In patients with pathologic nipple discharge, US is capable of visualizing ductal structures located in the subareolar region that can be easily obscured on mammography in patients with dense breasts. One drawback of breast US is that it is operator-dependent, and visualization of the subareolar portion of the breast can be difficult, requiring experience in breast imaging. Ductal diseases are a major challenge in terms of diagnostic imaging, since visualizing ductal structures is particularly difficult, especially if the pathologic entity involves small distal ducts, and does not produce sufficient material to dilate the ducts. Adding to this, the acoustic shadowing that is commonly seen beneath the nipple-areolar complex due to the gathering of the major ducts, the uneven skin surfaces of nippleareolar complex, or the protuberance of the nipple itself interferes with clear visualization of the ductal structures.
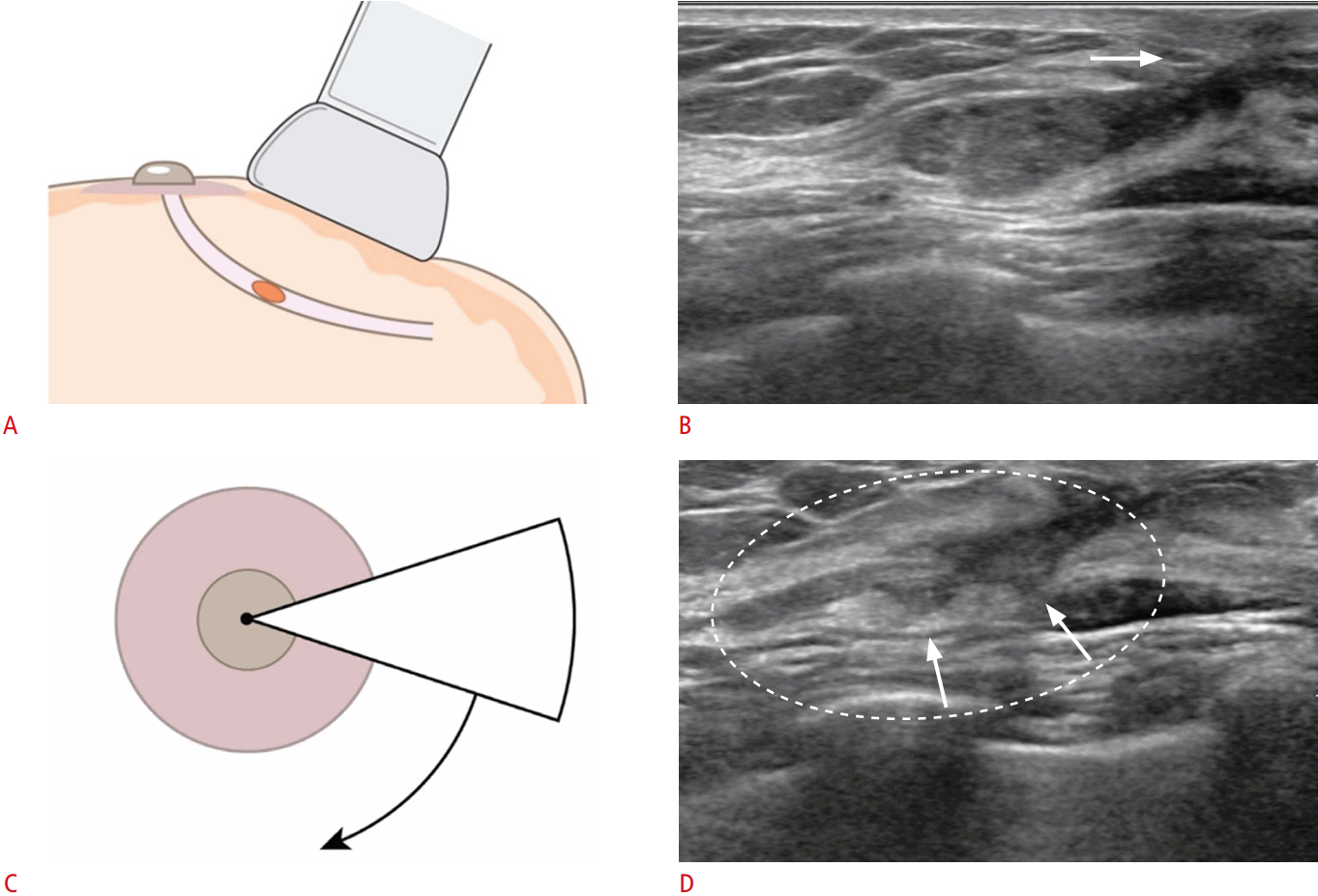
Radial Scans
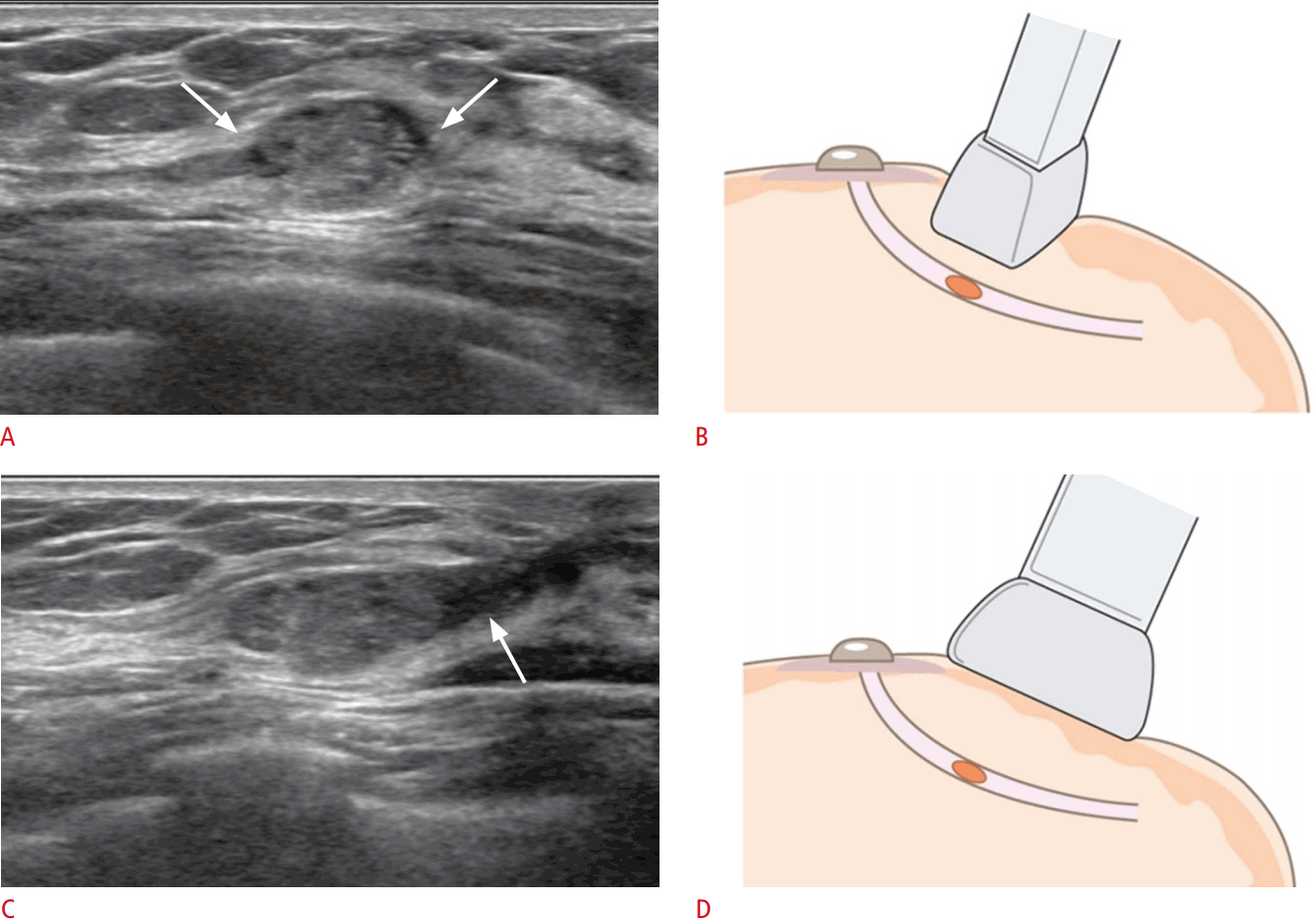
Applying the US probe at different angles helps to accurately detect the intraductal mass and/or to delineate the ductal structure involved. When an intraductal lesion is suspected, radial scans are helpful in visualizing the extent and direction of the duct involved, by positioning the US probe parallel to the long axis of the detected intraductal lesion (Fig. 4). Additionally, radial scans are helpful in detecting the ductal structures involved near the intraductal lesion causing the pathologic discharge, as well as lesions located in the peripheral ducts (Fig. 5).
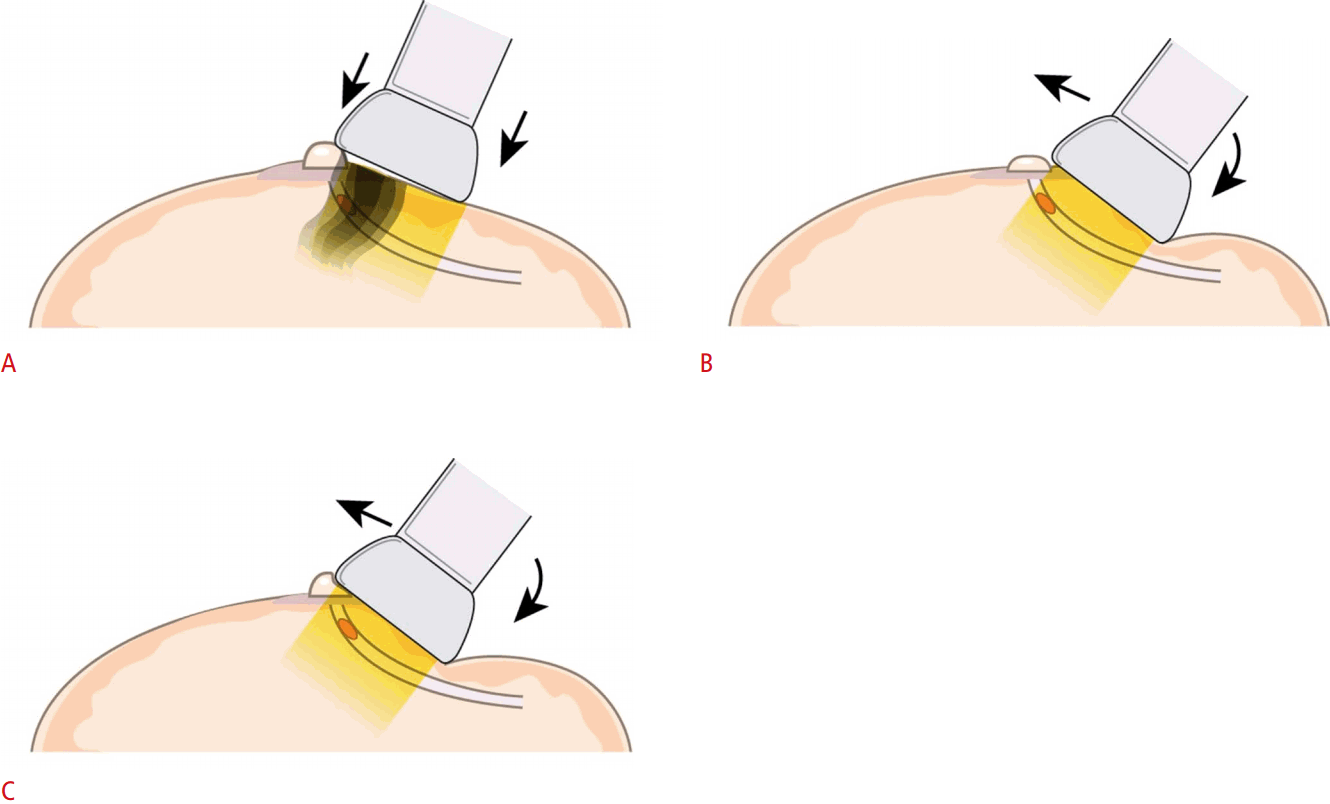
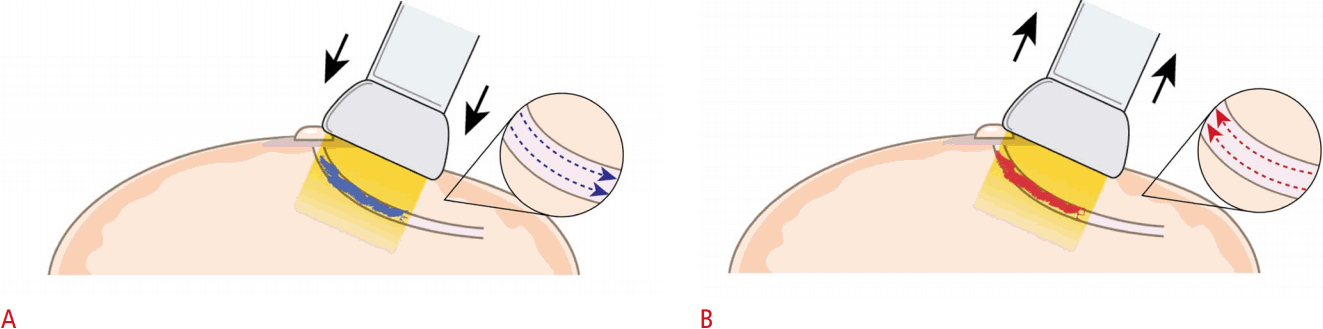
Peripheral Compression Technique
The nipple or areolar tissues may hinder the detection of lesions due to acoustic shadowing, so techniques for maneuvering the nipple area to reduce the shadowing helps in visualizing subareolar ductal structures. The transducer is positioned radially, parallel to the long axis of the diseased duct. Compression is then applied to the lateral end of the transducer, flattening out the nipple-areolar area and bending the nipple to the other side (Fig. 6).
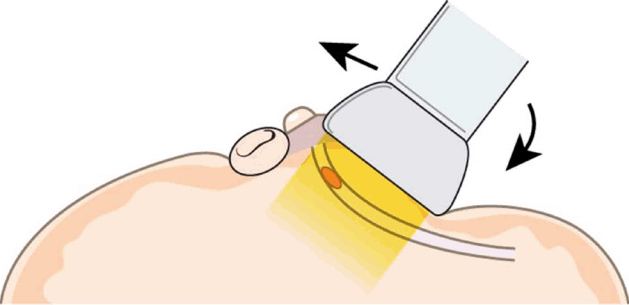
Rolled-Nipple Technique
While the peripheral compression technique involved bending the nipple with the transducer, the rolled-nipple technique requires manual compression by the performer. As the targeted duct is localized with the transducer positioned parallel to its long axis, the index finger of the free hand of the performer is positioned at the nipple, opposite from the transducer. By sliding the transducer towards the nipple, the nipple is gradually rolled over the index finger, producing firm adherence of the probe to the skin and flattening out the area that needs visualization (Fig. 7). When using this technique, performers must be cautious to apply light compression with the transducer to avoid collapsing the dilated ductal structures.
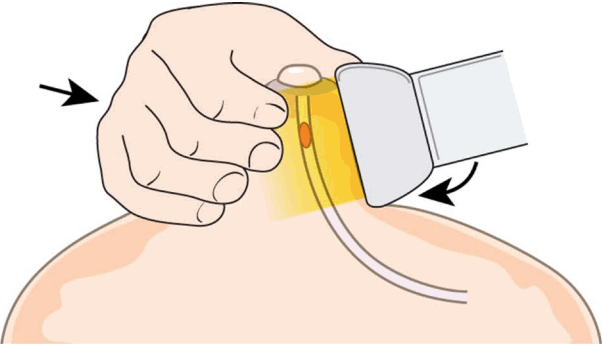
Two-Handed Compression Technique
Intraductal lesions often produce secretions that fill up the ducts, causing duct dilatation and nipple discharge. On breast US, these secretions appear as echogenic material within the ducts, mimicking intraductal solid masses. The two-handed compression technique is useful in differentiating intraductal debris from true masses, as the external compression can collapse ducts containing only secretory material, but not ducts containing masses. With the transducer positioned in the radial axis, the performer applies compression to the nipple region with both the transducer and the free hand not used for scanning (Fig. 8). The transducer is slid distally to include the nipple, and changes in the targeted duct reveal whether the lesion is a true mass or secretory debris.
Dynamic Maneuvers Using Doppler Imaging
Another technique that can be used to distinguish between true intraductal masses and secretory material is the use of Doppler images. Dilated ductal structures filled with secretory materials easily collapse when external compression is applied, and displacement and/or changes in the echogenicity of such materials can be seen on real-time US imaging, whereas the location and US characteristics of intraductal masses do not change due to compression. When Doppler scans are applied, the displacement of intraductal debris produces Doppler signals, as the secretory materials swish back and forth according to the alternating compression and release caused by applying the probe (Fig. 9).
Management of Women with Pathologic Nipple Discharge
At present, the management of women with pathologic nipple discharge varies across institutions, and there are no solid data regarding which clinical or radiological features accurately distinguish malignancies from lesions with a benign etiology [6,7]. Among the methods of evaluating patients with pathologic nipple discharge, breast US has the major advantage of enabling imaging-guided percutaneous biopsy, which is less invasive than surgical excision and facilitates making a preoperative diagnosis that substantially affects decision-making about management. Duct excision is an invasive procedure that can be difficult in patients in whom the affected duct cannot be localized or if the affected duct is located posteriorly, and has the risk of postoperative complications [12]. Since most patients with pathologic nipple discharge ultimately have benign conditions, a less invasive diagnostic procedure is more favorable for both the patient and clinician. Based on the pathologic diagnosis on US-guided percutaneous biopsy, conservative follow-up or subsequent vacuum-assisted excision can be considered for patients diagnosed with a benign lesion, while direct curative surgery can be planned for patients with a malignancy, avoiding additional surgery for diagnostic purposes.
As the accurate preoperative differential diagnosis of women with pathologic nipple discharge is difficult, diagnostic strategies combining clinical, radiological, and cytopathologic information have been proposed for diagnostic purposes and for planning the further management of patients with pathologic nipple discharge (Fig. 10) [6,16,20]. Among the causes of pathologic nipple discharge, intraductal papillomas, mostly in solitary form, are known to be the most common cause of pathologic nipple discharge [21]. Intraductal papilloma is part of a spectrum of papillary neoplasms ranging from benign papillomas to papillary carcinoma [22]. Distinguishing malignant papillary neoplasms from benign lesions is very difficult, even with additional immunohistochemistry staining of a biopsy specimen [23], and the significance of papillary neoplasm as a risk for breast cancer and the proper management of such neoplasms are still under debate. The rate at which papillomas are upgraded to malignancies after surgical excision has been reported to be 5%- 21% [24-28], and although various factors such as older age, size, and the presence of suspicious US features have been reported to predict such an upgrade, no solid evidence exists regarding which factors can be used to predict the diagnosis of benign papillomas. Based on these upgrade rates, most studies support complete surgical excision after diagnosis [24-28]. Still, more than 80% of these masses will be confirmed as benign; therefore, more and more studies have reported the use of less invasive excision strategies, such as US-guided vacuum-assisted excision, for benign papillomas [22,29-33].
In the evaluation of patients with pathologic nipple discharge, it is not rare to encounter patients with no specific findings on any sort of examination. Based on the low risk of underlying malignancy (0%-5%), several recent studies have proposed conservative follow-up for patients with a physical examination suggesting a benign condition, negative mammography results, and negative features on breast US [3,10,16,32]. Close observation and regular check-up with mammography and US for these patients are warranted; approximately 80% of cases of pathologic nipple discharge resolve spontaneously within 2 years in patients with a benign etiology [6]. If pathologic nipple discharge is persistent or recurrent after 2 years of monitoring or if the patient is unable to follow the monitoring schedule, duct exploration or excision must be considered to detect a malignancy that is occult on mammography or US [3,12,31,32]. In addition, using additional imaging modalities such as breast MRI, with its high sensitivity in detecting occult malignancies [12,14,34], or US elastography [35] has been proposed since they may enable localization and/or provide further information regarding the causative lesion. However, at present, no strict guidelines exist regarding the evaluation or management of patients with pathologic nipple discharge, and large prospective studies are warranted to develop reasonable guidelines for these patients.
Summary
Nipple discharge results from a range of causes, which may be either physiologic or pathologic. Combining the clinical, radiological, and cytopathologic features of the patient may be useful for predicting malignancy in these patients. Breast US enables the visualization of the ductal structure and intraductal causes of pathologic nipple discharge, and facilitates a subsequent imaging-guided percutaneous biopsy. Being familiar with the various US techniques that assist in the detailed visualization of subareolar ductal structures is helpful for accurately detecting and diagnosing lesions. Further prospective studies are warranted to establish standard guidelines for the management of patients with pathologic nipple discharge.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




