Synchronous multicentric small hepatocellular carcinomas: defining the capsule on high-frequency intraoperative ultrasonography with pathologic correlation
Article information
Abstract
Purpose
The aim of this study was to define the capsules of synchronous multicentric small hepatocellular carcinomas (HCCs) with use of high-frequency intraoperative ultrasonography (IOUS).
Methods
Among the 131 consecutive patients undergoing hepatic resection and high-frequency IOUS for HCC, 16 synchronous multicentric small HCCs in 13 patients were histologically diagnosed in the resected specimens. High-frequency IOUS and pathologic findings of these lesions were compared, with particular focus on the presence and appearance of the capsule in or around each lesion.
Results
Synchronous multicentric small HCCs were pathologically classified into distinctly nodular (n=12) or vaguely nodular (n=4) types. All 12 distinctly nodular HCCs including six subcentimeter lesions showed detectable capsules on high-frequency IOUS and pathology. The capsules appeared as a hypoechoic rim containing hyperechoic foci (n=6), hypoechoic rim (n=5), or hyperechoic rim (n=1) with varying degrees of coverage around each lesion. Histologically, the capsules were composed of a combination of one to four layers consisting of a fibrous capsule, peritumoral fibrosis, prominent small vessels, and entrapped hepatic parenchyma.
Conclusion
Synchronous multicentric small HCCs with distinctly nodular type, even at subcentimeter size, can show capsules with varying coverage and diverse echogenicity on high-frequency IOUS.
Introduction
During hepatic resection for hepatocellular carcinoma (HCC), intraoperative ultrasonography (IOUS) screening detects new nodules in 13.1% to 30% of patients [1,2]. The differential diagnosis of these new nodules is particularly difficult in the cirrhotic liver, where regenerative nodules can be almost the same size as small HCCs. HCC frequently shows a nodular appearance with a fibrous capsule (FC), whose presence is regarded as a helpful diagnostic clue on imaging, particularly ultrasonographic diagnosis, of HCC. Capsules are exceptional in small HCCs less than 1.5-2.0 cm in diameter, many of which are not growing expansively and show a vaguely nodular appearance [3,4]. However, a small HCC of the distinctly nodular type frequently presented as a clear nodule with a FC on pathology [3].
Meanwhile, early recurrence of HCC within 1 year after curative resection appears to arise from intrahepatic metastasis or a synchronous multicentric small HCC [3]. Discrimination between intrahepatic metastasis and synchronous multicentric occurrence is difficult, but it is important for clinical management and post-therapeutic prognosis. It has been reported that the prognosis for patients with synchronous multicentric recurrence after curative resection is significantly better than that for patients with recurrence due to intrahepatic metastasis [5]. Detection and characterization of synchronous multicentric small HCCs in the operative field is critical for curative resection and the post-therapeutic prognosis of HCC.
To our knowledge, there have been no reports on high-frequency IOUS (7-17 MHz) findings with pathologic correlation of the capsule in synchronous multicentric small HCCs detected by IOUS during operation on HCC. Through our early high-frequency IOUS experiences, we have recognized that the presence of a sonographically detectable capsule, even in a small HCC with a diameter of less than 1 cm, is a helpful feature differentiating HCC from cirrhosis-related benign nodules. This study aimed to evaluate the high-frequency IOUS findings focused on the capsules of synchronous multicentric small HCCs. We addressed the following two issues: (1) whether high-frequency IOUS can detect the capsule of a synchronous multicentric small HCC and (2) high-frequency IOUS findings of the capsule with pathologic correlation.
Materials and Methods
Patient Populations and Nodule Selection
The local Institutional Review Board approved the study protocol. Between January 2007 and December 2012, 131 patients underwent hepatic resection for HCC at our institution. From January 2007 to December 2008, high-frequency IOUS and pathologic findings in resected liver specimens were retrospectively reviewed. Only new nodules confirmed by excision and included in the resected specimen were investigated. New nodules were defined as nodules that were not detected by any preoperative diagnostic imaging modalities and were newly found on high-frequency IOUS. From January 2009 to December 2012, high-frequency IOUS and pathologic findings of new nodules in resected hepatic specimens for direct pathologic correlation were prospectively analyzed. For direct correlation between new nodules detected by high-frequency IOUS and on pathologic specimens, ultrasonography (US)-guided hookwire localization for the nodules within the resected specimen was done using a 9 cm 20-gauge Kopans hookwire needle. When more than five nodules were detected on IOUS, US-guided hookwire localization was performed for one to four dominant nodules. Dominant nodules were defined as nodules that were the largest, had suspicious capsules, or showed a mosaic pattern within the nodule. Seventy-nine of 131 patients were excluded based on the following: no newly detected nodules (n=59), incomplete records of high-frequency IOUS and pathologic findings (n=9), radiofrequency ablations performed (n=6), or only biopsies performed for new nodules detected by high-frequency IOUS (n=5). Of these 131 patients, 78 nodules in 52 patients met the study-inclusion criteria and were included.
In order to focus on synchronous multicentric small HCC and exclude intrahepatic metastasis, the pathologic diagnosis of new nodules was based on pathologic findings of synchronous multicentric small HCC, using the Liver Cancer Study Group of Japan morphologic criteria for synchronous multicentric small HCC as follows: well-differentiated HCC and dysplastic nodule containing well-differentiated HCC foci, or well-differentiated HCC containing moderately- or poorly-differentiated cancerous tissues that are considered to have originated and proliferated in situ [6]. The cellular differentiation of HCC was graded as well-, moderately-, or poorly-differentiated on the basis of the International Working Party criteria [7]. Of these 78 nodules, 62 were pathologically excluded based on the following criteria: regenerative nodules (n=35), suspected intrahepatic metastases (n=17), dysplastic nodules (n=6), bile duct adenoma (n=2), focal necrosis (n=1), and sclerosing hemangioma (n=1). The study group consisted of 16 nodules in 13 patients diagnosed as HCCs with synchronous multicentric development (Fig. 1).

Flow chart of the study population selection.
Study group consists of 16 nodules diagnosed as hepatocellular carcinomas (HCCs) with synchronous multicentric occurrence in 13 patients. IOUS, intraoperative ultrasonography.
The study cohort consisted of 12 males and one female. The mean age was 59 years (range, 39 to 75 years). The size of primary HCCs in 13 patients ranged from 1.7 to 6.0 cm (mean diameter, 3.1 cm). Ten patients had mixed macronodular and micronodular cirrhosis secondary to the hepatitis B virus. Two patients had mixed macronodular and micronodular cirrhosis due to hepatitis C. One patient had mixed macronodular and micronodular alcoholic cirrhosis. Thirteen patients with mixed macronodular and micronodular cirrhosis were Child-Pugh class A (n=10) and B (n=3).
Preoperative Imaging and Surgery
Preoperatively, all 13 patients underwent dynamic computed tomography (CT). CT exams were performed by one of two 16- or 64-detector CT scanners (Light Speed, GE Medical Systems, Milwaukee, WI, USA). For the contrast-enhanced portion of the examination, the patients received approximately 80-130 mL of iohexol (Omnipaque 300, GE Healthcare, Princeton, NJ, USA) or iodixanol (Visipaque 320, GE Healthcare) intravenously by means of a mechanical power injector (Stellant Injector System, Medrad Inc., Warrendale, PA, USA) administered at a rate of 3-4 mL/sec, followed by a 15 to 20 mL saline flush. The standard protocol for triphasic CT consisted of an unenhanced, arterial phase with a scanning delay of 30-40 seconds and a portal phase with a scanning delay of 60-80 seconds. Magnetic resonance imaging (MRI) was performed in 11 of 13 patients. MRI studies were performed on a 3.0T MR unit (Intera Achieva 3T, Philips Medical Systems, Best, Netherlands) with use of the body coil for transmission and reception of the signal. The pulse sequences used were calibrated to obtain magnetic resonance (MR) images of the liver with optimal anatomic resolution. Standard technique was used for each scan, T1-weighted precontrast and postcontrast Turbo FLASH with coronal oblique and axial scans and fat-saturated T2-weighted axial scans. All MR studies included dynamic studies with the administration of extracellular gadolinium contrast agent.
The surgical procedures were performed by two surgeons with more than 10 years of experience in hepatic surgery. Procedures included right hepatectomy, left hepatectomy, extended resection, segmentectomy, and tumorectomy. If new lesions discovered at high-frequency IOUS were confined to the lobe or hepatic segment designated for resection, the planned surgical procedure was not changed. If new lesions were discovered in a lobe or segment other than that designated for resection, either tumorectomy or intraoperative radiofrequency ablation were performed on the basis of the pathologic result of an intraoperative biopsy.
High-Frequency IOUS and Pathologic Correlation
The examination protocol was standardized as follows. After complete hepatic mobilization by dissecting hepatic attachments and careful palpation of the liver, the liver was thoroughly scanned in a consistent order: (1) tracing all hepatic veins and their tributaries, taking care not to overlook the short hepatic veins, (2) tracing all portal venous branches, and (3) examining the liver parenchyma for a primary lesion and for new nodules by means of systematic and repeated longitudinal and transverse scanning. This scanning sequence was briefly repeated for resected specimens. A subspecialty-trained gastrointestinal radiologist (J.H.A.) with more than 10 years’ experience performed all IOUS examinations. A high-resolution US system (iU 22 or HDI 5000, Philips Medical System, Bothell, WA, USA) was used with a 7-17 MHz linear array transducer and a 7-15 MHz compact linear probe.
After hepatic resection, we performed US-guided hookwire localization for the detected nodules within the resected specimen. Pathologic correlation was made by US-guided hookwire localization using a 9 cm 20-gauge Kopans hook needle for nodules within the resected specimen. The present investigation was a direct analysis of high-frequency IOUS and pathologic findings (n=15) rather than a retrospective review of high-frequency IOUS and pathology reports (n=1). All resected specimens were fixed in formalin, sectioned based on US-guided hookwire localization, stained with hematoxylin and eosin, and examined by a pathologist according to the criteria of the International Working Party [7].
Data Analyses
High-frequency IOUS findings and pathologic findings of 16 synchronous multicentric small HCCs, focused on the capsule, were analyzed by two subspecialty-trained gastrointestinal radiologists (J.H.A. and S.M.J.) with more than 10 years of experience and a pathologist (D.-W.E.) with 9 years of experience who paid special attention to the tumor capsule. The pathologist was not blinded to high-frequency IOUS results and was directed to assess what was seen pathologically in patients with a capsule appearing on high-frequency IOUS. Analysis included the following: (1) the size of synchronous multicentric small HCC nodules measured; (2) the pathologic classification of synchronous multicentric small HCC nodules by type as vaguely nodular or distinctly nodular [3,4]; (3) the histological grade of tumors; (4) when the capsules were present on high-frequency IOUS and the pathologic specimen, the frequency and thickness of capsules; (5) whether high-frequency IOUS could detect the capsule of a synchronous multicentric small HCC; and (6) high-frequency IOUS and pathologic findings of the capsules. High-frequency IOUS findings of capsules were classified into the following three types on the basis of the difference in echogenicity among capsules, the lesion, and the surrounding liver parenchyma: hypoechoic rim (echogenicity of the tumor capsule lower than that of the lesion and surrounding liver parenchyma); hyperechoic rim (echogenicity of the capsule higher than the lesion); and hypoechoic rim containing hyperechoic foci. The degree of coverage by the capsule on high-frequency IOUS was also analyzed. The thickness of capsules was recorded at the thickest point of the capsule for both high-frequency IOUS and pathological analyses.
Results
Tumor Size and Histological Grade
The size of synchronous multicentric small HCCs on high-frequency IOUS ranged from 6.0 to 12.3 mm (mean, 9.8±1.4; subcentimeter HCCs, n=6). Histologically, 16 synchronous multicentric small HCC nodules were classified by type as distinctly nodular (n=12) and vaguely nodular (n=4). The histological grade of the tumors showed well-differentiated HCCs (n=2), dysplastic nodules containing well-differentiated HCC foci (n=2), and well-differentiated HCCs containing moderately-differentiated foci (n=12). Four vaguely nodular HCCs revealed well-differentiated HCCs (n=2) and well-differentiated HCCs containing moderately-differentiated foci (n=2). Twelve distinctly nodular HCCs were diagnosed as dysplastic nodules containing well-differentiated HCC foci (n=2) and well-differentiated HCCs containing moderately-differentiated foci (n=10). The architectural patterns of the tumors were trabecular (n=14) and pseudoglandular (n=2).
High-Frequency IOUS and Pathological Findings of Capsules
Four vaguely nodular HCCs had no capsule on high-frequency IOUS and pathology. High-frequency IOUS findings of four vaguely nodular HCCs showed an isoechoic nodule (n=1) and ill-defined echogenic nodules with internal nodule-in-nodule appearance (n=2) and internal heterogeneity (n=1). In twelve distinctly nodular HCCs with capsules on pathology, high-frequency IOUS detected all capsules. The high-frequency IOUS findings of capsules in 12 distinctly nodular HCCs were a hypoechoic rim containing hyperechoic foci (n=6), hypoechoic rim (n=5), and hyperechoic rim (n=1). The degree of coverage by the rim was more than 90% (n=3), from 50% to 90% (n=6), and less than 50% (n=3) (Fig. 2). The one HCC with a hyperechoic rim showed 75% coverage (Fig. 3). Histologically, capsules were composed of a combination of one to four layers, consisting of a FC, peritumoral fibrosis (PF), prominent small vessels, and entrapped or compressed hepatic parenchyma (EP) (Figs. 3, 4). The histological combinations of tumor capsules at the thickest point were classified into the following: FC, PF, and EP (n=4); FC, PF, EP, and small vessels (n=3); PF and EP (n=3); PF, EP, and small vessels (n=1); and FC, PF, and small vessels (n=1) (Fig. 4). Capsule thickness on high-frequency IOUS ranged from 0.70 to 1.60 mm (mean, 1.00±0.08 mm). Capsule thickness on microscopic examinations ranged from 0.51 to 1.40 mm (mean, 0.89±0.09 mm). These results are summarized in Table 1.
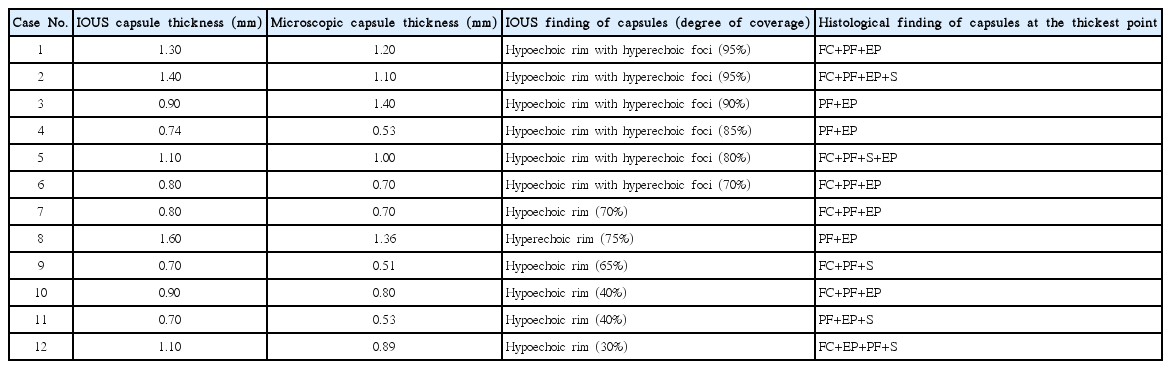
Example of the varying coverage of the capsules around synchronous multicentric small hepatocellular carcinomas (HCCs).
A. In case 7 in Table 1, high-frequency intraoperative ultrasonography (IOUS) shows a hyperechoic HCC with detectable hypoechoic rim (arrowheads). The coverage of the hypoechoic rim is more than 50%. B. In case 10 in Table 1, high-frequency IOUS shows a hyperechoic HCC with detectable hypoechoic rim (arrowheads). The coverage of hypoechoic rim is less than 50%.
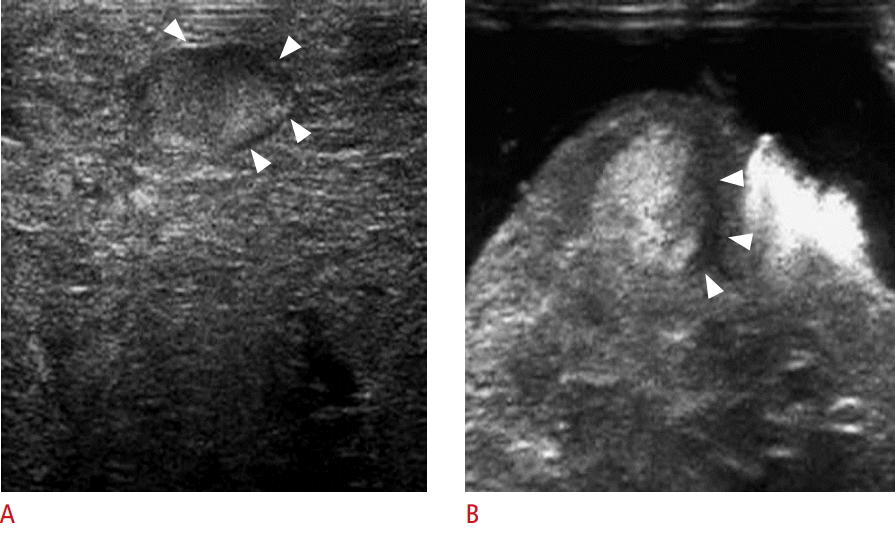
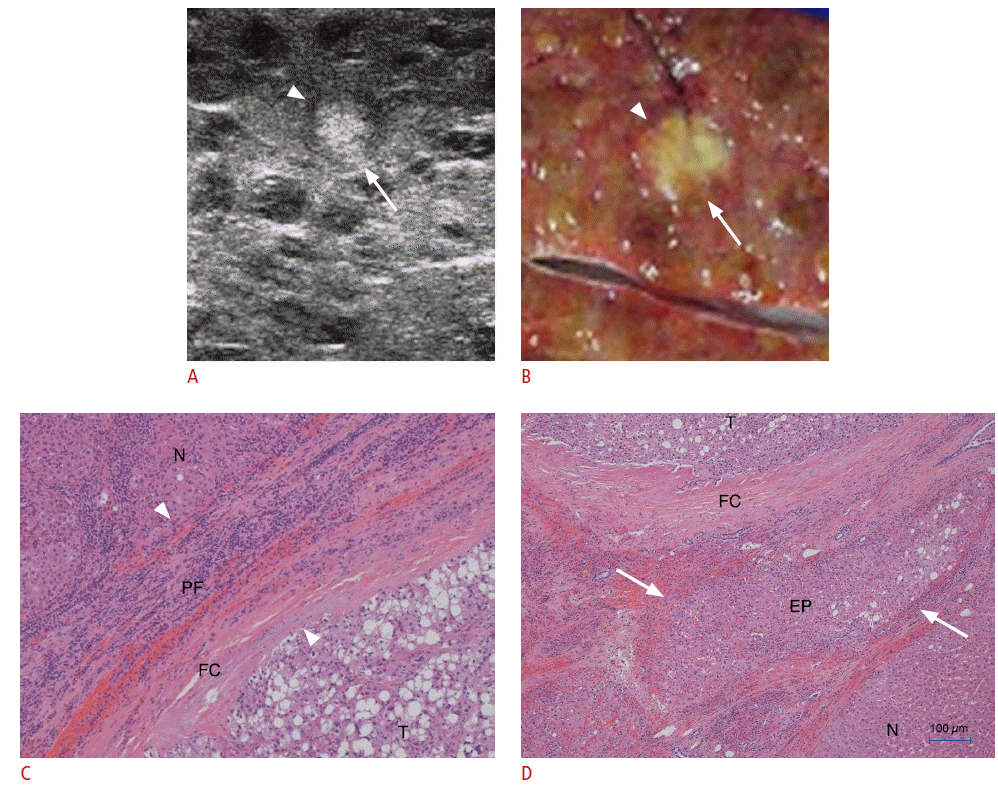
Synchronous multicentric small hepatocellular carcinoma (HCC) with hyperechoic rim in a 52-year-old male (case 8 in Table 1).
A. High-frequency intraoperative ultrasonography (IOUS) shows a hypoechoic HCC with detectable hyperechoic rim (arrowheads and arrows). The coverage of the hyperechoic rim is approximately 75%. B. Photograph of a resected specimen shows a distinctly nodular HCC. C, D. Photomicrographs (C, H&E, ×40; D, H&E, ×100) of the capsules in an anatomic location corresponding to the white arrows in A and B show that the capsule consists of the peritumoral fibrosis (PF) and entrapped hepatic parenchyma (EP). The hyperechoic area (arrows in A) on high-frequency IOUS is mainly composed of EP (arrows). T, tumor; N, nontumorous liver tissue.

Composition of the capsules around synchronous multicentric hepatocellular carcinomas on photomicrographs.
A. In case 1 in Table 1, a photomicrograph shows that the capsule (arrows) consists of a single layer of fibrous capsule (FC) (H&E, ×100). B. In case 4 in Table 1, a photomicrograph shows that the capsule (arrows) consists of a single layer of peritumoral fibrosis (PF) (H&E, ×100). C. In case 3 in Table 1, a photomicrograph shows that the capsule (arrows) is composed of three layers of FC, PF, and entrapped hepatic parenchyma (EP) (H&E, ×100). D. In case 10 in Table 1, a photomicrograph shows that the capsule (arrows) is composed of three layers of FC, PF, and prominent small vessels (S) (H&E, ×100). T, tumor; N or NT, nontumorous liver tissue.
The histological findings of hypoechoic rim in 11 HCCs with hypoechoic rim containing hyperechoic foci (n=6) and hypoechoic rim (n=5) were mainly composed of FC and/or PF (Figs. 4B, 5, 6). The histological finding of hyperechoic areas in the six HCCs with hypoechoic rim containing hyperechoic foci and one HCC with hyperechoic rim with 75% coverage was EP (Figs. 3, 4B, 5, 6).
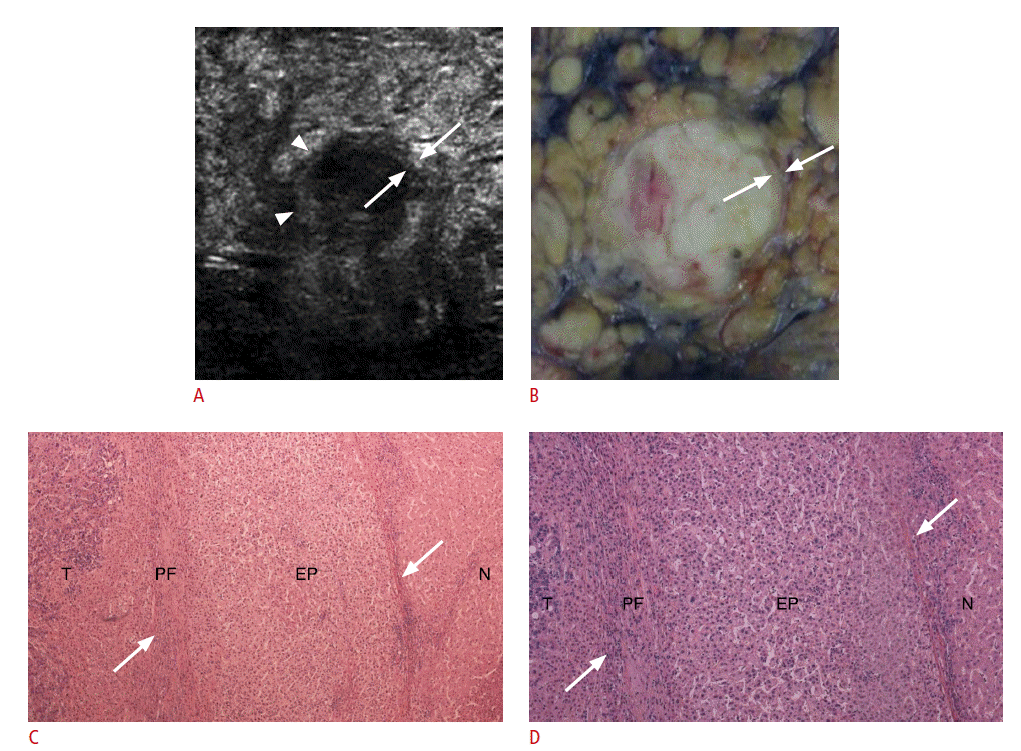
Correlation of high-frequency intraoperative ultrasonography (IOUS) and histological findings of a capsule in a 62-year-old male (case 4 in Table 1).
A. High-frequency IOUS shows a hyperechoic hepatocellular carcinoma (HCC) with detectable hypoechoic rim (arrowhead) containing hyperechoic foci (arrow). B. Photograph of a resected specimen shows a distinctly nodular HCC. C. In photomicrographs of the tumor (T) (H&E, ×40) and the capsule in an anatomic location corresponding to the arrowheads in A, B, and C, the hypoechoic area of the capsule on high-frequency IOUS is composed of peritumoral fibrosis (Fig. 4B). D. Photomicrograph of the capsule in an anatomic location corresponding to the arrows in A, B, and C shows that the hyperechoic focus of the capsule on high-frequency IOUS is mainly composed of entrapped hepatic parenchyma (EP) (arrows) (H&E, ×100). NT, nontumorous liver tissue; PF, peritumoral fibrosis.
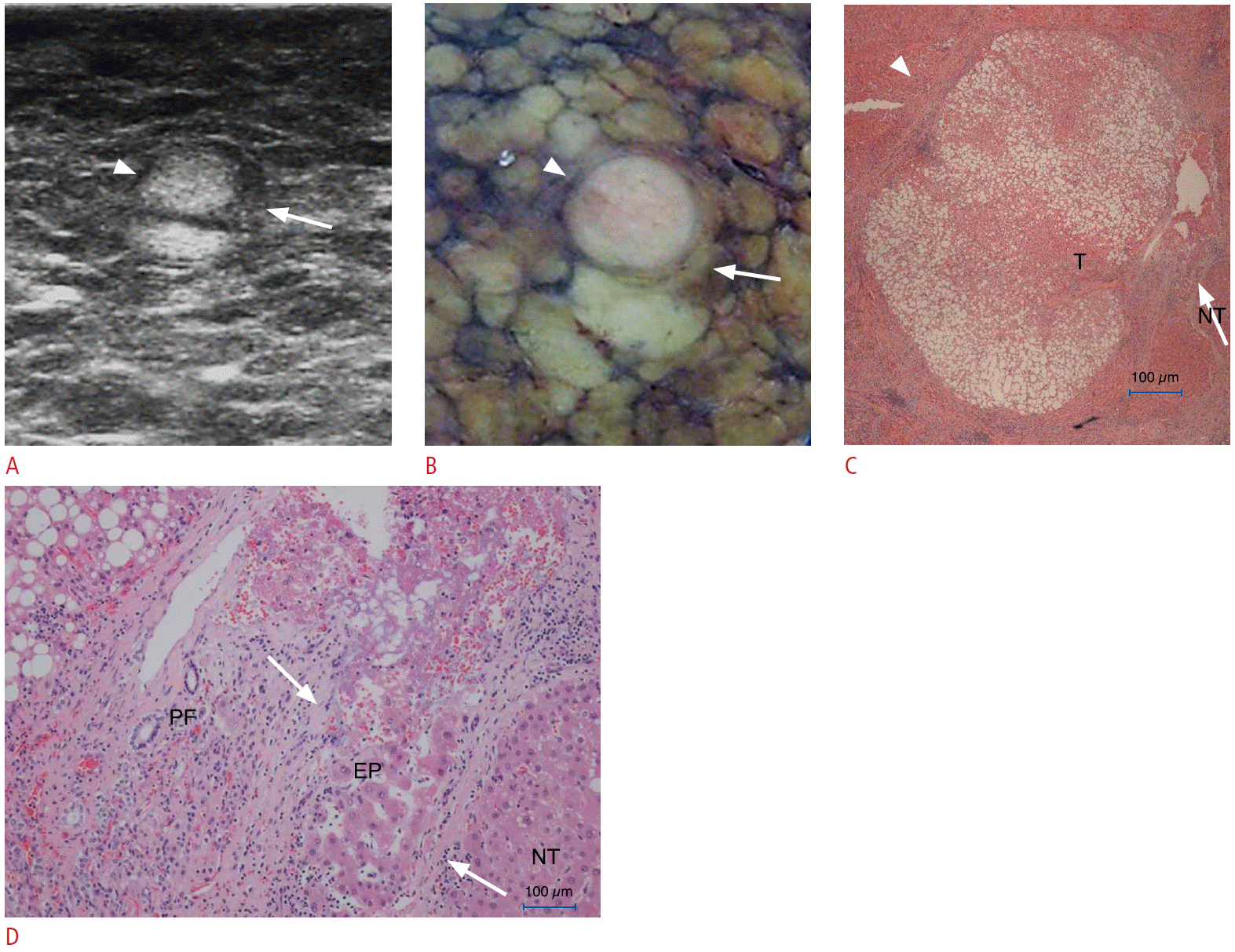
Correlation of high-frequency intraoperative ultrasonography (IOUS) and the histological findings of a capsule in a 64-year-old male (case 1 in Table 1).
A. High-frequency IOUS shows a hyperechoic hepatocellular carcinoma (HCC) with detectable hypoechoic rim (arrowhead) containing hyperechoic foci (arrow). B. Photograph of a resected specimen shows a distinctly nodular HCC. C. Photomicrograph of the capsule in an anatomic location corresponding to the arrowheads in A and B shows that the hypoechoic areas of the capsule on high-frequency IOUS consist of a fibrous capsule (FC) and peritumoral fibrosis (PF) (arrowheads) (H&E, ×40). D. Photomicrograph of the capsule in an anatomic location corresponding to the arrow in A and B shows that the capsule consists of FC and entrapped hepatic parenchyma (EP) (H&E, ×40). The hyperechoic focus of the capsule in A is mainly composed of EP (arrows). N, nontumorous liver tissue.
Discussion
In the setting of liver cirrhosis, many studies using morphologic examination of resected and biopsy specimens of small HCCs, and follow-up studies after curative treatment of HCC cases, have suggested that many HCCs are multicentric in origin. The reported frequency of synchronous multicentric HCCs in surgically resected cases ranges from 15% to 30% [8,9]. Previous reports have indicated that the recurrence rate after resection of HCC is 20%-40% within a year and about 80% in 5 years [10-12], and early recurrence appears to arise from intrahepatic metastases and missed early-stage HCC with synchronous multicentric occurrence. With the aim of reducing the early recurrence of HCC, the detection of synchronous multicentric small HCC during surgery for HCC using IOUS that provides high spatial resolution without interference from the surrounding structures is clinically important [13].
The present study focused on synchronous multicentric small HCCs detected during surgery for HCC. We demonstrated that synchronous multicentric small HCCs with a distinctly nodular type, even at subcentimeter size, had detectable capsules on high-frequency IOUS. We also found that most of the capsule could be identified by the hypoechoic rim with or without hyperechoic foci. Regenerative nodules are surrounded by thin fibrous septa. However, distinctly nodular HCCs have relatively thick capsules. Unlike the thin fibrous septa of regenerative nodules, we found that capsules of distinctly nodular HCCs consist of a FC, PF, small vessels, and EP. Our five distinctly nodular HCCs had capsules greater than 1 mm in thickness. These capsules contained the entrapped parenchymal layer; this layer may be associated with the evolution of the regenerative nodule to early and subsequently advanced HCC. To our knowledge, this is the first report of high-frequency IOUS findings focusing on the capsule of synchronous multicentric small HCCs.
Small HCCs of the distinctly nodular type show expansive growth, and many are encapsulated [3]. The main mechanism of capsule formation is thought to be the condensation of the fibrous elements of the surrounding noncancerous liver tissue due to the mechanical pressure of expansive tumor growth [3,4]. The distinct subnodular HCC within regenerative or dysplastic nodules as a nodule-in-nodule appearance gradually increases in size and eventually replaces the maternal regenerative or dysplastic nodule, and thus the capsule may contain entrapped parenchyma. The subnodular HCCs within regenerative or dysplastic nodules can show different tumor differentiations, and thus variations in the rate of expansive growth. The difference in growth rates of subnodular HCCs within a regenerative nodule or dysplastic nodule may reflect the various degrees of capsule coverage [3]. Therefore, the various degrees of capsule coverage in our cases may be associated with the evolution of a regenerative nodule to early and advanced HCC.
Histologically, capsule formation was confirmed in about 53% of distinctly nodular HCCs, even in relatively small tumors of less than 2 cm in diameter [3]. Fibrous capsules with two layers were reported: an inner layer rich in a fibrous component and an outer layer containing various numbers of small vessels and newly formed bile ducts [14]. A report demonstrated that some HCCs showing an enhanced rim on dynamic MRI do not actually have a true FC histologically [15]. In these cases, the pseudocapsule seen on MRI represents prominent hepatic sinusoids and/or PF. In our cases, the outer layer and prominent sinusoids were not prominent, which likely reflected poor development of tumor vascularity and draining veins as a small, early-stage HCC. The bright-loop appearance as a sonographic finding for HCC with a hyperechoic rim in the late stages of dedifferentiation of well-differentiated HCC has been demonstrated [16]. The bright-loop appearance represented the fatty change of well-differentiated HCC containing low echoic HCC with moderate differentiation. However, the main histological finding of hyperechoic areas in the six HCCs with a hypoechoic rim containing hyperechoic foci and one HCC with hyperechoic rim among our cases was entrapped parenchyma.
This study has several strengths. The present investigation was a direct analysis of high-frequency IOUS findings and pathologic specimens rather than a retrospective review of high-frequency IOUS and pathologic reports; pathologic correlation was made using US-guided hookwire localization for nodules of interest within the resected specimens. Although the focus on synchronous multicentric small HCC has both merits and demerits, there was a selection bias. The reported incidence of capsules in small distinctly nodular HCCs is about 53% of 80 cases. In this series, high-frequency IOUS detected all capsules of 12 synchronous multicentric small HCCs with distinctly nodular type. These results may be considered as another selection bias, occurring because we defined dominant nodules as nodules that were the largest, had suspicious capsules, or showed a mosaic pattern within the nodule.
There are also several limitations to this study. First, our sample number was small. Second, results were descriptive rather than analytic because of the small sample size. It is unclear whether the histological finding of entrapped parenchyma represents dysplastic nodules. Some EP showed increased cellular density. Third, MRI with extracellular contrast agent was only used for preoperative evaluation. This evidences a selection bias and methodological limitation because MRI performed with the hepatobiliary phase agent gadoxetate had high per-lesion sensitivity for lesions ≤20 mm [17]. Finally, the logical next step may be to evaluate whether the detectable capsules of synchronous multicentric small HCCs with distinctly nodular type on high-frequency IOUS could be considered an important finding differentiated from benign regenerative nodules. Thus, further study may be needed.
In summary, high-frequency IOUS detected all capsules of 12 synchronous multicentric small HCCs with distinctly nodular type. The capsule of synchronous multicentric small HCCs with distinctly nodular type detected by IOUS showed a range of capsule coverage of the hypoechoic rim with or without hyperechoic foci (n=11) and the hyperechoic rim (n=1). The varying degrees of capsule coverage may be associated with the evolution of a regenerative nodule to early and advanced HCC. These detectable capsules should be considered another IOUS finding of synchronous multicentric small HCC with distinctly nodular type.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by Gangneung Asan Hospital Foundation. The authors thank the Gangneung Asan Hospital Foundation for their technical support.