Modern ultrasound imaging of pancreatic tumors
Article information
Abstract
In patients with solid pancreatic lesions (SPLs), the differential diagnosis must be evaluated to determine whether radical surgery, pancreatic parenchyma-saving strategies, or follow-up is indicated. Contrast-enhanced (endoscopic) ultrasonography and elastography facilitate the further characterization of SPLs. The majority of cases of pancreatic ductal adenocarcinoma exhibit hypoenhancement with contrast-enhanced ultrasonography. Elastographically soft SPLs are benign with very few exceptions, whereas stiffer SPLs can be malignant or benign. This article reviews the current use of modern ultrasound imaging techniques, including contrast-enhanced ultrasonography and elastography, for the detection and characterization of SPLs. In particular, the unexcelled diagnostic potential of multiparametric endoscopic ultrasonography to detect and characterize small SPLs is highlighted.
Introduction: the Smaller the Lesion, the Better the Prognosis
Symptomatic pancreatic ductal adenocarcinoma (PDAC), which is the most commonly diagnosed solid malignant tumor of the pancreas, is generally diagnosed at a late stage with or without metastases [1,2]. Most internationally recognized guidelines [3-6] recommend radical surgery for all small solid pancreatic lesions (SPLs) unless a strong suspicion of an etiology other than PDAC is suspected or contraindications are present. In earlier times, the preoperative diagnosis of PDAC <20 mm (T1) was reported to be <5%; in a large series including 13,131 patients, only 3.1% of cases were staged as stage T1a [1]. Very early diagnosis of this tumor in asymptomatic stages is crucial for improving its prognosis [7-11]. Data from the Surveillance, Etiology and End Results (SEER) program [12] as well as from the Japanese Pancreatic Cancer Registry [13] show that the smaller the lesion at time of diagnosis, the longer the expected 5-year survival rate. In the SEER database (2000-2010), PDAC ≤20 mm account for only 14.8% of patients with pancreatic cancer, but for 28.2% of 5-year survivors [12]. The 5-year survival rate may be as high as 30%-60% in very small PDAC with curative radical surgery [9,14-17], compared to a rate of <5% in general (https://seer.cancer.gov/). Progress from early PDAC to stage T4 may occur in less than 1 year [1]. In today’s view, an SPL diameter of ≥15-20 mm is approximately 80% predictive of PDAC [7,18,19]. Neuroendocrine neoplasia has a much better 5-year survival, depending on the specific histology and hormone production [20,21].
The accuracy of traditional imaging methods, including ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI), in the differential diagnosis of pancreatic masses was found to be disappointing [6,16,22]. CT was the recommended technique for diagnosis and staging of pancreatic cancer [14,16,22-24], but showed unsatisfying results for the detection of small SPLs (<20 mm) [7,25,26]. CT does not reliably allow the differential diagnosis of small SPLs [9,27]. Endoscopic ultrasonography (EUS) is considered to be the imaging method of choice to exclude PDAC [24,28,29]. Based on 22 studies including 1,170 cases, the pooled sensitivity of EUS for the detection of SPLs was 94%, which is markedly higher than the reported results for multidetector CT, MRI, and transabdominal US [30]. According to meta-analysis data, EUS has a diagnostic yield of 70% for detecting SPLs in patients with indeterminate results from multidetector CT scans, in 42% of whom the final diagnosis was PDAC [31]. Moreover, EUS offers the opportunity to detect asymptomatic PDAC [32]. A recent retrospective multicenter analysis of 200 small PDACs showed that in only 52.6% of cases the tumor diagnosis was possible due to direct visualization of the tumor by imaging techniques. In 74.8% of the cases, dilatation of the main pancreatic duct (MPD) was the clue for the diagnosis. The sensitivity of EUS for tumor detection was 92.4%, whereas transcutaneous ultrasonography (TUS), CT, and MRI had a sensitivity of only 67.3%, 65.8%, and 57.5%, respectively [33]. Other studies using TUS and EUS have highlighted the high diagnostic value of these techniques for the detection of MPD dilatation or stricture in order to diagnose small PDACs [34-36]. The unmatched diagnostic ability of EUS was also described for the detection of pancreatic neuroendocrine tumors (PNETs). A retrospective study showed that CT overlooked 68% of PNETs measuring <10 mm, whereas the sensitivity of EUS was 100% [37]. According to a recent meta-analysis, EUS has an additional diagnostic yield of 28% over radiological imaging and up-to-date scintigraphic techniques to detect PNETs [38]. Several studies have shown EUS to be superior for the characterization of SPLs [25,39-43]. EUS is recommended by the National Comprehensive Cancer Network guidelines [14]. Contrast-enhanced imaging techniques allow improved characterization before radical surgery and fine-needle biopsy successfully enables the preoperative differential diagnosis in many circumstances [2,7]. EUS-guided tissue sampling is 85%-92% sensitive and nearly 100% specific for the diagnosis of pancreatic malignancy [44-47]. However, a recent study showed that the sensitivity of EUS-guided fine-needle aspiration significantly decreased with decreasing mass size [48]. In conclusion, for the detection and characterization of small SPLs, EUS is the imaging technique of choice.
Contrast-Enhanced Ultrasonography and/or Contrast-Enhanced Endoscopic Ultrasonography
The introduction of contrast-enhanced EUS (CE-EUS) has improved the performance of endoscopic imaging [49,50]. A multicenter pancreatic US study (PAMUS) with more than 1,000 patients and other studies using contrast-enhanced ultrasonography (CEUS) and CE-EUS showed an improved diagnostic accuracy for the characterization of focal pancreatic lesions [29,51-53]. Meta-analyses demonstrated a 90% accuracy of CEUS and CE-EUS for differentiating PDAC from other etiologies of SPLs [54-57]. Recent data from 394 asymptomatic patients or patients with unspecific symptoms, with incidentally found small solid SPLs ≤15 mm and a definite histological or cytological diagnosis, were retrospectively evaluated. Patients with significant weight loss, jaundice, or a history of chronic pancreatitis were excluded [32]. Furthermore, patients with defined hormone production, genetically determined diseases, and cystic or semisolid lesions were excluded from those guidelines [21,58,59]. Patients with neuroendocrine neoplasia and hormone production were analyzed as well and the results were published in a separate paper [21]. The first imaging methods used for detection according to a prior consensus were TUS, EUS, CT, and MRI, with varying work-ups depending on the availability of imaging techniques, biopsy, and surgery. Only 146 of 394 small SPLs (37%) were finally diagnosed as PDAC. In the subgroup of SPLs measuring exactly 15 mm (n=83), 51 lesions proved to be PDAC (62%). In contrast, only 95 of 311 SPLs <15 mm (31%) were diagnosed as PDAC (P<0.01) [32]. The most important differential diagnosis of PDAC is PNET. In fact, 156 of the 394 small SPLs (40%) turned out to be typically hyperenhancing [51,52] PNETs, of which 129 PNETs (83%) were benign and 27 (17%) malignant [32]. The third most common etiology was pancreatic metastases (n=28, 7%). Other differential diagnoses included often hypervascular serous microcystic cystadenoma, solid pseudopapillary tumor, non-Hodgkin lymphoma, focal pancreatitis, intrapancreatic accessory spleen, and hamartoma [28,29,51,52], whereas mucin-filled intraductal papillary mucinous neoplasia and isolated necrosis are non-enhancing [32]. It can be concluded that the smaller an SPL, the less likely the diagnosis of PDAC and the more frequent the diagnosis of PNET and other rare etiologies.
CEUS and/or CE-EUS was performed in 219 of 394 patients using an intravenous injection of 2.4 mL (CEUS) or 4.8 mL (CE-EUS) of SonoVue according to the guidelines of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) [28,60-63]. Isoenhancement, hyperenhancement, or hypoenhancement in comparison to the surrounding pancreatic parenchyma was documented [32,64]. In 57 of 62 patients (92%), PDAC exhibited hypoenhancement, whereas in non-PDAC patients 132 of 157 SPLs (84%) showed isoenhancement or hyperenhancement in comparison to the surrounding pancreatic parenchyma. In addition, 91 of 102 PNETs (89%) were hyperenhancing or isoenhancing, resulting in a correct differential diagnosis of PDAC and non-PDAC in 189 of 219 patients (86%) [32]. The results using CEUS were better compared to CT; CT did not delineate a focal pancreatic lesion in 14 of the 38 patients with complete reports of CE-EUS and CT (37%; PDAC, n=6 and PNET, n=8; median diameter 8 mm [range, 4 to 12 mm]) [32] and the ultrasound contrast agent SonoVue was highly sensitive due to being strictly intravascular [65]. These results are in concordance with the findings published for more than 1,000 histologically proven focal pancreatic lesions and in earlier studies using CEUS techniques [29,49]. Eye-catching features have been published for the imaging of serous microcystic neoplasia with only microscopically detectable cysts mimicking a solid lesion [66].
In conclusion, and in accordance with the published literature, CEUS and CE-EUS allow the differential diagnosis of solid pancreatic tumors in about 90% of cases. This knowledge has been reflected in recent guidelines [60,61] and should be applied for cost-effectiveness reasons as well [67].
EUS versus TUS
In addition to the recently published results for small SPLs using conventional US, TUS, and EUS [32], we herein report data on the comparative results obtained using TUS and EUS. TUS was performed prior to EUS in 45 patients (median age: 59 years; range, 18 to 81 years; 20 males and 25 females) with 25 (56%) malignant and 20 (44%) benign SPLs. In 5 of 45 patients (11%), the SPL was not detected by TUS prior and after EUS. In six of 45 patients (13%), detection of the lesion on TUS and CEUS were only possible with knowledge of the EUS findings. In 34 of 45 patients (76%), the SPL was detected by TUS prior to the EUS examination and CEUS was performed as described. The CEUS results were concordant except in one patient with a hyperenhancing lesion using EUS that was shown as hypoenhancing by TUS. We conclude that most SPLs can be detected by TUS, and CEUS evaluation is possible for further characterization (Figs. 1, 2) [63]. The value of handheld point-of-care devices remains to be determined [68-72].
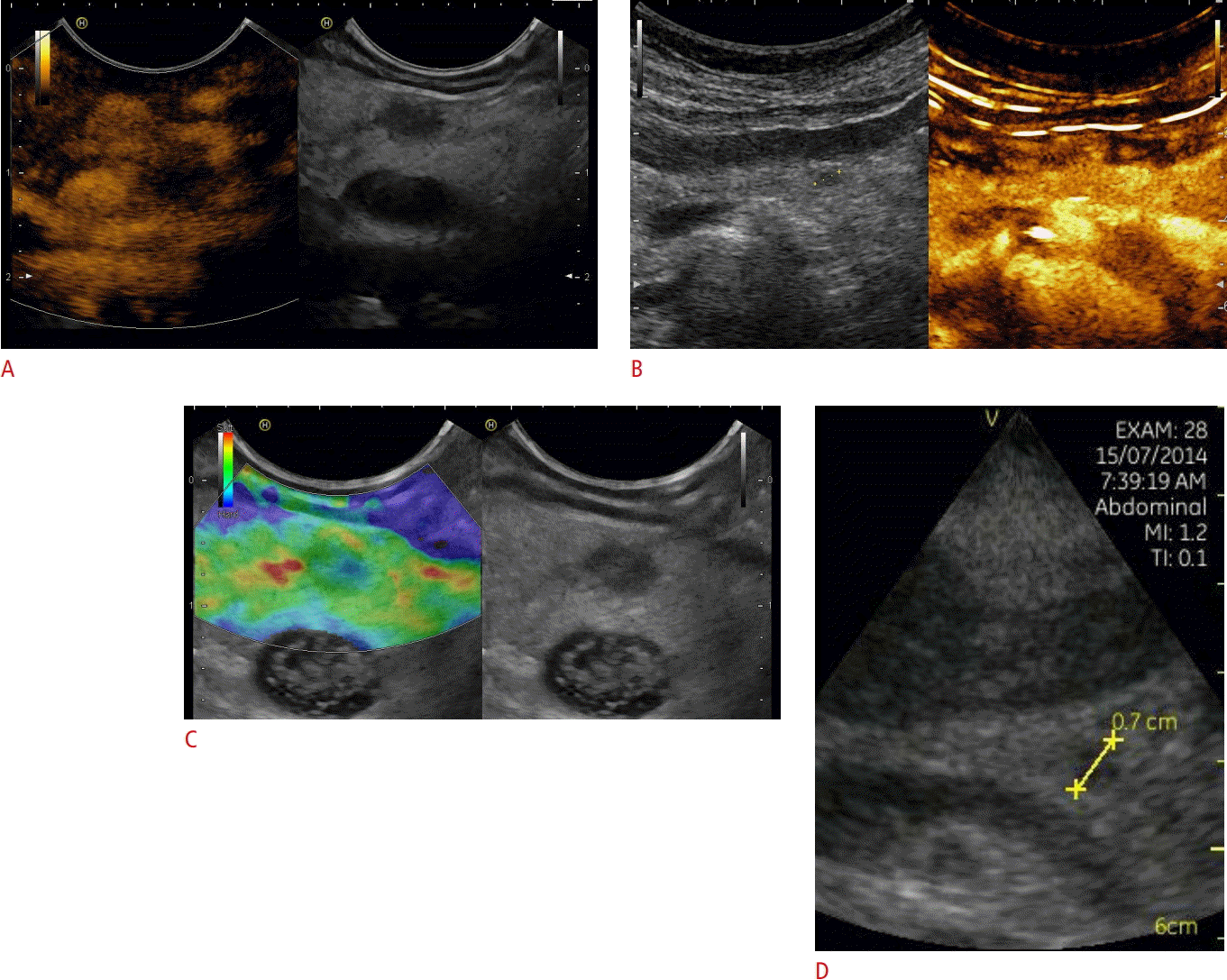
Neuroendocrine neoplasia.
A, B. Focal pancreatic lesions that were hyperenhancing on contrast-enhanced imaging techniques (7×6 mm, between markers) using endoscopic ultrasonography (A) and transcutaneous contrast-enhanced ultrasonography (B) are shown. C. The soft elastographic image is shown as well, indicating a benign lesion. D. The handheld device Vscan also demonstrates the lesion. A neuroendocrine neoplasia was diagnosed by biopsy and histopathological evaluation.
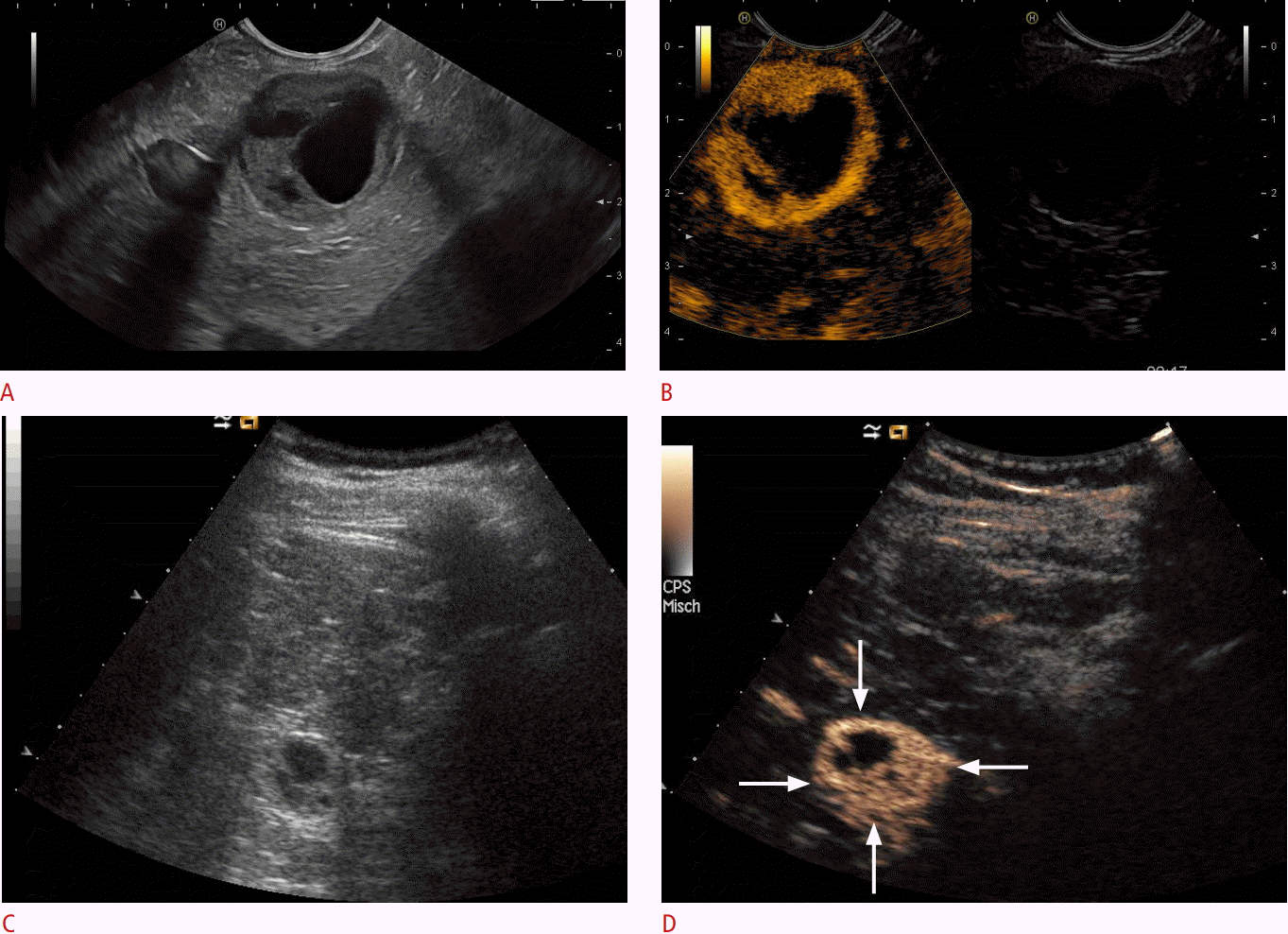
Neuroendocrine mixed solid-cystic neoplasia.
Solid-cystic focal pancreatic lesions (marked with arrows) using B-mode (A) that were hyperenhancing on contrast-enhanced endoscopic ultrasonography (B) and transcutaneous B-mode (C) and contrast-enhanced ultrasonography (D) are shown. A neuroendocrine neoplasia was diagnosed by biopsy and histopathological evaluation.
Ultrasound Elastography
Ultrasound elastography (USE) allows the assessment of tissue stiffness by virtual palpation. Two main types of USE are used for the evaluation of the pancreas and of other organs [73-83]. Ultrasound-based strain elastography using EUS has been established for the assessment of small focal pancreatic lesions and the examination technique has been described in detail, including the appropriate transducer, frequency selection, frame rate, line density, palpation speed and amplitude, noise filters, persistence, dynamic range of elasticity, and other quality parameters (e.g., strain graph display) [80,81,83-86]. Soft small SPLs are typically benign, whereas stiffer (harder) SPLs in otherwise healthy pancreatic parenchyma can be malignant or benign. Recently a study was performed of 218 patients with SPLs ≤15 mm and a definite histological diagnosis [87]. In this particular group of small SPLs, 50% turned out to be soft compared to the surrounding pancreatic parenchyma. It could be shown that especially in patients with small pancreatic lesions, EUS elastography can rule out malignancy with a high level of certainty if the lesion is displayed as soft. In larger SPLs (>30 mm) the results are less convincing mainly due to the heterogenicity of the lesions but also because of concomitant changes of the surrounding pancreatic parenchyma [87]. The examination technique must follow certain rules, which have been described in detail [85,86]. Elastography is not able to decisively differentiate focal pancreatitis from PDAC, since chronic focal pancreatitis can also be stiffer than the otherwise healthy pancreatic parenchyma. Strain elastography is also useful in diagnosing autoimmune pancreatitis since the entire organ shows stiffer tissue properties before B-mode changes are visible [88-92]. Circumscript pancreatic tuberculosis is also stiffer than the surrounding pancreatic parenchyma [93,94], whereas the application and correct interpretation of elastography in chronic pancreatitis is more difficult and semiquantitative strain-exploiting elastographic techniques are preferred.
The current role of shear wave elastography remains to be determined. Shear wave measurements are higher in PDAC, with Shear wave velocities >3 m/sec [95-98], compared to the surrounding pancreatic parenchyma.
Conclusion
In patients with SPLs, etiological differentiation is necessary to facilitate reasonable decisions on further management: radical surgery in patients with resectable PDAC, oncological treatment in patients with non-resectable malignancy, pancreatic parenchyma-saving strategies or surveillance in benign neuroendocrine neoplasia or follow-up in small benign lesions (Fig. 3) [32].

Diagnostic algorithm.
Diagnostic algorithm for small pancreatic lesions is shown. CT, computed tomography; MRI, magnetic resonance imaging; EUS, endoscopic ultrasonography; MEN, multiple endocrine neoplasia; CE-EUS, contrast-enhanced EUS; EUS-FNA, EUS with fine-needle aspiration; PDAC, pancreatic ductal adenocarcinoma; NET, neuroendocrine tumor. Adapted from Dietrich and Burmester, Endosc Ultrasound 2017;6:S106-S110 [50], according to the Creative Commons license [50].
Based on the enhancement pattern in CEUS and elastography findings, further characterization of SPLs is possible. Hypovascularity is observed in approximately 90% of PDACs. Soft SPLs are benign, with very few exceptions, whereas stiffer (harder) SPLs in otherwise healthy pancreatic parenchyma can be malignant or benign.
Approximately 60% of small SPLs (≤15 mm) are diagnosed with etiologies other than PDAC [28,51,52]. In patients with hypervascular and/or soft SPLs, tissue acquisition is therefore recommended prior to treatment decisions, as radical surgery might not be appropriate. Nevertheless, about 40% of patients with small SPLs revealed PDAC at a very early stage, with a better prognosis. Patients with a hypovascular SPL ≤15 mm should be primarily and radically operated since this finding is indicative of PDAC.
In patients with serous cystadenoma, mesenchymal lesions, intrapancreatic accessory spleen, and non-functional PNETs <10 mm with a Ki67 index <3%, follow-up may be recommended, whereas PNETs >10 mm with a Ki67 index >3% will often be operated due to their malignant potential [99].
Notes
Author Contributions
Conceptualization: Dietrich CF, Jenssen C. Drafting of the manuscript: Dietrich CF, Jenssen C. Critical revision of the manuscript: Dietrich CF, Jenssen C. Approval of the final version of the manuscript: all authors.
The authors thank the Bad Mergentheimer Leberzentrum e.V. for support.
