Introduction
Liver cirrhosis is a major cause of morbidity and mortality and is currently the 14th leading cause of death worldwide. Early diagnosis may help prevent the associated detrimental effects, including variceal bleeding, hepatic encephalopathy, and portal vein thrombosis. Staging of liver cirrhosis helps with prognostic information and directs appropriate therapy. Parenchymal changes seen in patients with diffuse liver disease and cirrhosis include surface nodularity, increased echogenicity, coarsened echotexture, and a high caudate-to-right hepatic lobe size ratio. Secondary signs such as splenomegaly and varices represent sequelae of portal hypertension in the case of cirrhosis [1,2]. Spectral and color Doppler ultrasonography can demonstrate many characteristic flow patterns from hepatic vasculature in patients with liver cirrhosis with or without portal hypertension. In this article, we discuss the many characteristic flow patterns identified by Doppler ultrasonography within the portal veins, hepatic veins, and hepatic arteries, as well as the pathophysiology and secondary signs and pitfalls in this patient population.
Portal Venous Flow Patterns in Cirrhosis
Portal Vein Pulsatility
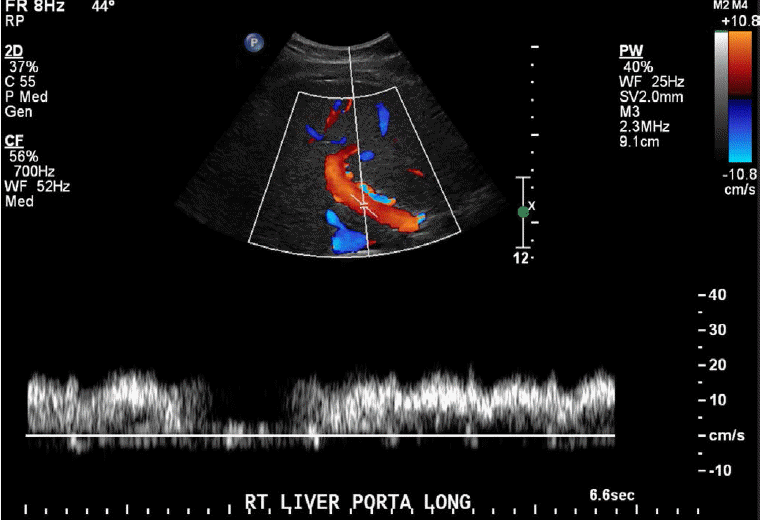
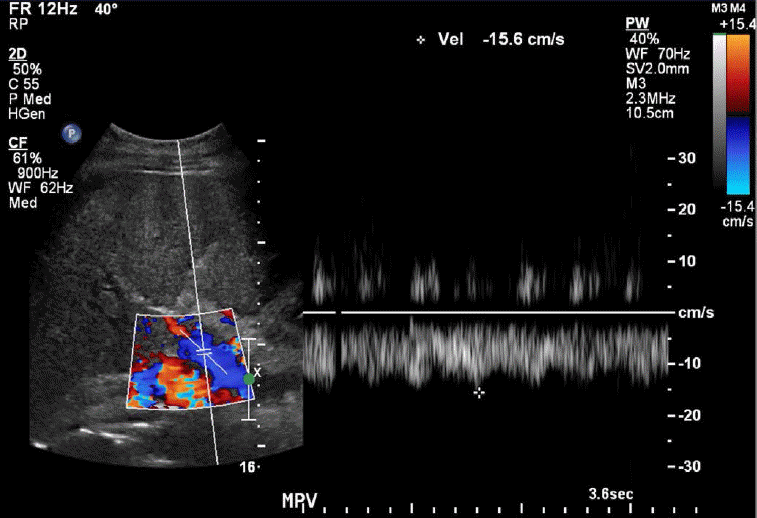
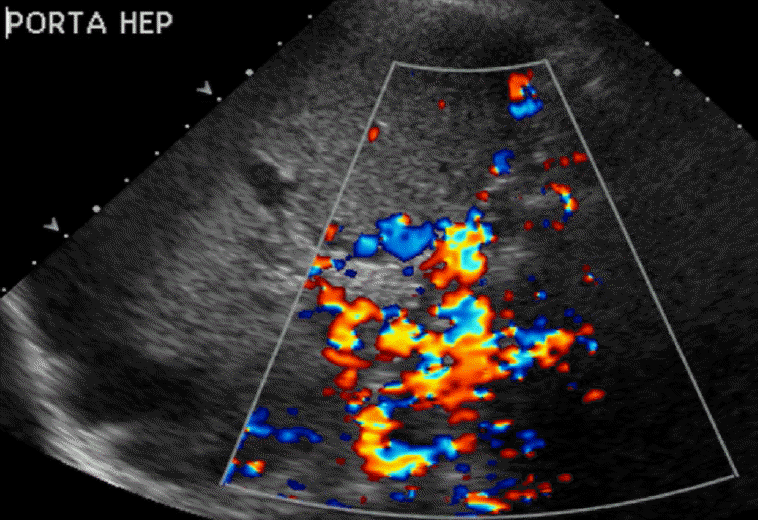
The normal portal vein waveform normally shows gentle undulations (Fig. 1). In patients with cirrhosis, arterio-portal shunting is a major cause for pulsatility. Increased pulsatility may be measured by calculating the pulsatility index, which is measured as a fraction of the difference between the peak systolic velocity and the end diastolic velocity divided by the average velocity. Increased pulsatility, defined as an increase in the pulsatility index, occurs due to the transmission of pressure across the hepatic sinusoids during the end diastole [3,4]. Alternatively, an increased pulsatility index (>0.5) may occur with high right atrial pressure, which can be seen with right heart failure and tricuspid regurgitation. The basis of arterio-portal shunting is the structural distortion that may be seen in patients with cirrhosis in combination with a reversed portal venous flow and increased hepatic venular resistance. Further, hepatocellular carcinoma, which is commonly seen in cirrhotic patients, may cause peri-lesional shunting via draining veins. Liver biopsies performed on cirrhotic patients may also result in arterio-portal fistula formation. Arterio-portal shunting in cirrhosis cases can result in increased portal vein pulsatility (Fig. 2) [5]. Therefore, increased portal vein pulsatility in cirrhotic patients, depending on the clinical history, may prompt an evaluation for the presence of a vascular lesion or arterio-portal fistula. A secondary sign that may help in diagnosing arterio-portal shunts is pulsatility that is in sync with the adjacent hepatic artery waveforms [6].
Sluggish or Slow Portal Venous Flow
Normal main portal vein (MPV) peak systolic velocities range between 20 cm/sec and 40 cm/sec. A low flow velocity of <16 cm/sec in addition to a caliber increase in the MPV are diagnostic features of portal hypertension [7]. Cirrhosis results in intrahepatic portal hypertension secondary to the increased resistance in hepatic venules caused by the intrahepatic fibrosis [8]. Accurate velocity measurements within the MPV depend on many parameters, including the Doppler/beam-flow angle. False-positive velocities may be secondary to a Doppler angle that is closer to 90┬░ with respect to the flow direction [9]. The choice of Doppler frequency also affects velocity measurements, as higher frequencies produce more accurate velocities while lower frequencies allow for better penetration. Finally, the presence of a turbulent flow and sampling error may result in the under- or overestimation of the flow velocity [10].
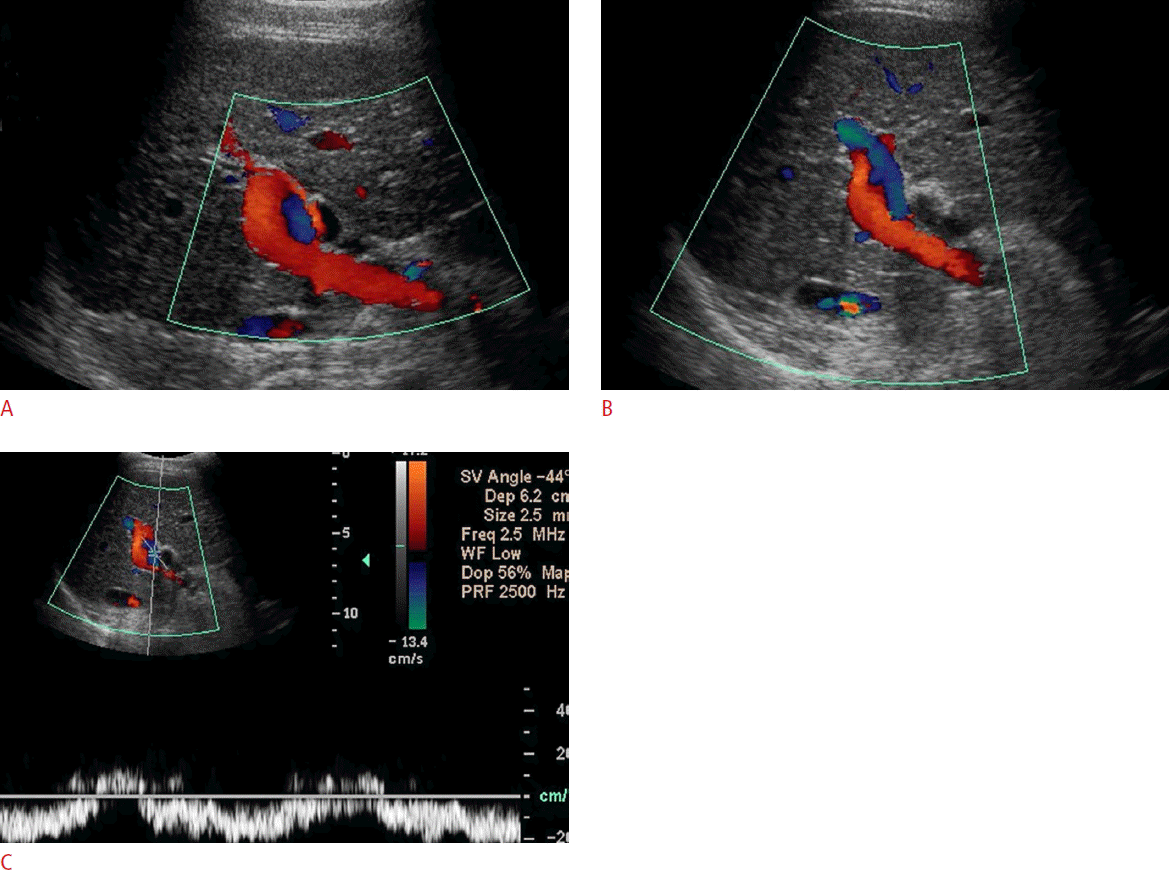
Helical Flow Pattern
Helical blood flow is a type of secondary flow (defined as a minor flow superimposed on the primary flow pattern). A helical flow is caused by a disturbance in the laminar flow [11,12]. A change in viscosity, vessel geometry, local asymmetries along the vessel wall, and changes in the flow direction and speed can cause accentuated helical patterns [13]. Color Doppler imaging shows alternating red and blue bands in a spiral-like pattern (Fig. 3). Helicity was first characterized within a region of caliber change at the level of the carotid bulb. Studies of the carotid artery indicate that a helical flow may be a marker of exposure to disturbed shear or turbulence [14]. Further, some researchers have hypothesized that this pattern may play a significant role in preventing atherogenesis and fibrointimal hyperplasia [15].
Helicity, although usually transient, has been seen in both liver transplant and transjugular intrahepatic portosystemic shunt (TIPS) recipients. If there is prolonged persistence of the helical flow, it is associated with an increased portal venous velocity and portal vein stenosis (Fig. 4). The helical flow in transplant patients is due to a change in the portal vein diameter between the donor vessel and the native vessel and is accentuated when the discrepancy between the portal veins is greater than 50%. This is in keeping with other authors who have reported that an expansion of the cross-sectional area of a vessel caliber causes flow separation, inducing a circular pattern [16]. Other studies have shown that shortly after liver transplantation, the helical flow within the portal vein resolves due to the decreased flow velocity and local turbulence [17]. The helical flow has been seen in TIPS recipients as well. In a prospective analysis, the prevalence of the helical flow after TIPS placement was reported to be 28% with a variable timeline after placement. The TIPS procedure changes the hemodynamic parameters of the flow, including the portal vein velocity, flow direction, and flow pulsatility [18]. The amount of turbulence created by these dynamic changes is variable among patients and presumably accounts for the unpredictability of whether a helical flow is present postoperatively.
To-and-Fro Portal Venous Flow Pattern
With increasing portal venous pressure, there is a progressive decrease in the portal venous flow velocities approaching the level of stagnation. As this occurs, the phenomenon of a to-and-fro flow can be encountered whereby the nearly stagnant blood column in the portal veins is seen to shift into and out of the liver with the respiratory cycle. The effect of transient flow reversal or cessation of the forward flow during inspiration can be simulated with the Valsalva maneuver, which also results in a transient hepatofugal flow (Fig. 5). With worsening portal hypertension, stagnation of the blood column can lead to thrombosis or progress to a frank flow reversal.
Flow Reversal
A normal portal venous flow is hepatopetal. A flow reversal (or a hepatofugal flow) is seen in the case of portal hypertension (Fig. 6). In particular, in patients with cirrhosis, obstruction of the hepatic venules and sinusoids by fibrosis, substantiated by arterio-portal and porto-systemic shunting, eventually leads to flow reversal. Flow reversal is detected using spectral and color Doppler. Sonographic pitfalls may occur due to the presence of variant portal vein anatomy, resulting in an apparent, false-positive flow reversal depending on the relative location of the ultrasound probe. Atypical intrahepatic arterial collaterals, which are mainly seen after liver transplantation rather than in patients with liver cirrhosis, show a flow from the hepatic capsule to the hilum and may be confused with a hepatofugal flow within the portal venous system [19]. Further, aliasing due to a low pulse repetition frequency setting is a sonographic artifact that may be misinterpreted as a reversed portal venous flow.
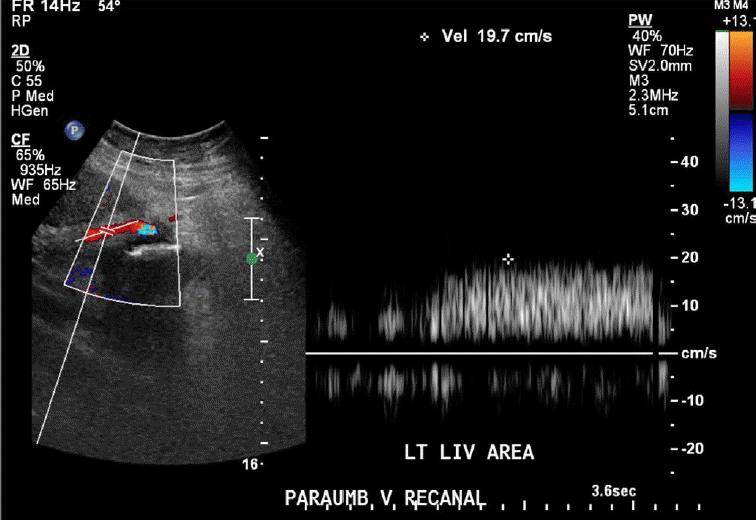
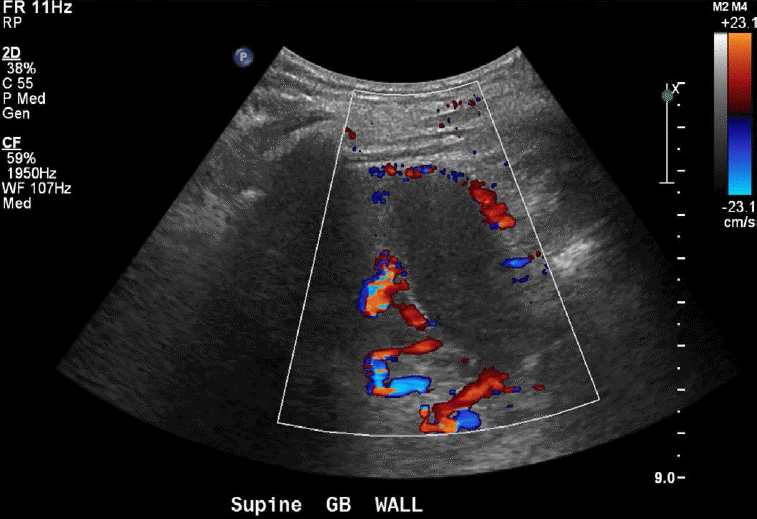
Porto-systemic shunting or porto-systemic collateral formation is caused by the opening of normally collapsed porto-systemic anastomoses. Some common porto-systemic collateral vessels include coronary (left gastric) venous collaterals, gastric varices, esophageal and paraesophageal varices, abdominal wall varices, and recanalized paraumbilical veins (Fig. 7), retroperitoneal-paravertebral varices, and pericholecystic (Fig. 8), periportal (Fig. 9), and perisplenic varices. Gastrorenal and splenorenal shunts are other examples of varices [20].
Stagnant Portal Flow and Thrombosis
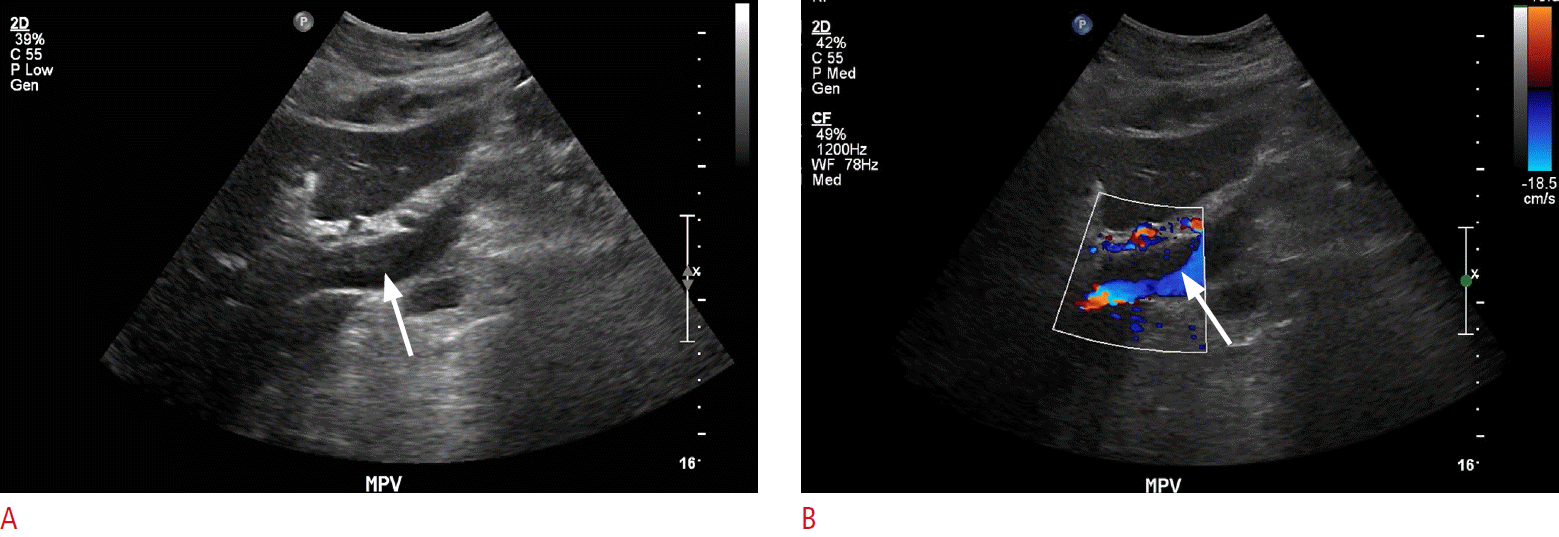
Complete stagnation of portal venous flow is a function of advanced portal hypertension and is associated with severe liver fibrosis such that forward flow is virtually absent (Fig. 10). Further, the portal vein thrombus is usually hypoechoic and can be difficult to discern on grayscale imaging alone. Therefore, evaluation of the MPV on both grayscale and color flow Doppler is necessary (Fig. 11). An apparent absence of flow may also be seen within areas of occlusion secondary to a bland or tumor thrombus. A helpful differentiating factor between a bland thrombus and either a tumor thrombus or recanalization of an occlusive bland thrombus is the identification of whether the blood flow is pulsatile, as in the case of tumor thrombi, or of a waveform similar to that of portal venous flow, as seen in the case of recanalized bland thrombi. The secondary signs of portal vein thrombosis observed by Doppler include the presence of periportal collaterals, representing cavernous transformation, with a flow in the hepatopetal direction (Fig. 12) [21].
Portal Vein Aneurysm
The normal MPV has a caliber of up to 13 mm, and a portal vein aneurysm is a focal saccular or fusiform dilatation of the portal venous system. A portal vein aneurysm can be congenital or acquired and occurs due to vein wall weakening with a caliber of at least 20 mm. The acquired causes include portal hypertension, pancreatitis, trauma, and post-surgical etiologies [22]. Grayscale images typically show a focally enlarged MPV, while Doppler imaging shows a turbulent or yin-yang flow within the aneurysm, a stagnant flow, or a thrombus associated with the aneurysm (Fig. 13).
Pseudothrombosis
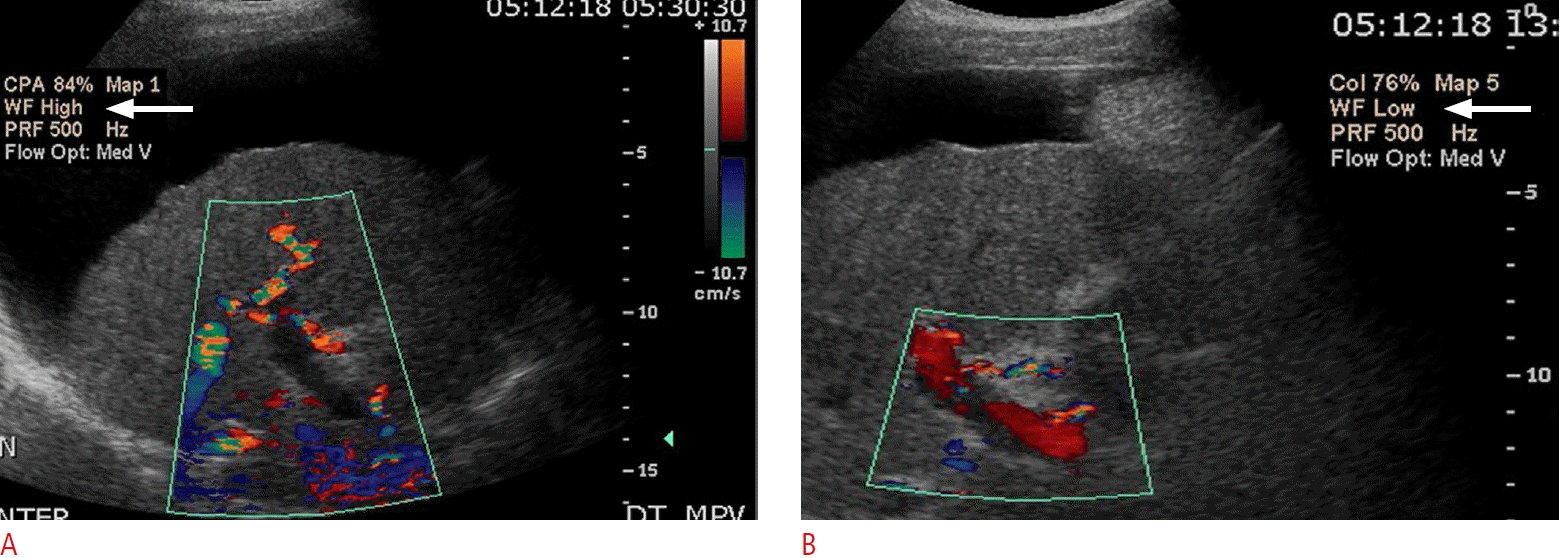
An apparent absence of flow may actually be artifactual, secondary to the Doppler settings. For example, a high velocity scale setting may miss velocities below the threshold value, resulting in an apparent absent flow. The transducer probe type (i.e., frequency setting), depth of the vessel, Doppler angle/probe position, or the presence of any overlying, obstructing structure may prevent an accurate velocity measurement, resulting in a false-positive area of absent flow [23]. Therefore, attention to Doppler gain settings, wall filter, frequency of probe used, and Doppler angle of insonation is critical to avoid this issue (Fig. 14).
Hepatic Vein Flow Patterns in Cirrhosis Patients
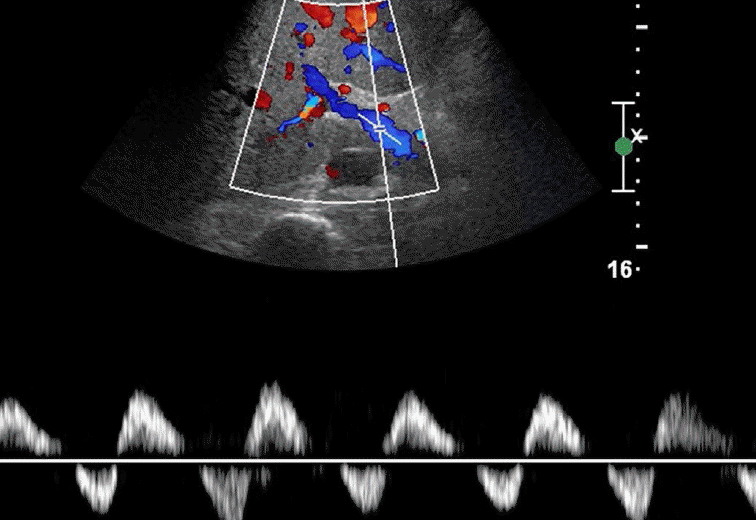
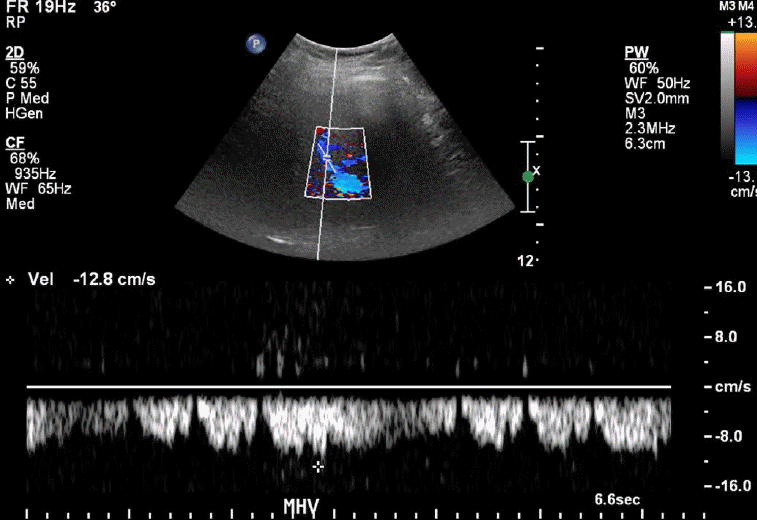
The normal waveform within the hepatic veins is triphasic with two hepatofugal phases related to the atrial and ventricular diastole (Fig. 15). Fibrotic or inflammatory changes as well as fat deposition in the liver may create a monophasic flow pattern [24]. In the case of end-stage cirrhosis, distorted architecture with changes in the underlying liver architecture can cause a striking reduction in the caliber or absence of the visualization of the hepatic veins [25]. Early waveform changes in cirrhosis patients include spectral broadening and dampening of the normal, retrograde, pre-systolic wave of the hepatic vein waveform [26]. Later, the normal triphasic waveform pattern may be diminished or replaced with a monophasic pattern (Fig. 16). Therefore, the monophasic hepatic vein waveform indicates relatively high portal pressures.
The changes in collaterals may also affect hepatic vein pulsatility. The patency of the paraumbilical vein is a rather frequent finding in cirrhosis patients, which alters the hepatic hemodynamics. The portal venous flow has also been reported to be affected by the patency of the paraumbilical vein, but hepatic arterial resistance and flow are not affected by such patency [27]. A decrease in the phasicity of the hepatic vein is also associated with hepatic vein stenosis or thrombosis. There are additional physiologic factors such as high intra-abdominal pressure due to the Valsalva maneuver or suspended respiration in the case of end expiration, which may result in monophasic waveforms.
Hepatic Arterial Changes
Unlike the portal vein flow patterns in cirrhosis patients, neither the hepatic artery nor the superior mesenteric artery resistive indices correlate with the cirrhosis stage. However, in patients with cirrhosis, hepatic artery compensation occurs when the portal venous flow is compromised [28]. Further, the hepatic arterial flow regulation in cirrhosis patients is maintained via a mechanism called the hepatic arterial buffer response, which increases the flow in this case [29].
Increased Resistive Index
The hepatic arterial resistance changes with increasing portal pressure values [30]. Unfortunately, it has been determined that hepatic arterial resistive indices do not correlate with the actual severity of cirrhosis. It is unclear whether there is a predictable chronological order between changes in the hepatic artery and changes in the portal venous flow [31,32]. The observed specificity and sensitivity of utilizing a hepatic arterial resistive index (RI) cutoff of >0.77 to predict cirrhosis were low at 70% and 68%, respectively [33]. A unique circumstance of the utility of RI is found in the case of a fulminant hepatic failure with acute viral hepatitis. It was found that an RI of >0.74 had an 84% sensitivity and 94% specificity for a fulminant hepatic failure in this case [34]. Unfortunately, no predictive values were found in the case of chronic cirrhosis. We know that there is significant variability among RI sampling, which may contribute to the lack of predictability and association with the degree of cirrhosis [35]. RI evaluation in the case of liver transplant rejection was also found to have a poor predictive value of worsening rejection versus improvement [36].
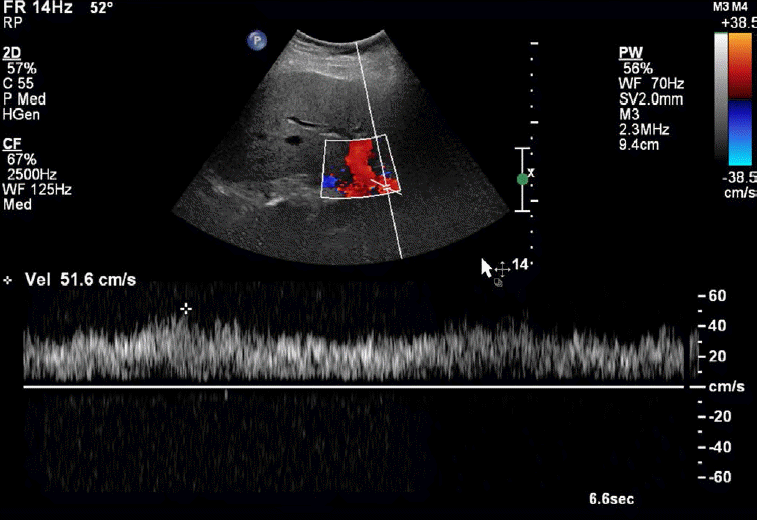
Increased Hepatic Artery Velocity
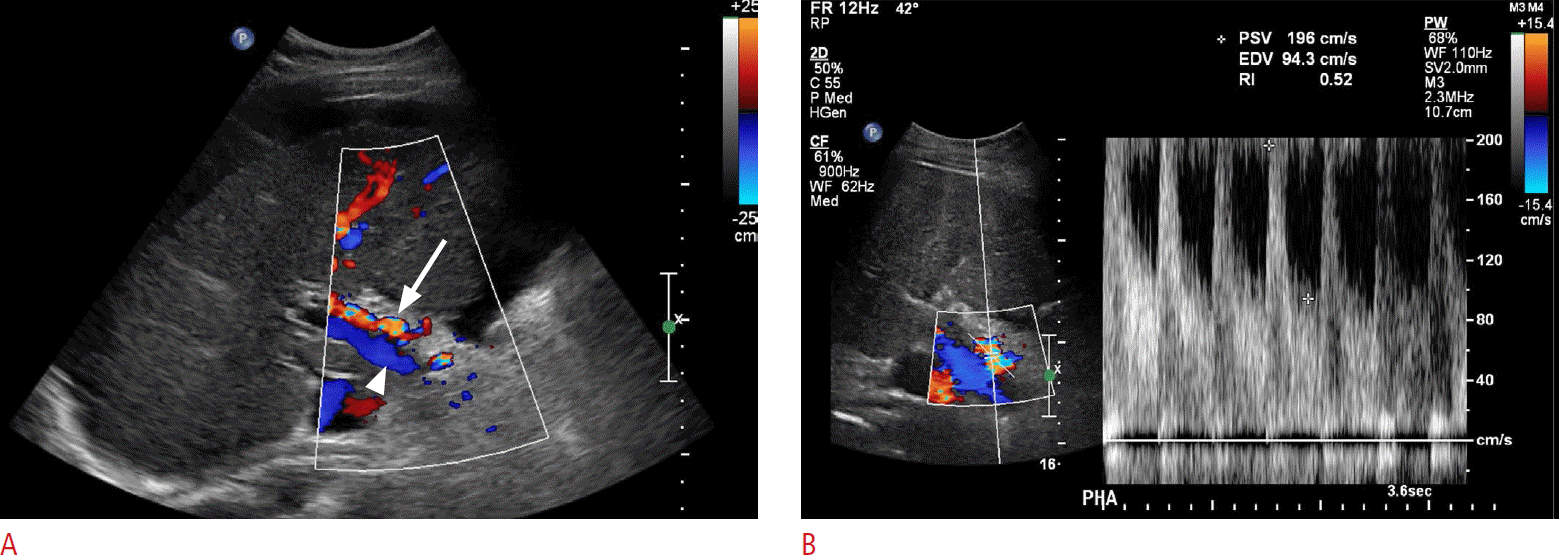
In normal patients, the hepatic artery is a low-resistance systemic artery with low-resistance monophasic waveforms. Peak systolic velocities approximate 100 cm/sec. Although not an entirely reliable finding, in the case of portal hypertension or portal vein thrombosis, both of which decrease the portal venous flow within the liver, the arterial flow rate and velocity may increase as a compensatory response (Fig. 17) [3]. Patients with portal vein thrombosis have been shown to have decreased hepatic arterial resistive indices, in some cases less than 0.50. Decreased portal vein flow velocities with the resultant hepatic arterial flow and velocity increase cause a decrease in the liver vascular index, which has been shown to have a high sensitivity and specificity in the diagnosis of cirrhosis and portal hypertension [37].
Summary
Knowledge of vascular flow patterns in cirrhosis patients provides invaluable information for patients with cirrhosis and portal hypertension. Aberrant portal venous flow patterns have the most significant impact and associative value with cirrhosis given that most of the blood supply is from the portal system. The hepatic arterial flow and the portal venous flow show a correlation in spite of the fact that they are independently regulated. Flow pattern changes within the portal side do not seem to develop in a chronological order. However, in a cirrhotic patient undergoing a routine imaging follow-up, the radiologist is in a perfect position to monitor pattern changes, which may show hemodynamic alterations, indicative of worsening or improving liver function.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+





 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




