Chung and Cho: Ultrasonography of soft tissue “oops lesions”
Abstract
In this article, I would like to define “oops lesions” as soft tissue mass-like lesions that involve surprise or embarrassment for radiologists following the final diagnosis. Examples of “oops lesions” include malignant tumors that appear benign, malignancy-mimicking benign tumors, incorrect identification of epidermal inclusion cysts, and soft tissue pseudotumors. Ultrasonography (US) findings are very helpful in the diagnosis of soft tissue tumors; however, the diagnosis of soft tissue tumors on the basis of US findings alone has some limitations. Therefore, clinical findings, laboratory data, findings from additional imaging modalities, and demographic data of patients should be considered together with US findings.
Keywords: Soft tissue neoplasms; Musculoskeletal diseases; Ultrasonography
Introduction
“Oops lesions” could be defined as soft tissue mass-like lesions that involve surprise or embarrassment for radiologists following the final diagnosis. The surprise might represent good or bad news; regardless, it is due to obtaining results that differ from clinical expectations. When a final diagnosis is reached, different responses are possible: “oops” or not; good or bad. If a lesion suspected to be benign is actually confirmed as a malignancy, it is an “oops lesion” and is also sad news. The opposite situation is also an “oops lesion” but is good news. In contrast, when an expected malignancy is confirmed, it is not an “oops lesion” but just sad news. In this article, such surprising or embarrassing cases of soft tissue mass-like lesions will be presented in detail. Examples of “oops lesions” include malignant tumors that appear benign, malignancy-mimicking benign tumors, incorrect identification of epidermal inclusion cysts, and soft tissue pseudotumors. “Oops lesions” could be defined as soft tissue mass-like lesions that involve surprise or embarrassment for radiologists following the final diagnosis. The surprise might represent good or bad news; regardless, it is due to obtaining results that differ from clinical expectations. When a final diagnosis is reached, different responses are possible: “oops” or not; good or bad. If a lesion suspected to be benign is actually confirmed as a malignancy, it is an “oops lesion” and is also sad news. The opposite situation is also an “oops lesion” but is good news. In contrast, when an expected malignancy is confirmed, it is not an “oops lesion” but just sad news. In this article, such surprising or embarrassing cases of soft tissue mass-like lesions will be presented in detail. Examples of “oops lesions” include malignant tumors that appear benign, malignancy-mimicking benign tumors, incorrect identification of epidermal inclusion cysts, and soft tissue pseudotumors.
Benign-Appearing Malignant Tumors
The ultrasonography (US) findings that might suggest benignancy of a soft tissue mass are a small size, superficial location, homogeneous echo pattern, and hypovascularity. However, these findings are not sufficiently reliable to definitively characterize the nature of a lesion [ 1, 2]. US findings can be misleading, since similar findings also occur in synovial sarcoma ( Fig. 1), liposarcoma, melanoma, lymphoma, myeloid sarcoma ( Fig. 2), and small metastasis ( Fig. 3). Superficial location refers to the subcutaneous tissue and skin above the superficial investing fascia, which separates the subcutaneous tissue layer from the underlying muscle [ 3]. For soft tissue tumors, large size, deep location, heterogeneous signal intensity and echo, and internal hemorrhage or necrosis suggest the possibility of malignancy rather than a benign tumor [ 4- 7]. To date, the soft tissue sarcoma clinical practice guidelines (National Institute for Health and Care Excellence [NICE] and European Society for Medical Oncology [ESMO]) have used 5 cm as the cut-off size for referral, together with other features such as depth in relation to fascia and increasing size. Other tertiary referral institutions use a 3-5 cm size as a cut-off for risk of malignancy in superficial masses [ 8]. For superficial masses, there are some factors indicative of malignancy. Unlike deep-seated lesions, size is not an important factor because a significant proportion of superficial malignant tumors measure less than 5 cm in maximal diameter. Fascial edema, skin thickening, skin contact, hemorrhage, and necrosis are highly significant factors indicative of malignancy. Lobulation and peritumoral edema are also significant features [ 9]. Obtuse angles between the superficial investing fascia and the subcutaneous mass crossing the fascia strongly suggest malignancy [ 3]. Galant et al. [ 3] described these fascia-tumor relationships with schematic representation ( Fig. 4). Malignant tumors of the subcutaneous compartment have a higher tendency to develop a close relationship with the fascia than benign lesions ( Figs. 1B, 5).
Malignancy-Mimicking Benign Mass Lesions
Malignancy should be considered when imaging findings are as follows: complex peri-lesional or intra-lesional changes, internal hemorrhage, hypervascularity, or large-sized mass [ 1, 2, 10]. However, these findings can also be present in benign tumors. Ruptured masses or combined infection can cause difficulty in the differential diagnosis of benign and malignant tumors. For cases of ruptured epidermal inclusion cysts, a thick and irregular peripheral rim and septa could be reminiscent of inflammatory lesions such as an abscess or a neoplastic lesion ( Fig. 6) [ 11]. Thus, benign masses can mimic malignancy in cases of post-traumatic hematoma ( Fig. 7), myositis ossificans, ruptured benign masses, immature granulomatous lesions, and fibrous lesions ( Figs. 8, 9). Usually, a large-sized, deep-seated mass has a high probability of malignancy; however, this link does not occur in all cases [ 4- 7]. Benign soft tissue tumors such as schwannoma ( Fig. 10), nodular fasciitis, lipoma, and hibernoma can be large enough to be misinterpreted as a malignancy.
Incorrect Identification of Epidermal Inclusion Cysts
The US findings of epidermal inclusion cysts are well known. An epidermal inclusion cyst appears as a small, well-defined subcutaneous or cutaneous mass. The sonographic appearance of epidermal inclusion cysts varies with the contents of the cyst, from an anechoic lesion to a hyperechoic, solid-appearing mass [ 12, 13]. The possibility of an epidermal inclusion cyst should be considered when a patient presents with a mass located at the epidermis and subcutaneous tissue that has a demonstrated subtle heterogeneous echo pattern. Most radiologists are familiar with these typical US findings indicating an epidermal inclusion cyst; however, there are sometimes tricky cases that lead to a pathologic diagnosis contrary to expectation that can cause embarrassment. The imaging findings of an unruptured epidermal inclusion cyst may be similar to other subcutaneous cystic masses, some solid tumors, and vascular lesions. Thus, differential diagnoses include ganglion or bursitis with internal hemorrhage or debris, neurogenic tumors, nodular fasciitis, myxoid tumors, dermatofibrosarcoma protuberans, and hemangioma [ 11]. The diagnosis of an epidermal inclusion cyst is often confused with perineurioma (an uncommon benign tumor of peripheral nerves composed primarily of perineurial cells [ 14]), schwannoma ( Fig. 11), dermatofibrosarcoma protuberans, and fibrosarcoma arising from dermatofibrosarcoma protuberans ( Fig. 12).
Soft Tissue Pseudotumors
Soft tissue pseudotumors include non-neoplastic lesions that are mistaken for neoplastic lesions following imaging studies or clinical examination. Moreover, exophytic bone lesions such as osteochondroma can be misinterpreted as soft tissue tumors. Therefore, many conditions could be involved in a diagnosis of a pseudotumor: inflammatory lesions (cellulitis, abscess, subcutaneous granuloma annulare, parasite infestation); vascular/lymphatic lesions (aneurysm, thrombus, lymphadenitis); posttraumatic lesions such as muscle tear ( Fig. 13), muscle herniation, hematoma, fat necrosis, myositis ossificans; a foreign body reaction such as foreign body granuloma ( Fig. 14); crystal deposition disease (gouty bursitis); bone tumor (osteochondroma); and normal variation (accessory muscle, sesamoid bone, carpal boss) [ 15]. Upon imaging, many cases of soft tissue pseudotumors appear cystic in nature and occur at common sites. However, they can show a mixed or solid echo pattern and be detected at unusual sites. In these cases, lesions could show atypical imaging findings and therefore be difficult to diagnose correctly.
Conclusion
US findings are helpful in the diagnosis of soft tissue tumors; however, the diagnosis of soft tissue tumors on the basis of US findings alone has some limitations. Thus, it is not unexpected that “oops lesions” are encountered during clinical practice. Therefore, when a differential diagnosis for a soft tissue mass is made, all available information should be considered, such as demographic data, laboratory findings, and findings from other imaging modalities. In addition, practitioners should be familiar with the variability in US imaging findings.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
1. Lagalla R, Iovane A, Caruso G, Lo Bello M, Derchi LE. Color Doppler ultrasonography of soft-tissue masses. Acta Radiol 1998;39:421–426.   2. Bodner G, Schocke MF, Rachbauer F, Seppi K, Peer S, Fierlinger A, et al. Differentiation of malignant and benign musculoskeletal tumors: combined color and power Doppler US and spectral wave analysis. Radiology 2002;223:410–416.   3. Galant J, Marti-Bonmati L, Soler R, Saez F, Lafuente J, Bonmati C, et al. Grading of subcutaneous soft tissue tumors by means of their relationship with the superficial fascia on MR imaging. Skeletal Radiol 1998;27:657–663.   5. De Schepper AM, De Beuckeleer L, Vandevenne J, Somville J. Magnetic resonance imaging of soft tissue tumors. Eur Radiol 2000;10:213–223.   6. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol 2000;175:575–587.   8. Morel M, Taieb S, Penel N, Mortier L, Vanseymortier L, Robin YM, et al. Imaging of the most frequent superficial soft-tissue sarcomas. Skeletal Radiol 2011;40:271–284.   9. Calleja M, Dimigen M, Saifuddin A. MRI of superficial soft tissue masses: analysis of features useful in distinguishing between benign and malignant lesions. Skeletal Radiol 2012;41:1517–1524.   10. Hwang S, Panicek DM. The evolution of musculoskeletal tumor imaging. Radiol Clin North Am 2009;47:435–453.   11. Hong SH, Chung HW, Choi JY, Koh YH, Choi JA, Kang HS. MRI findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am J Roentgenol 2006;186:961–966.   12. Vincent LM, Parker LA, Mittelstaedt CA. Sonographic appearance of an epidermal inclusion cyst. J Ultrasound Med 1985;4:609–611.   13. Lee HS, Joo KB, Song HT, Kim YS, Park DW, Park CK, et al. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J Clin Ultrasound 2001;29:374–383.   14. Lazarus SS, Trombetta LD. Ultrastructural identification of a benign perineurial cell tumor. Cancer 1978;41:1823–1829.   15. Navarro OM, Laffan EE, Ngan BY. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics 2009;29:887–906.  
Synovial sarcoma in a 40-year-old woman.
A. Ultrasonography shows a 1.5 cm homogeneously hypoechoic subcutaneous mass lesion in the lateral aspect of the left knee joint. B. Another image shows the tadpole-shaped area of the mass attached to the deep fascia (arrows), indicating a close relationship with the fascia and the mass. This mass was confirmed as a synovial sarcoma.
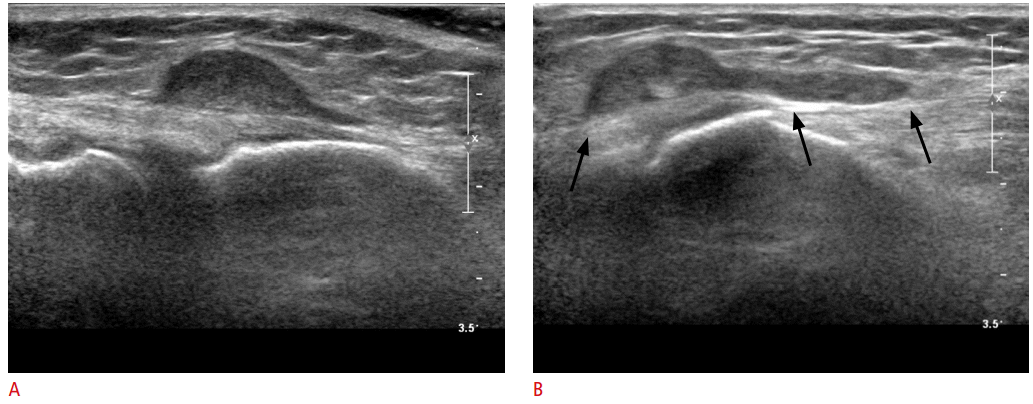 Fig. 1.
Myeloid sarcoma in a 56-year-old man.
A. Upon positron emission tomography imaging, multiple intra-abdominal hypermetabolic masses (arrows) are demonstrated. Additionally, another mass is noted at the subxiphoid area (arrowhead). B. Longitudinal sonogram reveals a mass-like lesion with a heterogeneous and mixed echo pattern, but no discrete margin at the sub-xiphoid area. This was confirmed as myeloid sarcoma in an acute myeloid leukemia patient after ultrasound-guided needle biopsy.
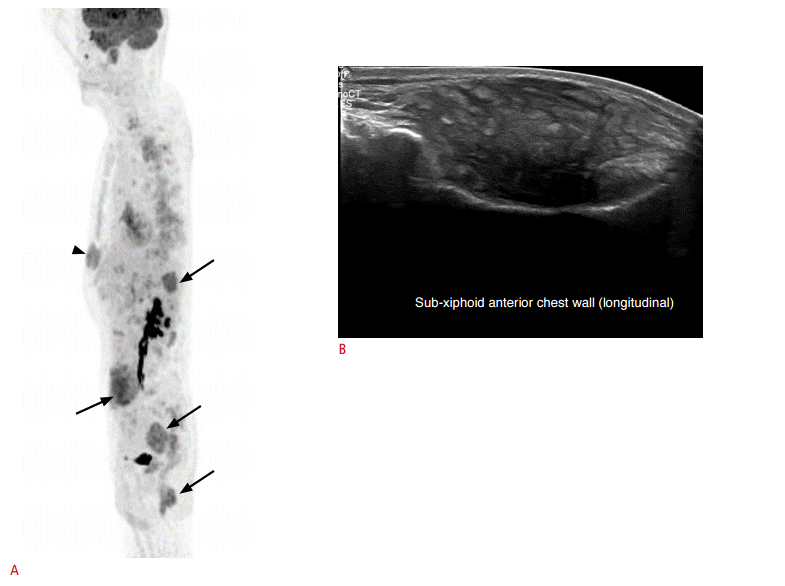 Fig. 2.
Metastasis from renal cell carcinoma in a 60-year-old man.
A. Ultrasonography (US) shows a small, well-defined intramuscular mass in the buttock with homogeneous echo pattern. B. However, the mass shows internal vascularity upon a Doppler US examination. The final diagnosis was metastasis from renal cell carcinoma.
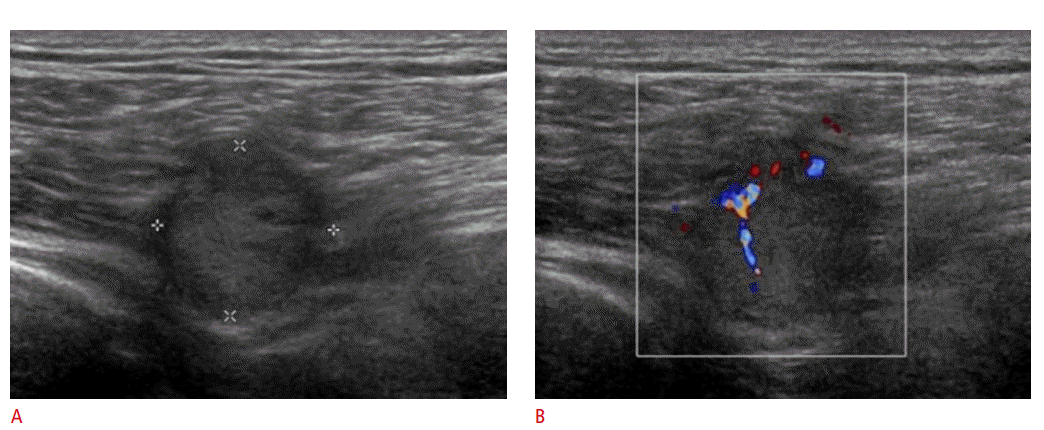 Fig. 3.
Schematic representation of fascia-tumor relationships.
Tumors are classified into five groups. Tumors without fascial contact (group 1) or with minimal fascial contact (group 2) could be benign, while lesions that contact the fascia widely with obtuse contact angles (group 4) or cross the fascia (group 5) strongly suggest malignancy. Modified from Galant et al. Skeletal Radiol 1998;27:657-663 [ 3], with permission of Springer.
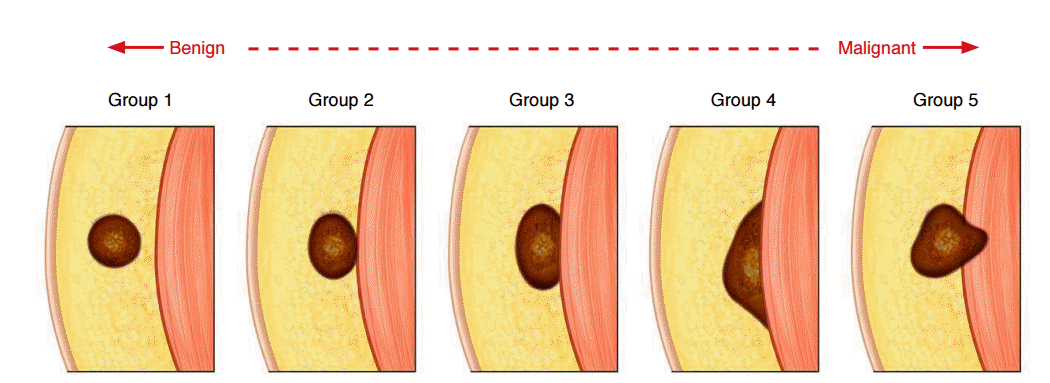 Fig. 4.
Undifferentiated pleomorphic sarcoma in a 73-year-old man experiencing mild swelling and discomfort in his lateral leg.
A, B. Two axial magnetic resonance images (A, T2-weighted; B, contrast-enhanced T1-weighted) show regional thickening and enhancement along the superficial investing fascia of the leg (arrows). Muscles deep to this fascia lesion also show signal change and enhancement. A nodular area is seen on the enhanced image (arrowhead). The bright round structure protruding from skin surface is a skin marker (asterisks). The initial impression was fasciitis. C. Upon ultrasonography examination, the nodular area is seen as a solid hypoechoic mass broadly attached to the fascia. The fascia itself is diffusely thickened along its course (arrows), which was histopathologically revealed to be a diffuse infiltration of sarcoma. Muscles below the fascia also showed tumor infiltration. No inflammation was found.
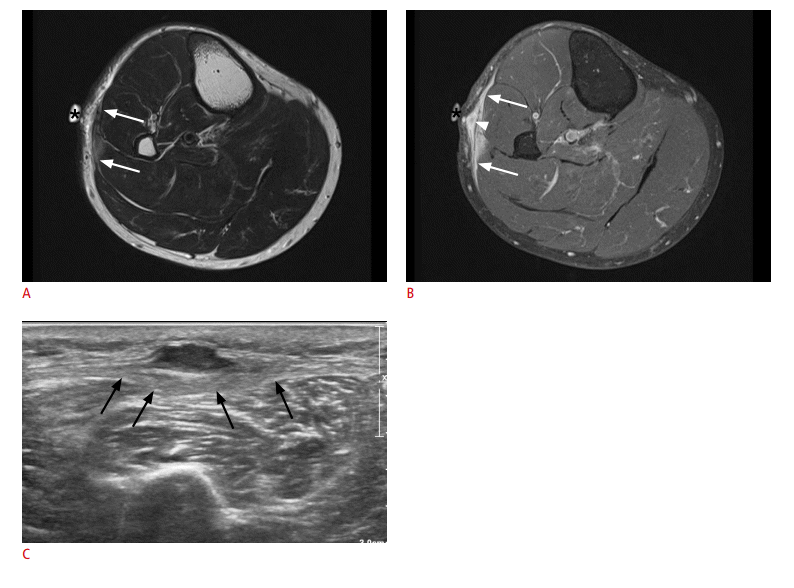 Fig. 5.
Ruptured mass in the subclavicular area in a 23-year-old woman.
A. Ultrasonography (US) shows an irregularly marginated hypoechoic lesion mainly located in the left subclavian subcutaneous tissue layer. B. On Doppler US, blood flow looks increased in and at the periphery of the mass. The mass was diagnosed as a ruptured epidermal inclusion cyst.
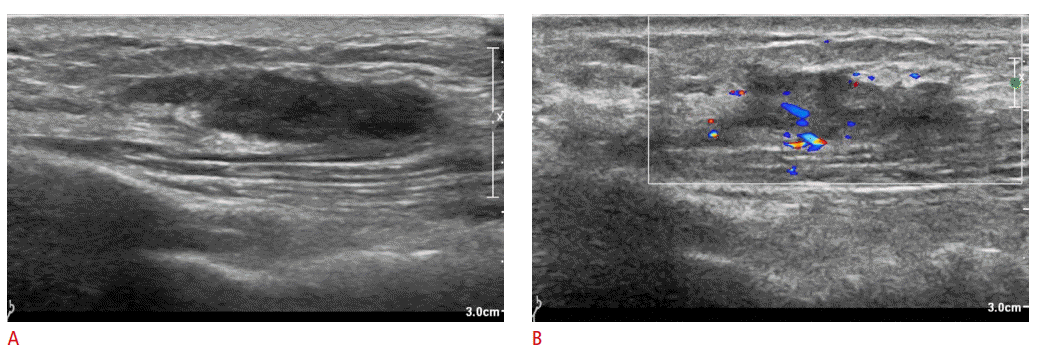 Fig. 6.
Hematoma in a 57-year-old woman.
A. Ultrasonography shows a well-defined heterogeneously hyperechoic mass on the abdominal wall. At the corner of the mass biopsy a needle is seen (arrow). B. Computed tomography shows a well-defined mass with a postsurgical clip at the skin. This was a postoperative hematoma.
 Fig. 7.
Nodular fasciitis in a 40-year-old man.
A. There is a well-defined intramuscular mass at the shoulder with peritumoral edema on the T2-weighted magnetic resonance image. B, C. This mass shows heterogeneous echogenicity on ultrasonography (US) (B) and internal vascularity on Doppler US (C). The initial impression was a malignant tumor. However, the pathologic diagnosis was nodular fasciitis.
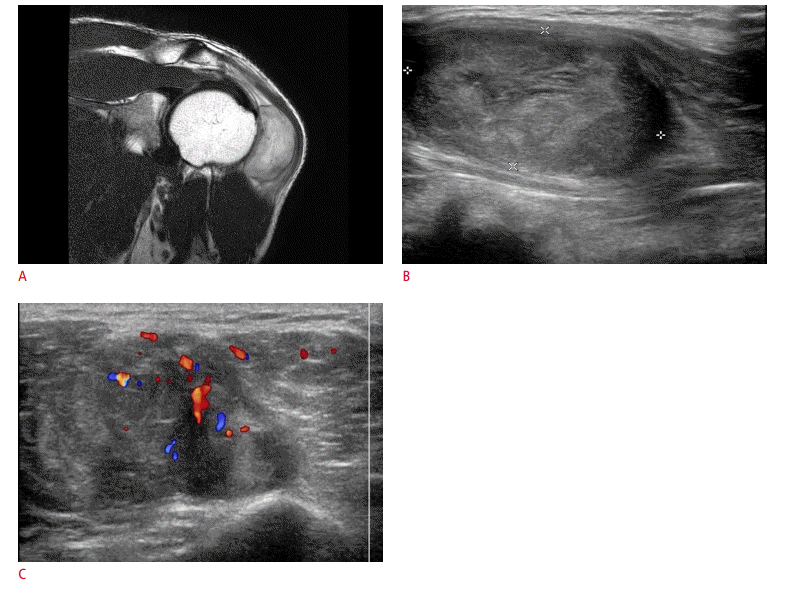 Fig. 8.
Fibromatosis in a 22-year-old woman presenting with an accidentally detected mass in her calf.
A. Ultrasonography demonstrates a solid mass with heterogeneous echo pattern (arrows) in the gastrocnemius muscle, showing a somewhat aggressive appearance. B. However, a T2-weighted magnetic resonance image shows a soft tissue mass with an infiltrating margin and low signal areas within the mass, suggesting fibromatosis. Fibromatosis was histopathologically confirmed.
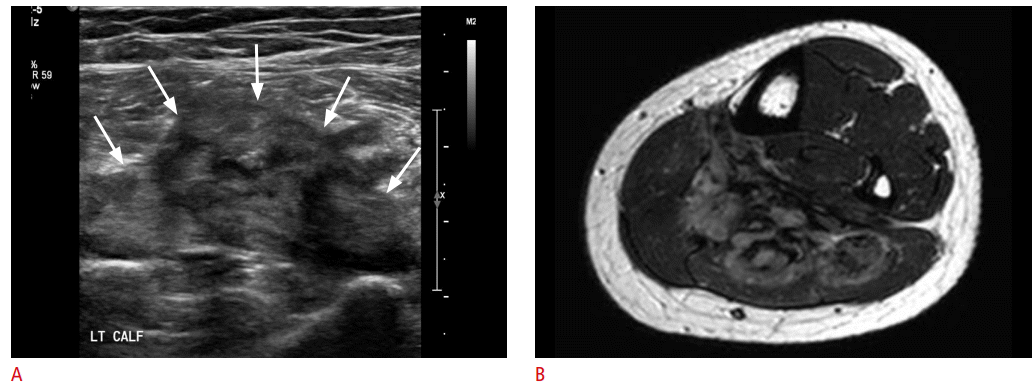 Fig. 9.
Schwannoma of a 47-year-old woman.
A. A large lobulated hyperintense mass is seen in the gluteus maximus muscle on a T2-weighted short tau inversion recovery magnetic resonance image. B. Upon ultrasonography, this mass shows a slightly heterogeneous echo pattern and there shows mild blood flow to the mass. The mass was confirmed as a schwannoma after excision.
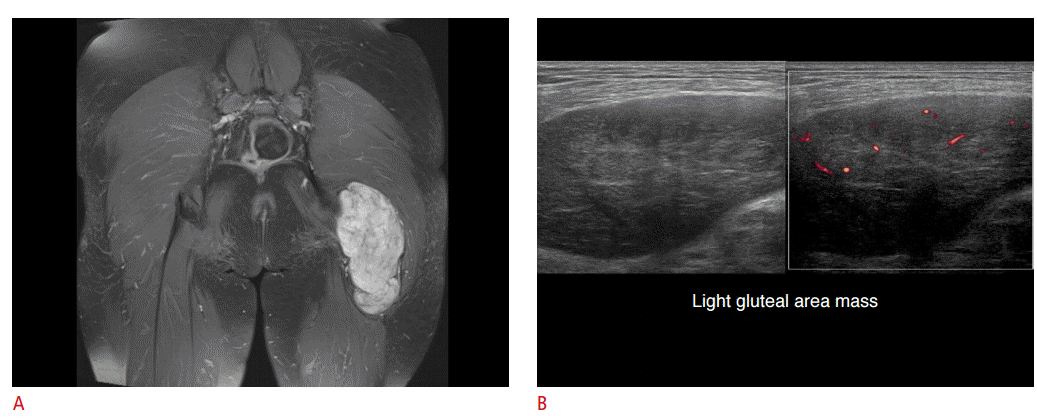 Fig. 10.
Schwannoma located in the skin and subcutaneous tissue of a 15-year-old boy.
Ultrasonography shows a well-defined mass located at the skin and subcutaneous tissue. This mass shows slightly hyperechoic areas and a peripheral hypoechoic band with associated posterior enhancement. After excision, it was confirmed as a schwannoma.
 Fig. 11.
Fibrosarcoma arising from dermatofibrosarcoma protuberans.
A. Ultrasonography shows an epidermal inclusion cyst-like soft tissue mass identified in the subdermal tissue of the back. B. However, a gross specimen shows a solid mass, distinct from an epidermal inclusion cyst. The histopathologic diagnosis was dermatofibrosarcoma protuberans with focal areas of fibrosarcoma in the mass.
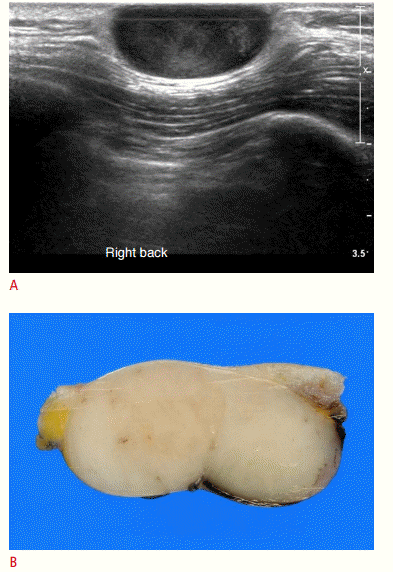 Fig. 12.
Sonogram of a 41-year-old man with a chronic muscle tear presenting with a pseudotumor in the right upper arm.
There is no soft tissue tumor, but a mild bulging contour (arrowheads) in the upper arm. A localized hyperechoic region (arrows) is noted at the musculotendinous junction of the biceps brachii, just distal to the bulging contour of the upper arm. These findings suggest a chronic tear at the musculotendinous junction.
 Fig. 13.
Foreign body granuloma in a 67-year-old man.
A. A rim-enhancing cystic mass (arrow) is identified at the sacral level on axial fat-suppressed contrast-enhanced T1-weighted magnetic resonance imaging. The patient had a history of posterior decompression and fixation surgery of the lumbar spine due to spinal stenosis 18 months prior. B. Ultrasonography shows a cystic mass containing hyperechoic nodular areas (arrows). C. A folded sheet of gauze was found during subsequent surgery. The pathological diagnosis was a foreign body granuloma and a sheet of gauze, leading to a diagnosis of gossypiboma.
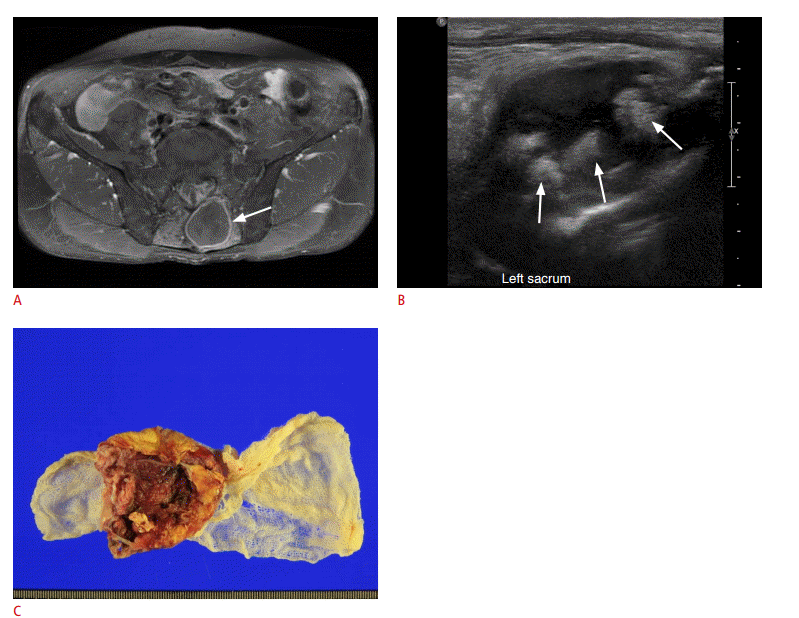 Fig. 14.
|
|
| TOOLS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
METRICS

|
|
|
|
|
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+














 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




