Benefits of contrast-enhanced ultrasonography for interventional procedures
Article information
Abstract
For evaluating unclear tumorous lesions, contrast-enhanced ultrasonography (CEUS) is an important imaging modality in addition to contrast-enhanced computed tomography and magnetic resonance imaging, and may provide valuable insights into the microvascularization of tumors in dynamic examinations. In interventional procedures, CEUS can make a valuable contribution in pre-, peri-, and post-interventional settings, reduce radiation exposure and, under certain circumstances, decrease the number of interventions needed for patients.
Introduction
Contrast-enhanced ultrasonography (CEUS) enables physicians to dynamically assess the vascularization of tissues and vessels in a real-time manner. For this purpose, a contrast agent is injected intravenously, which allows an assessment of the precise contrast dynamics of suspicious lesions. The lesions are evaluated during the arterial phase (15-30 seconds after injection), the portal venous phase (30-70 seconds after injection) and the late phase (>70 seconds after injection) [1-3], which can be transferred as cine loops (5-10 second intervals) to the local memory of the ultrasound unit and subsequently to the local institutional archiving system. The typical malignant characteristics of suspected hepatic metastasis are pronounced early arterial hypervascularization, early wash-out in the portal venous phase, and persistent wash-out in the late phase (Fig. 1). The higher spatial resolution of ultrasound imaging than computed tomography (CT) and magnetic resonance imaging (MRI) enables physicians to safely characterize lesions even smaller than 1 cm, which can be useful for pre-interventional planning, peri-interventional monitoring, and post-interventional treatment response assessment [4].

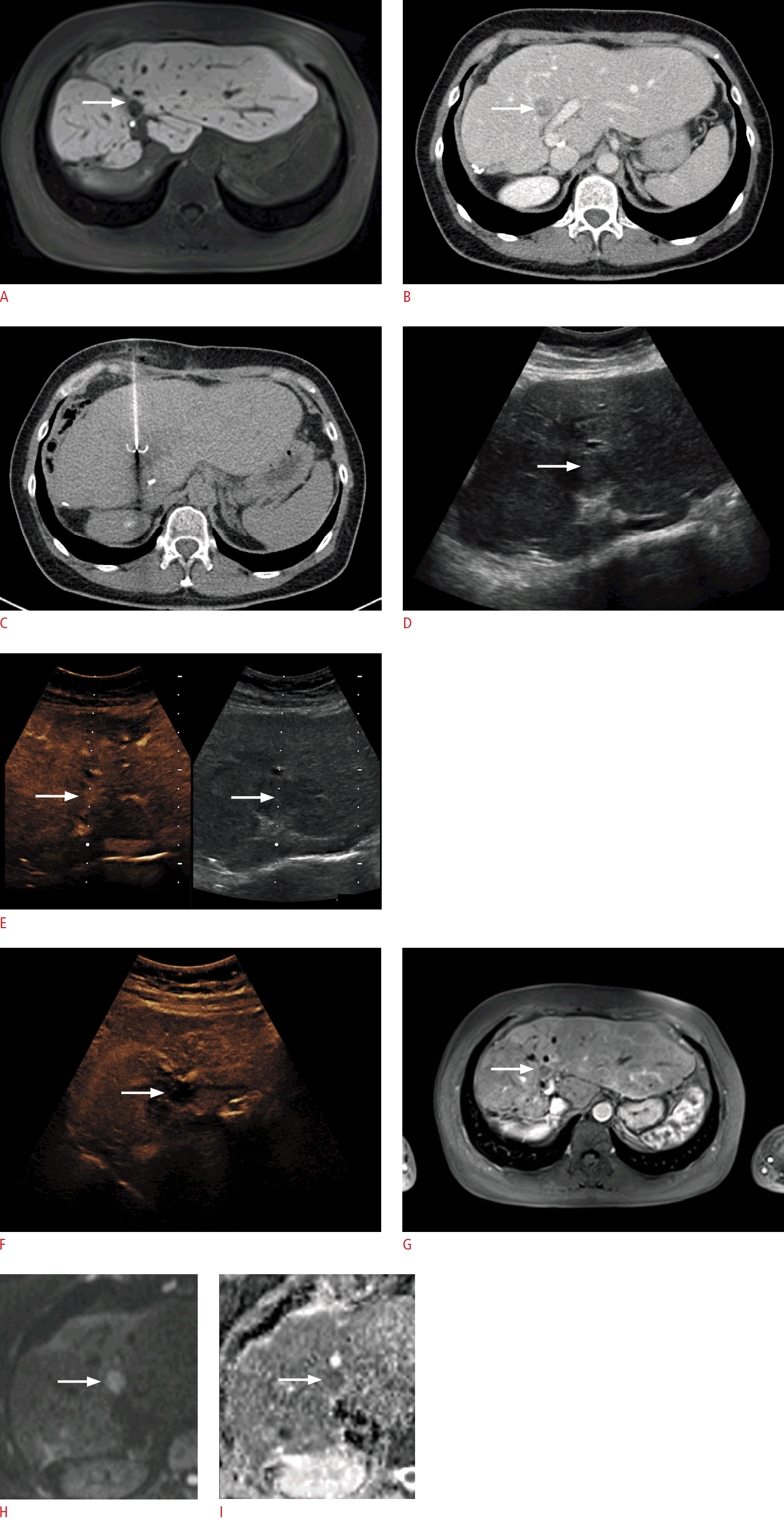
A 54-year-old woman with metastatic colorectal cancer.
A, B. B-mode ultrasonography reveals a 12-mm peripheral hypoechoic lesion (arrow) in liver segment IVa, cranially adjacent to the portal trunk (A) without evidence of hypervascularization (arrow) in duplex sonography (B). C, D. Contrast-enhanced ultrasonography (CEUS) shows an early arterial contrast medium (arrow) (C) with wash-out (arrow) in the portal venous and late phase (D) (5 minutes after injection of contrast medium) indicating a singular liver metastasis. Image fusion of the same patient (E-G) comparing B-mode ultrasonography and CEUS to previous magnetic resonance imaging (3 months ago) reveals a new demarcated liver lesions (arrow) with the ultrasonographic and morphological features described above (A, C, D).
Overview of the Use of Contrast Media with a Specific Focus on Sonazoid
The recommended volume of contrast medium varies between 1.0 and 2.4 mL, depending on the ultrasound system and the tissue to be examined, and is followed by a bolus of up to 10 mL of sterile saline (0.9% NaCl) [1,5-7]. Modern contrast agents (e.g., SonoVue, Bracco, Milan, Italy) are small gas-filled microbubbles that generate a nonlinear tissue-independent contrast, allowing dynamic evaluations at the level of the capillary microcirculation with a spatial resolution superior to that of CT and MRI [4,8,9]. In order to obtain sufficient imaging quality, a low mechanical index (<0.2) is preferably used to prevent early destruction of the oscillated microbubbles induced by the emitted ultrasound waves. Unlike contrast agents for CT or MRI, which are usually based on iodine or gadolinium, respectively, most ultrasound contrast agents (e.g., SonoVue) have a purely intravascular distribution pattern, which underscores the benefits of CEUS, especially for small lesions [10-13]. In addition, ultrasound contrast agents have extremely low risk profiles and can be administered to a wider range of patients due to their lack of influence on kidney or thyroid gland function [14,15]. Early studies demonstrated the safe application of intravenous contrast agents during CEUS examinations in pregnant women [16,17]. These agents are also safe for application in children and young adults, and are already widely used in routine practice [18-20].
Sonazoid (GE Healthcare, Waukesha, WI, USA) has unique characteristics as a second-generation ultrasound contrast agent. It was first approved and launched in Japan in 2007 and is currently also available in Korea, Norway, Singapore, Taiwan, and China. Norway was the first (and currently, the only) country in Europe to approve Sonazoid in 2014. The lack of broad availability can be explained by the number of adverse events (AEs) in early studies. In a prospective study from 2009, AEs were registered in 49.2% of cases, with fever, nausea, and diarrhea as the three most common clinical symptoms. Adverse drug reactions occurred in 10.4% of patients; however, all of them were only mild [21]. In a study from 2019 with an investigation period between 2014 and 2015, a markedly lower rate of AEs was described (24.1%), but this rate is still higher than that of approved contrast media [22].
Sonazoid, a perflubutane-based contrast agent, enables the acquisition of a parenchyma-specific Kupffer phase. The 2- to 3-µm microbubbles are phagocytosed by Kupffer cells, which are liver-specific macrophages, allowing the examiner to evaluate the hepatic parenchymal enhancement for up to 60 minutes [23]. Lesions such as hepatocellular carcinoma (HCC) or abscesses show no or markedly decreased enhancement in the post-vascular phase due to the lack of regular Kupffer cells [24]. The physician can also generate a defect reperfusion image in which both the arterial and the Kupffer phase can be assessed by repeated administration of Sonazoid in the same slice [11,25]. In comparison of their diagnostic value, current studies indicate that Sonazoid is noninferior to established contrast media such as SonoVue [26].
In a consensus statement and recommendation for the clinical practice of contrast-enhanced ultrasound using Sonazoid in 2020, the Asian Federation of Societies for Ultrasound in Medicine and Biology indicated that the further introduction of Sonazoid in the rest of Europe could be expected in the near future due to its low rate of AEs (0.5% and 6.3%), its advantages regarding a stable time window of up to 60 minutes with a possible improvement of the whole-liver imaging quality, and its benefits within the framework of liver interventions [27].
CEUS in the Framework of Liver Interventions
Puncture and Biopsy
The advantages of ultrasound-assisted biopsy compared to CT or MRI interventions include the possibility of acquiring real-time imaging, multiplanar image acquisition, and superior cost-effectiveness. One of the main benefits of performing CEUS-guided punctures or biopsies is the ability to obtain morphological information regarding the microvascular blood supply and blood flow in lesions that could otherwise only be obtained by contrast-enhanced CT (CECT) or contrast-enhanced MRI (CEMRI) [27-29]. This advantage enables the physician to differentiate between vascularized and non-vascularized tumor tissue in order to perform a targeted puncture/biopsy and, conversely, to obtain a higher quantity of tissue for histopathological analysis. This fact is highly relevant for partially necrotic lesions, which can be analyzed more accurately than in non-dynamic examinations like CT or MRI. Modern ultrasound devices also provide additional hardware that enables automatic needle guidance to the target lesion, thereby increasing safety, especially for less-experienced examiners [30]. For this purpose, a steering device can be attached to the transducer containing a channel for the needle that can be aligned at different angles. For safe performance of the interventional procedure, it is recommended to first mark the location on the skin, which allows a good access path while avoiding risky structures such as vessels. In comparison to the free-hand technique, the reduced mobility and adjustment of the needle position during the procedure may be regarded as disturbing. Overall, however, there is a significant time advantage with similar success rates, especially for less-experienced examiners [31-33].
In CEUS, the contrast medium can be administered either via peripheral venous access or directly via the inserted needle in order to increase the diagnostic accuracy (e.g., in cystic transformed lesions). In addition, CEUS has a positive impact on diagnostic accuracy in fusion imaging and may be used to safely visualize lesions that are not visible on native B-mode ultrasonography [34,35].
Minimally Invasive Ablative Therapies
The primary goal of thermal ablation is to induce cell death by intense heat or high radiation induction to achieve devascularization of the suspected lesion with subsequent necrosis. This requires a targeted placement of an ablation probe under imaging guidance, which can, using grayscale ultrasonography, cause difficulties in purely visualized small lesions for even the most-experienced examiners [36]. According to a study by Rim et al., tumors could not be visualized by conventional ultrasonography in 30% of patients referred for percutaneous radiofrequency ablation (RFA) [37] which underscores the importance and additive value of CEUS.
In the context of interventional therapy planning, CEUS can enable real-time imaging of the topographic anatomy of the lesion to be treated, such as the distance to the surrounding vessels, the liver capsule or other intra- or retro-peritoneal organs such as the stomach, intestine, or kidney. Regarding the latter consideration, peri-interventional insertion of an inflatable balloon could be used to displace the intestines from the radiation field [38]. The possibility of real-time imaging and image fusion can also provide the interventionalist with important information during the intervention [39,40]. While conventional B-mode ultrasonography can sometimes be of limited value in the peri-interventional setting, for example due to intralesional gas formation (e.g., during RFA), CEUS can provide additional information if there are contraindications to the use of contrast media for CT or magnetic resonance tomography imaging [41]. In a previous study, the use of Sonazoid led to a significant reduction in RFA sessions when the hepatic lesion was not well-delineated on native B-mode ultrasonography and ablation would have been performed based on the information from CT [42]. Peri-interventional CEUS can also have a positive effect on the detection of residual tumors, which makes it possible to treat these lesions during the same session [43]. However, the investigator must carefully differentiate between residual tumor and peri-ablational hyperemia or gas bubbles at the ablation site in the course of RFA [44].
After the histopathological acquisition of tumorous material and an interdisciplinary decision regarding the implementation of locally invasive therapy, CEUS offers the possibility of a radiation-free procedure to evaluate the success of therapy. Early post-interventional CEUS imaging in combination with preoperative CECT or CEMRI can determine the safety margin after, for example, RFA or transarterial chemoembolization, with a high sensitivity of 80%-100%. Thus, CEUS can provide information on the success of local ablative therapy (Fig. 2) [45-47]. Thus, the investigator may be able to preclude tumor recurrence by assessing the lack of revascularization in follow-up examinations [48]. According to the literature, several reports have raised concerns about tumor recurrence after RFA in HCC, the exact underlying mechanism of which is not yet fully understood. One possible explanation is intravascular tumor spread due to a sudden increase of the intracellular pressure in the ablated tissue [49,50]. According to the research of Jeong et al. [51], Sonazoid shows a significant suppressive effect on the popping phenomenon, without affecting the clinical outcomes. In general, ultrasound imaging is useful in all pre-, peri-, and post-interventional settings and may provide valuable information on the success of local ablative therapy.
The same patient as in Fig. 1.
A. Magnetic resonance imaging (MRI) shows, similarly to ultrasonography, a liver metastasis in segment IVa with a defect in the hepatobiliary phase (arrow) (contrast agent: Primovist). B, C. On computed tomography, the lesion morphologically shows washout in the venous phase (arrow) (B), which was followed by radiofrequency ablation (RFA) with RFA probe placement (C). D. On B-mode ultrasonography 5 minutes after RFA, the lesion shows a slightly progressive hypoechoic rim (arrow). E, F. In a follow-up examination 5 months after performing RFA, contrast-enhanced ultrasonography (CEUS) shows early arterial enhancement (E) and wash-out (F) at the cranial rim of the treated lesion (arrows). G-I. On MRI, the lesion shows (analogous to CEUS) shallow contrast-medium enhancement (G) with restricted diffusion (H, I), revealing local recurrence (arrows).
Drainage in Biliary Interventions
In biliary interventional therapy, CEUS can be utilized for the therapeutic placement of a percutaneous transhepatic biliary drainage (PTBD), which is a common procedure in benign or malignant biliary diseases (Fig. 3). CEUS-PTBD was first described in 2009 and displays typical indications for ultrasound-guided diagnostics [52]. Both native B-mode imaging and CEUS provide high anatomical accuracy in the evaluation of the correct drainage position. However, the field of view can sometimes be limited in native B-mode ultrasonography since an intraluminal tip position can lead to artifacts caused by physiological intestinal gas accumulation. In many cases, additive fluoroscopic cholangiography is required for correct visualization of the catheter tip, which is associated with radiation exposure to the patient. To avoid exposure to ionizing radiation, CEUS can overcome the limitations of native B-mode imaging and thus provide additional information on the drainage position. For this purpose, contrast medium can be applied via the primarily placed drainage, thereby enabling the location of the tip to be directly established in relation to the bile ducts or the small intestine. This is particularly useful if the bile ducts are not dilated or if the question of accidental dislocation of the drainage arises (Fig. 3C) [53]. Another potential complication that can be assessed by CEUS is the presence of a biliary-arterial fistula [54]. In summary, CEUS represents a radiation-free examination technique that can provide valuable information on the correct drainage position and possible complications, and enables the physician to perform the examination at the patient’s bedside [36].

A 62-year-old woman with percutaneous transhepatic biliary drainage (PTBD) (arrowhead).
A. Contrast uptake in the biliary system (arrow, arrowhead) after injection of contrast agent via the drainage, indicates the correct position of the PTBD. After 7 seconds, there is increasing contrast of the central and peripheral bile ducts (B). C. Ultrasonography of the same patient shows displacement of the PTBD after 5 days (arrow).
Handling of Image Fusion in Ultrasound Imaging
In order to expand the scope of applications, image fusion has been implemented in modern ultrasound devices. By enabling the sonographer to better visualize small lesions on B-mode ultrasonography, image fusion facilitates pathological clarification or, in the interventional setting, punctures or biopsies of these small lesions [55,56]. Further areas of application include post-interventional perfusion analyses for the assessment of local ablative procedures [41].
In the early stages, co-registration in the context of image fusion was considered difficult for inexperienced users, but now runs largely automatically in up-to-date ultrasound machines, often by using a rigid transformation matrix [13]. In the case of manual co-registration, clear and easily found anatomical structures such as large vessels or cysts can be used for better handling. The necessary equipment includes an extra-magnetic field generator and an additional position sensor. The sensor registers the position of the ultrasound probe in interaction with the extra-magnetic field generator and sends the acquired data to the ultrasound device to enable the most accurate co-registration possible. In addition to B-mode imaging, the ultrasound imaging system can also make use of Doppler ultrasonography and CEUS, which covers the entire range of ultrasound imaging and thus allows optimal detection and examination of the microvascularization of the tumorous tissue. The display of the images after successful image fusion can be differentiated between a side-by-side mode (Fig. 1E-G), in which the cross-sectional CT or MRI examination is compared to the ultrasound imaging, and an overlay technique, which combines the CT or MRI examination with the currently generated ultrasound examination to bundle the image information from the different examination modalities into one image. Despite its partially automated procedure, image fusion ultrasound examinations require a certain degree of experience in handling the technical precautions and protocols. A disadvantage of the present method is that image fusion adjustment during patient movement or breathing is not always possible to an adequate extent, which may result in ineffective co-registration. Therefore, it is recommended to perform the examination in a breathing position identical to that of the examination used for co-registration.
Conclusion
CEUS is a diagnostic procedure that can play an important role in planning, performing, and monitoring interventions, as well as in follow-up imaging. Thus, this technique represents an examination with a wide range of possible clinical applications in routine daily practice. CEUS and image fusion combine the general advantages of CEUS imaging with the possibility of combined access to information from additive CT or MRI for detection, therapy, and follow-up.
Notes
Author Contributions
Conceptualization: Marschner CA, Rübenthaler J, Clevert DA. Data acquisition: Marschner CA, Rübenthaler J, Schwarze V. Data analysis or interpretation: Marschner CA, Schwarze V, Froelich MF. Drafting of the manuscript: Marschner CA, Schwarze V, Froelich MF. Critical revision of the manuscript: Rübenthaler J, Clevert DA. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
