Introduction
The role of radiologists in the treatment of parathyroid disease was previously limited to the preoperative localization of hyperfunctioning parathyroid lesions [1]. However, radiologists have been required to play a more active role, because it is increasingly necessary to distinguish parathyroid incidentalomas (PTIs) detected during thyroid ultrasonography (US), from thyroid lesions [2-5]. Moreover, some alternative nonsurgical treatments for parathyroid lesions have also emerged [6-14]. This article reviews evolving role of radiologists in the treatment of parathyroid disease.
Normal Anatomy and US Examination of Parathyroid Glands
Normal parathyroid glands are very small, measuring approximately 6 mm in the craniocaudal dimension and 3-4 mm in the transverse dimension, with shape like a flattened disk. Normal-sized parathyroid glands are not usually identified by most imaging modalities. Therefore, a parathyroid gland that is visible in US is very suspicious for the presence of a pathological entity [15]. The superior parathyroid glands are typically located on the posterior aspect of the upper thyroid lobes, with little anatomic variation in the population. The inferior parathyroid glands have a more variable location due to their embryologic relationship to the thymus. The inferior parathyroid glands are located along the lateral lower pole of the thyroid gland in 50% of the population, whereas they are located 1 cm below the lower thyroid lobe in 15% of the population [1], although they can be located anywhere between the angle of the mandible and the upper mediastinum. The incidence of intrathyroidal parathyroid tissue is approximately 2% [16]. Most people have four parathyroid glands, although supernumerary glands have been found in 13% of the population, most commonly in the thymus [1]. An examination for suspected parathyroid enlargement should include bilateral longitudinal and transverse images from the carotid arteries to the midline, with the carotid artery bifurcation as the superior border and the thoracic inlet as the inferior border [17,18].
Localization of Hyperfunctioning Parathyroid Lesions
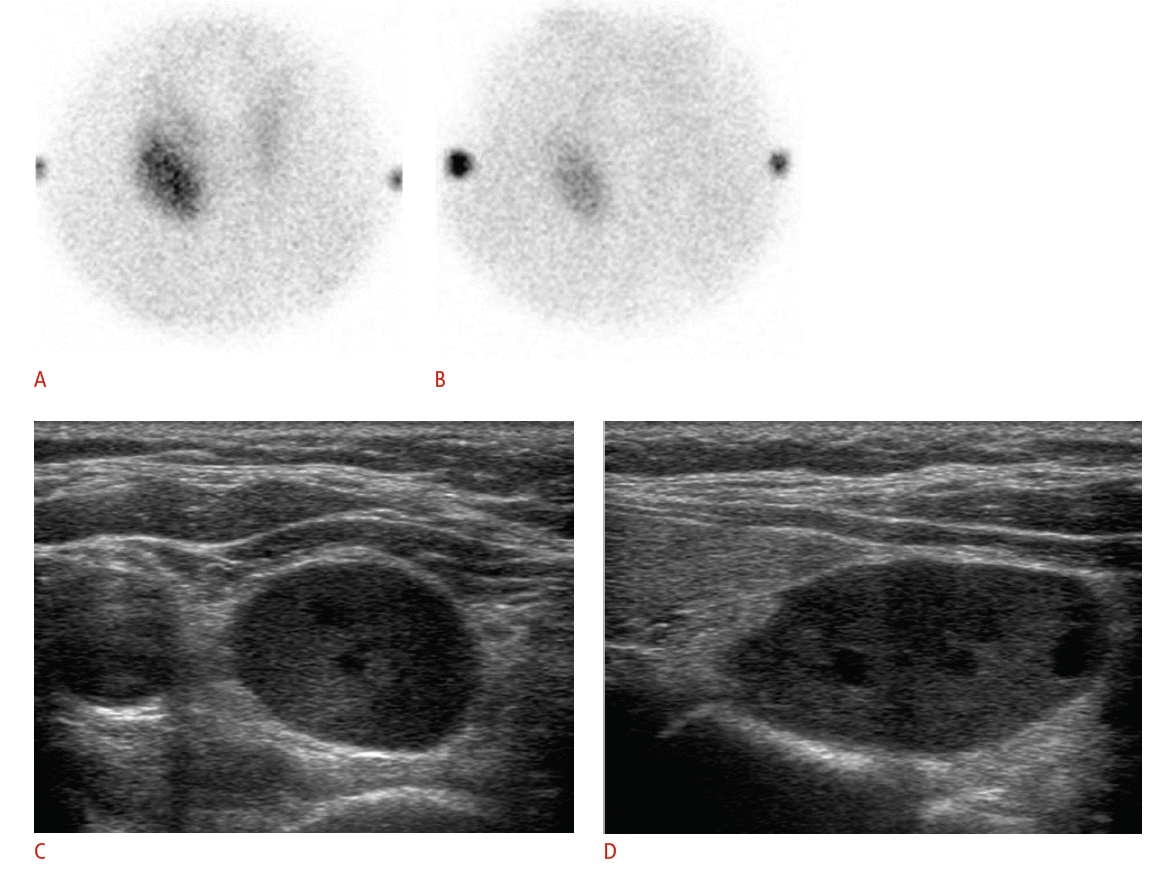
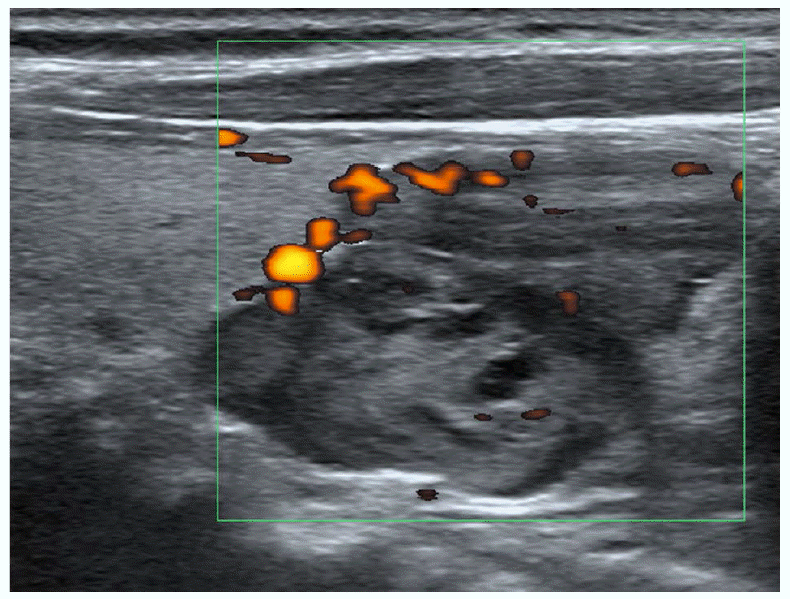
The pathologic lesions associated with primary hyperparathyroidism (HPT) include solitary adenoma (80%-85%), multiglandular disease (15%-20%), and carcinoma (<1%). Multiglandular diseases of the parathyroid are due to hyperplasia of all of the parathyroid glands or, occasionally, double adenomas [19]. Single adenomas can be cured by excision of the adenoma through unilateral neck exploration. However, patients with suspected multiglandular disease, as well as those with ambiguous localization of the lesion in preoperative imaging, may require bilateral neck exploration, because the sensitivity of imaging in the detection of multiglandular disease is lower [20]. Although exceptions exist to this generalization, parathyroid carcinomas tend to be larger than adenomas, with an average size of 3 cm. The only reliable imaging feature indicating malignancy is invasion of the surrounding structures [1,21]. The majority of primary HPT patients have single-gland disease, which has led to a shift from traditional, bilateral neck exploration involving the evaluation of all four glands to minimally invasive parathyroidectomy [22]. This change was driven by the potential of achieving decreased patient morbidity and lowering costs, with similar rates of surgical success [23,24]. Minimally invasive parathyroidectomy requires preoperative localization studies, in which US and technetium-99m sestamibi scintigraphy (SS) are traditionally employed [25,26]. SS and US are the major imaging techniques for the preoperative localization of hyperfunctioning parathyroid lesions. Using a dual-phase technique, SS represents hyperfunctioning parathyroid lesions as areas of sustained increased uptake on the delayed phase, in contrast the faster washout that is found in normal parathyroid and thyroid gland tissue (Fig. 1). US represents an abnormal parathyroid gland as an oval, bean-shaped, or infrequently, multilobulated hypoechoic mass with a well-defined margin, located posteriorly or inferiorly to the thyroid gland (Fig. 1) [27-29]. Parathyroid lesions are usually very vascular, typically showing a peripheral vascular arc and a prominent polar feeding vessel that arises from the branches of the inferior thyroidal artery (Fig. 2). The identification of a polar feeding artery can distinguish parathyroid glands from lymph nodes, which usually have a hilar blood supply. Other features include asymmetrically increased vascularity in the thyroid gland on the side of the lesion and in the hyperechoic capsule [30]. Numerous studies comparing SS and US suggest that they have similar sensitivities and specificities in the detection of solitary adenomas. The reported sensitivities of SS and US in the detection of single parathyroid adenomas are comparable, with a range of 68%-95% for SS and a range of 72%-89% for US. However these techniques have substantially lower sensitivities in the detection of multiglandular disease [1,24,27-29]. As expected, a preoperative approach that combines both SS and US is more accurate than either technique alone. De Feo et al. [31] reported a higher sensitivity of combined SS and US (96%) compared to the use of SS (71%) or US (67%) alone in the prospective subgroup of their study. SS and US can complement each other when both methods are applied [27,31-34]. While SS provides functional information about nodules and can visualize ectopic lesions, US can visualize the anatomic relationship of the enlarged parathyroid gland to surrounding structures in the neck.
Parathyroid Incidentalomas
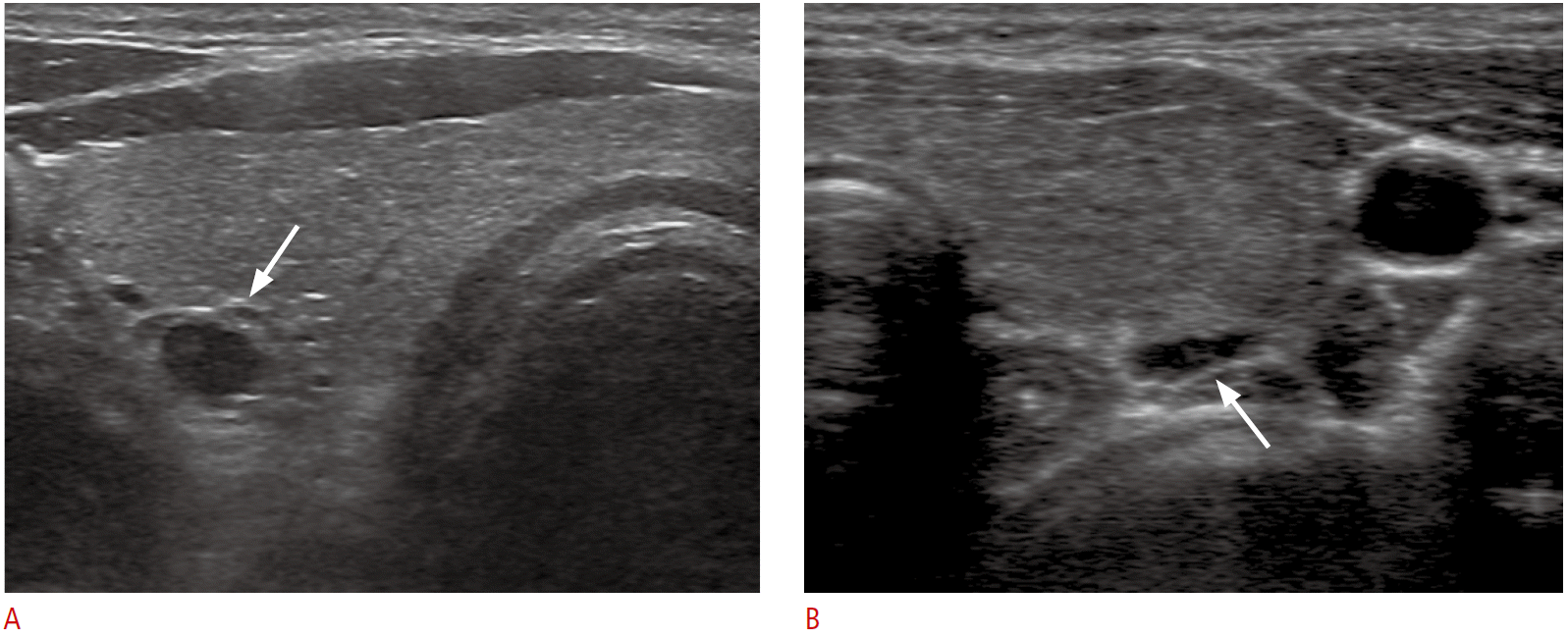
The term ‘parathyroid incidentaloma’ was previously used to refer to unexpected parathyroid adenomas that are encountered during surgery, but with the advent of high-resolution US, the term has also been applied to images that are discovered incidentally during thyroid US and raise the suspicion of a pathological parathyroid [2-4]. The reported intraoperative incidence of PTIs is between 0.2% and 7.6% [3,4], while the US incidence is <1% [4,35]. In autopsy studies of patients without primary HPT or thyroid disease, the incidence of parathyroid adenoma or hyperplasia varied from 1.9% to 7.6% of cases [36,37]. PTIs are most often found in younger patients, do not weigh as much, and are biochemically and pathologically less hyperfunctioning than lesions involved in parathyroid disease, which suggests that PTIs may represent an early stage of parathyroid disease [4]. The possibility of an enlarged parathyroid gland should always be considered when a homogeneous hypoechoic, well-defined, oval nodule is seen along the thyroid capsule (Fig. 3) [4,36]. Fine needle aspiration (FNA) of suspected parathyroid lesions and parathyroid hormone (PTH) (FNA-PTH) estimation from saline washing of the needle play an important role in localizing primary HPT [5]. If US evidence suggests the presence of a PTI, serum calcium and PTH levels, as determined by FNA-PTH, are used to determine whether the suspected lesion in a PTI [2,4]. In previous studies, approximately 20% of sonographically suspected lesions were proven to be PTIs, based on FNA-PTH, and the frequency of hyperfunctioning PTIs was reported to be 12.5%-37.5%, as determined by serum calcium and PTH levels [4,36]. Multinodular goiter or perithyroidal lymph nodes, which are often present in chronic lymphocytic thyroiditis, may be factors leading to false positive diagnoses of PTI (Fig. 3) [35]. A positive correlation was found between the size of the PTI and serum PTH levels in the patients studied by Frasoldati et al. [36], who found that larger PTIs are more likely to result in parathyroid hyperfunction [2,38]. Most patients with hyperfunctioning PTIs do not show symptoms or signs specific to HPT, and therefore are classified as having asymptomatic primary HPT. Recent guidelines for the management of asymptomatic primary HPT suggest that the decision between performing surgery and monitoring without surgery should be made based on serum calcium levels, bone density, and the results of renal evaluation [39]. Nonfunctioning PTIs in patients with normal serum calcium and PTH levels are considered to be the early stage of the development of primary HPT. However, no prospective studies have assessed what percentage of nonfunctioning PTIs become hypersecreting over time [2].
Nonsurgical Treatments of Parathyroid Lesions
Nonfunctioning Parathyroid Cyst: Simple Aspiration and Ethanol Ablation
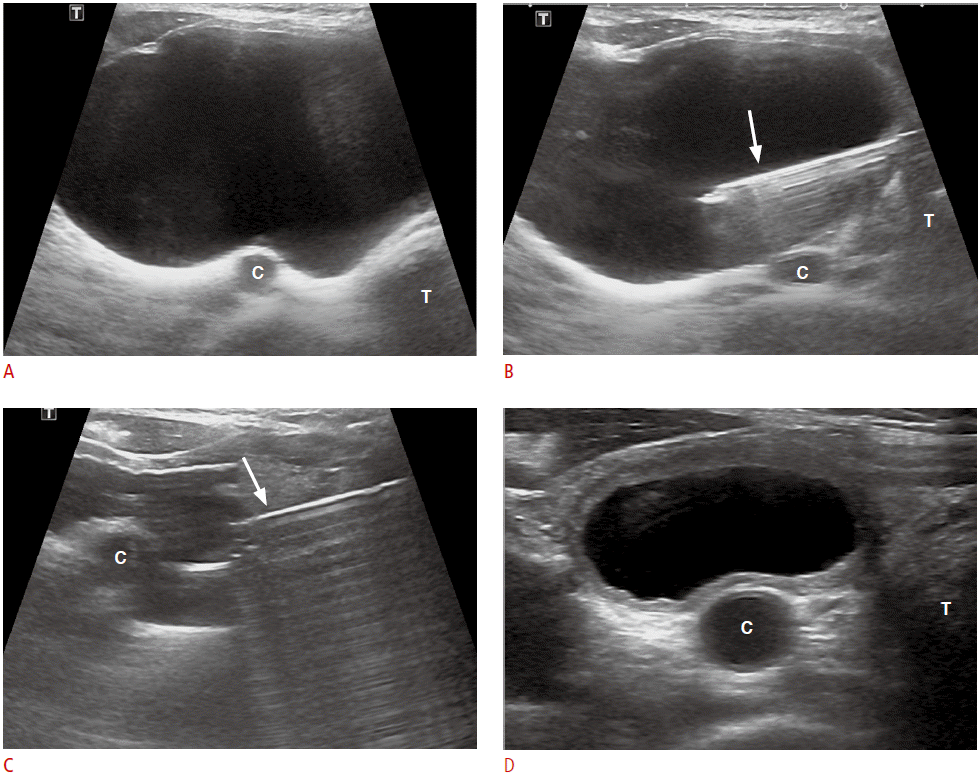
Nonfunctioning parathyroid cysts (PCs) are divided into two categories: functioning and nonfunctioning cysts. Nonfunctioning PCs are true cysts and usually asymptomatic, whereas large cysts can cause symptoms such as neck bulging, dysphasia, pain, tracheal compression, and recurrent laryngeal nerve palsy [14,40-42]. US-guided simple aspiration is the preferred technique for diagnosing nonfunctioning PCs [14,43]. The fluid aspirated from these cysts is usually clear and colorless [14,41]. Elevated PTH levels in aspirated fluid is indicative of a PC [4,5]. Although simple aspiration has been used as an initial diagnosis and treatment for symptomatic nonfunctioning PCs, cases of recurrence after aspiration have been reported [14,43,44]. In patients with recurrent cysts, repeat aspiration, surgical excision, tetracycline treatment, and ethanol ablation (EA) have been performed [41,43,44]. About 33% of PCs have been reported to be successfully treated by simple aspiration alone, while recurring cases can be successfully treated by EA without major complications (Fig. 4) [14]. In order to minimize ethanol leakage during EA of PCs, transisthmic approach should be employed, in which the needle is inserted through a sufficient amount of normal thyroid parenchyma (Fig. 4) [14]. For symptomatic nonfunctioning PCs, simple aspiration is a first-line procedure for diagnosis and treatment, whereas EA is a subsequent treatment modality suitable for recurrent PCs.
EA, Laser Thermal Ablation, Radiofrequency Ablation, and High-Intensity Focused Ultrasound for the Treatment of Hyperfunctioning Parathyroid Lesions
The conventional treatment of patients with primary HPT is neck surgery. However, some patients with HPT cannot undergo surgery due to the presence of unacceptable risks associated with the surgical procedure and/or anesthesia, while others refuse surgical treatment. Several nonsurgical treatment modalities have been developed to treat such patients. For over 20 years, EA has been used by specialists to treat patients with primary HPT as well as patients with secondary or tertiary HPT due to renal disease. The success rate of EA is inversely correlated with the size of the parathyroid tumor and the duration of follow-up. However, side effects are common, including pain, vocal cord palsy, and extraparathyroid fibrosis, which would be likely to interfere with any possible subsequent surgery [6,13]. Since 2001, laser thermal ablation has been suggested as a possible therapy, and has shown some efficacy in controlling serum levels of calcium and PTH in HPT patients [7-9]. In a recent study assessing the long-term effectiveness of laser thermal ablation, the serum PTH levels of the six patients included the study were temporarily reduced, but were above the normal range in all patients at the last follow-up examination, which took place at a mean of 54±34 months after the procedure [7]. Very limited information is available about the experiences of HPT patients treated by radiofrequency ablation, because only three case reports have been documented. However, in all three reports, serum levels of PTH and calcium were reduced after ablation [10,11]. High-intensity focused ultrasound is a noninvasive ablative method based on the generation of extracorporeal ultrasound waves focused on target tissue [12,13]. Kovatcheva et al. [13] reported results of high-intensity focused ultrasound treatment in 13 patients with primary HPT. EA, laser thermal ablation, radiofrequency ablation, and high-intensity focused ultrasound have been reported to show some efficacy in controlling serum calcium and PTH levels and in reducing the volume of the parathyroid. However, these treatments either involve some side effects or do not show evidence of good long-term efficacy [6-13]. More studies are needed to verify the utility and general applicability of these techniques.
Conclusion
This article has described the role of radiologist in the diagnosis and the treatment of parathyroid lesions. US examination plays an important role in patients with primary HPT in the preoperative localization of hyperfunctioning parathyroid lesions. Careful US examination and the use of an FNA-PTH assay can distinguish incidental parathyroid lesions from thyroid lesions. Symptomatic nonfunctioning PCs can be nonsurgically cured by US-guided simple aspiration and EA. More studies are needed to verify the utility of the nonsurgical treatment modalities that have been developed to treat hyperfunctioning parathyroid lesions.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




