Value of sagittal color Doppler ultrasonography as a supplementary tool in the differential diagnosis of fetal cleft lip and palate
Article information
Abstract
Purpose
The purpose of this study was to evaluate the feasibility and usefulness of sagittal color Doppler ultrasonography (CDUS) for the diagnosis of fetal cleft lip (CL) and cleft palate (CP).
Methods
We performed targeted ultrasonography on 25 fetuses with CL and CP, taking coronal and axial images of the upper lip and maxillary alveolar arch in each case. The existence of defects in and malalignment of the alveolus on the axial image, hard palate defects on the midsagittal image, and flow-through defects on CDUS taken during fetal breathing or swallowing were assessed. We compared the ultrasonography findings with postnatal findings in all fetuses.
Results
Alveolar defects were detected in 16 out of 17 cases with CP and four out of eight cases with CL. Alveolar malalignment and hard palate defects were detected in 11 out of 17 cases and 14 out of 17 cases with CP, respectively, but not detected in any cases with CL. Communicating flow through the palate defect was detected in 11 out of 17 cases of CL with CP. The accuracy of detection in axial scans of an alveolar defect and malalignment was 80% and 76%, respectively. Accuracy of detection of in mid-sagittal images of hard palate defect and flow was 80% and 86%, respectively. The overall diagnostic accuracy of combined axial and sagittal images with sagittal CDUS was 92%.
Conclusion
Sagittal CDUS of the fetal hard palate is a feasible method to directly reveal hard palate bony defects and flow through defects, which may have additional value in the differential diagnosis of fetal CL and CP.
Introduction
Orofacial clefts, including cleft lip (CL) with or without cleft palate (CP), are relatively common congenital anomalies, reported in approximately one in 700 live births [1]. The rates of CL with or without CP are higher in parts of Latin America and Eastern Asia and lower in South Africa and Southern Europe [2]. The accurate prenatal detection and differential diagnosis of CL and CP is of clinical importance because the type and extension of clefts are correlated with fetal outcomes and associated anatomical and chromosomal abnormalities [3,4]. CL without CP is generally known to show favorable prognosis compared to CL with CP. Reconstruction techniques, surgical implications, and risks for chronic otitis media, hearing loss, and abnormal speech are very different when the palate is involved. CL with CP includes greater morbidity and reparations resulting in more extensive surgeries.
Transabdominal ultrasonography (TA-US), performed in the second trimester of pregnancy, is the first choice of imaging modalities to screen for orofacial clefts due to its easy accessibility and the fact that it does not result in radiation exposure. In low-risk groups (unselected population-based groups of pregnant women), the overall detection rate for all types of clefts was reported as 0%-70%, for CL with or without CP it was 33%-88%, and for isolated CP it was 0%-22% [5]. There have been some prospective studies for the detection and identification of CL and CP in high-risk groups (i.e., referral cases because of abnormal findings during routine prenatal screening ultrasonography [US], family history of CL or CP). The reported detection rate in these cases using 2-dimensional (2-D) TA-US was 91%-96% for CL with or without CP and 41%-49% for CL with CP [6-8]. For the high-risk group in these studies, using 3-dimensional (3-D) TA-US improved the detection rate to 100% for CL with or without CP and 41%-46% of CL with CP [6-8]. Some additional scan techniques have been introduced for 3-D US, such as the reverse face view [9] or flipped face view [10], to improve the diagnostic accuracy of detection and identification of CL and CP.
However, 3-D US takes a longer time to execute a scan and, in the majority of cases, needs a customized volume transducer. Hence, conventional 2-D US is still important as the first-line imaging modality for the prenatal diagnosis of CL and CP. Efforts to enhance the accuracy of 2-D US, including the application of color or power Doppler have been reported [11-13]. A conventional grayscale ultrasound may miss the existence of CP, because acoustic shadowing from the bony alveolar ridge often obscures the palate defect. Previous communications reported that the color or power Doppler on the sagittal plane can improve the diagnostic accuracy of CP by detecting the slow amniotic fluid flows between the buccal space and the nasal fossa during breathing or swallowing, with representative cases at the level of case reports [11-13]. However, to our knowledge, there have been no previous studies validating the use of color Doppler ultrasonography (CDUS) on the sagittal plane in the diagnosis of CL and CP.
Thus, the aim of our study is to evaluate the feasibility and usefulness of sagittal CDUS for the diagnosis of fetal CL and CP.
Materials and Methods
This prospective study was approved by our Institutional Review Board and Ethics Committee. Informed consent was carefully obtained from all study participants.
Study Population
From January 2007 to January 2010, 25 pregnant women were consecutively enrolled in our study. The study participants were those patients whose fetuses were suspected to have CL or CP as a result of routine screening US scans in the obstetrics clinic of our center, followed by a referral to the radiology department for targeted US scans. There were no multiple gestation cases, so a total of 25 fetuses were evaluated in this study. The mean maternal age was 31.2 years old (range, 26 to 39 years). The mean gestational age at the time of the targeted US was 25.6 weeks old (range, 18.5 to 36.0 weeks). The characteristics of study participants are summarized in Table 1.
Targeted Ultrasound
All targeted US was performed by a radiologist (J.Y.C.) with 17 years of experience in maternal-fetal radiology and ultrasound examination using an Acuson Sequoia 512 ultrasound machine with a 2-4-MHz curved probe and a 5-8-MHz sector probe (Acuson Corp., Mountain View, CA, USA). In all cases, coronal and axial images of the upper lip were obtained. Axial images of the maxillary alveolar arch were analyzed for the existence of defects in and malalignment of the alveolar arch. Attempts were made to obtain mid-sagittal images to evaluate the existence of hard palate defects. This was followed by attempts to obtain sagittal color Doppler ultrasonograms during fetal breathing or swallowing to detect bidirectional amniotic fluid flow through the defect. Before evaluating the mid-sagittal plane, the upper lip was visualized and evaluated on the coronal plane. Then, the probe was rotated to the sagittal plane using the fetal nose as the pivot axis. The mid-sagittal plane was defined as the sagittal ultrasonogram of the fetal face containing forehead, nasal bone, vomer, philtrum, lower lip, and the tip of chin in one plane. To detect low-velocity flow through a palatine defect, the wall filter was set as low as possible, and color gain was set as high as possible without noise. The range of total examination time was from 8 to 53 minutes, and the mean examination time was 26.2±16.8 minutes. The examination time of the sagittal plane with color Doppler only was not recorded; however, this was assumed to have taken approximately 5 minutes.
Image Analysis
All targeted US findings were compared to postnatal findings based on hospital records made by experienced obstetricians. Clefts were categorized by location and extent (involving lips, alveolus, and palate) and by laterality (unilateral, bilateral, or midfacial) in both the targeted US and postnatal findings. Four imaging findings (i.e., alveolar defect in an axial scan, alveolar malalignment in an axial scan, palatal echo defect in a sagittal scan, and flow-through defect in a sagittal CDUS image) were documented in each case. The existence rates of each finding for each fetus with CL with CP or isolated CL were calculated. Finally, the accuracy of detecting CL with or without CP using a combined scan of axial and sagittal images, including CDUS images, was also calculated.
Results
All of the 25 fetuses were confirmed postnatally to have an orofacial cleft. Among them, 17 had CL with CP and eight had isolated CL only (CL without CP). Four of the eight isolated CL cases had a cleft alveolus. Due to weak fetal swallowing, a sagittal CDUS could not be obtained in four out of the 25 cases (three out of 17 CL with CP cases and one out of eight isolated CL case).
An alveolar defect in an axial image was detected in 16 out of 17 cases of CL with CP (94.1%), and in four out of eight (50%) isolated CL cases. The overall accuracy for diagnosing CL with or without CP using the finding of an alveolar defect in an axial image was 80% (20 out of 25 cases). Alveolar malalignment was detected in 11 out of 17 cases of CL with CP (57.8%); however, it was not detected in any cases of isolated CL. Palate echo defects in sagittal scans were detected in 14 of 17 cases (82.4%). Communicating flow-through defects in the sagittal CDUS were identified in 12 of 17 cases (70.6%) of CL with CP (Fig. 1).
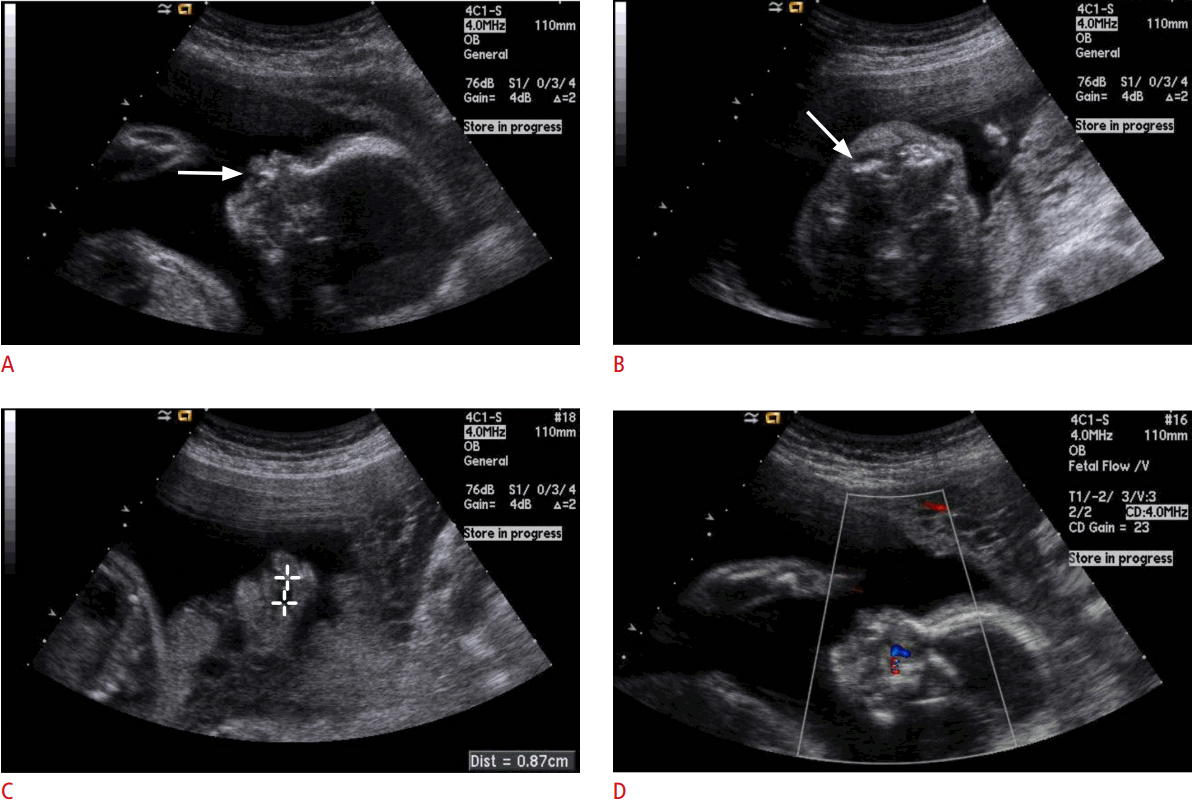
Prenatal ultrasonographic findings of cleft lip and palate in a 27-week fetus.
A. Sagittal image of upper lip shows narrow defect of upper lip (arrow). B. Axial image of upper alveolar shows a small alveolar defect and malalignment (arrow). C. Coronal image of the hard palate reveals approximately 0.9 cm long defect on the hard palate. D. Color Doppler sonogram shows flow through the hard palate defect.
Case 20 showed no definite defect in the hard palate, but a transpalatal communicating flow was detected in the sagittal CDUS. Therefore, we suggested the possibility of a diagnosis of CL with CP based on the finding in the sagittal CDUS, confirmed by postnatal neonate examination.
Case 9 showed a suspicious hard palate defect on the grayscale scan and was initially considered to be CL with CP. However, there was no definite transpalatal communicating flow on consecutive sagittal CDUS. The US diagnosis was reported as CL with alveolar involvement and confirmed by postnatal neonate examination (Fig. 2).

Prenatal ultrasonographic findings of cleft lip with alveolar involvement in a 26.4 week fetus.
A. Coronal image of the upper lip shows a defect of upper lip (arrow). B. Axial image of the alveolar arch shows a small alveolar arch defect (annotated). Arch malalignment is equivocal. C. Sagittal image of the hard palate reveals suspicious focal defect on the hard palate (arrow). D. Color Doppler sonogram shows no definite flow through the hard palate defect.
Case 3 showed a hard palate defect in the sagittal grayscale ultrasound and a transpalatal flow in the CDUS; these were confirmed as an isolated CL with alveolar defect. It was suggested that the flow through the alveolar cleft had been improperly identified as transpalatal flow in the CDUS.
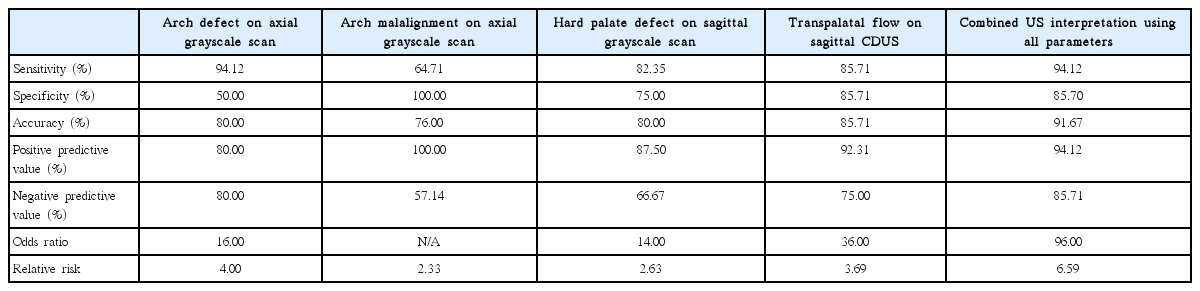
The sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and accuracy of each of the parameters (i.e., alveolar arch defect in an axial scan, alveolar malalignment in an axial scan, hard palate defect in a sagittal scan, transpalatal flow in a CDUS, and combined US interpretation using all parameters) for differentiating CL with CP from isolated CL are summarized in Table 2. Sagittal CDUS presented slightly improved sensitivity, accuracy, and PPV compared to sagittal grayscale scan (85.71% vs. 82.35% sensitivity, 85.71% vs. 80.00% PPV, and 92.31% vs. 87.50% accuracy, respectively). The combined US interpretation of all parameters showed good diagnostic performance, with 94.12% sensitivity, 94.12% PPV, and 91.67% accuracy.
Discussion
The standard US evaluation method of fetal upper lip and anterior palate for cleft consists of coronal and axial plane assessment of midfacial anatomy [14]. Although adding fetal facial scans to routine screening 2-D US has been considered optimal [15], the accuracy for detecting CL with or without CP is variable, especially in low-risk patients [5]. Hence, some efforts to improve the detection rate of CL with or without CP have been attempted by adding the sagittal view to the evaluation.
However, detection of a defected palate in a grayscale sonogram is difficult due to acoustic shadowing from the bony alveolar ridge and maxilla [13]. The importance of a sagittal scan, therefore, has been described with a limited view toward detecting “premaxillary protrusion(s)” when bilateral CL with or without CP was suspected in coronal or axial scans. Babcook et al. [16] reported that the echogenic fusion line of the secondary palate was seen in only 26% of in utero fetuses in sagittal sonograms and in 100% of normal fetal specimens.
Delineating the palatine defect by visualization of the slow amniotic flow through the defect using sagittal CDUS has been tried in some case reports [11-13] to increase the detection rate of CL with or without CP by collecting additional information through sagittal US scans. In this study, we extended the methods to a larger group of high-risk patients.
In our study, the accuracy of observing flow in sagittal CDUS to detect CL with CP was 85.7% (CL with CP, 12 out of 14 cases; CL without CP, one out of seven cases showed transpalatal flow in sagittal CDUS). This result is a high detection rate compared to previous studies showing 41%-46% for detection of CL with CP [6,7], which highlights the diagnostic value of adding sagittal CDUS to fetal facial US. For these previous studies, the CL with CP detection rate of 3-D US was 86%-90%. Thus, the results of our study suggest that by only adding sagittal CDUS to fetal facial US, are detection rates similar those achieved using 3-D US to detect CL with CP possible. Detection of palate defects directly in sagittal images or flow in sagittal CDUS showed comparable, or better, accuracy than axial scans for CL with CP (detecting alveolar defects or alveolar malalignment). We infer from this that the sagittal scan with CDUS is a feasible and useful scanning method to detect CL with CP; our high detection rate compared to previous studies can be explained by the use of sagittal CDUS.
We found that the accuracy of detecting CL with CP by delineating a hard palate defect on the grayscale sagittal US was successful (accuracy of 80%). Rotten and Levaillant [17] described sonographic characteristics of secondary palate clefts in sagittal US scans. They reported that viewing defects in hard palate continuity was possible and that the defects were asymmetric. However, they did not report the detection accuracy of a sagittal scan alone, and concluded that the secondary palate was best seen in coronal scans with 87% concordance between the US report and postnatal evaluation. In our study, we reported comparable accuracy as reported by Rotten et al. [17], supporting our hypothesis that sagittal US scans may have additional value in the diagnosis of CL with or without CP.
Though similar in accuracy, sagittal CDUS had the slight advantage of increasing specificity, PPV, and NPV compared to detecting hard palate defects in sagittal grayscale scans. A combined US interpretation of all parameters could show good diagnostic performance, with up to 94% of sensitivity, 94% of PPV, and 92% of accuracy. The odds ratio and relative risk of sagittal CDUS were better than that of sagittal grayscale scans; positive findings of sagittal CDUS were better correlated with the existence of actual CPs than those of sagittal grayscale scans. Thus, sagittal CDUS should be added to a routine sagittal grayscale sonogram. Occasionally, there can be cases of CL without CP that show alveolar arch defects or malalignment in sagittal grayscale sonogram. In those cases, performing sagittal CDUS for visualizing transpalatal flow can help in distinguishing true CP from artifacts (Fig. 1), strengthening the added value of sagittal CDUS.
By combining axial and sagittal sonograms with CDUS, the diagnostic accuracy was 92% (approximately 10% higher than using axial images alone). Our results had comparable accuracy with previous studies [6-8], in which the accuracy of 2-D US to detect CL with or without CP ranged from 90% to 96%. However, limiting the perspective to CL with CP, the accuracy of previous studies were relatively low (31%-46%) [6,8]. Our study showed 94.1% accuracy; using a combination of axial and sagittal images with CDUS had 16 accurate diagnoses out of 17 total. In a real clinical setting, differentiation from CL with alveolar defect or malalignment without CP and CL with CP is crucial; the former requires a less complex surgical correction (usually a one-stage operation) and shows better clinical outcomes in terms of permanent deformity, phonation, and velopharyngeal insufficiency compared to the latter. Based on our results, we suggest adding sagittal US with CDUS on conventional axial and coronal US of fetal faces to provide the additional benefit of a high NPV (85.7%) compared to grayscale scans, consequently increasing the overall accuracy for differentiating CL with alveolus involvement without CP and CL with CP.
Our study has several limitations. First, the population we studied was relatively small. Second, this population was limited to high-risk cases that had been referred to tertiary care centers due to abnormal findings on screening US. Future studies with larger, low-risk populations (those subject to routine screening) are needed to validate and generalize our results and conclusions. Third, although uncommon, there could be possibilities of false positives (i.e., mistaking flow-through alveolar clefts for transpalatal flow) or false negatives (i.e., due to insufficient fetal swallowing motion) of sagittal CDUS. Furthermore, the yield of sagittal CDUS can be variable based on whether the fetus swallows or breathes during the examination. We note that we were not able to obtain sagittal CDUS from 16% (four out of 25) of our study participants. The yield and average additional examination time of getting sagittal CDUS must be assessed through studies of large, low-risk populations to evaluate the validity of adding sagittal US with CDUS to routine fetal facial scan protocols.
Despite these limitations, we can conclude that sagittal US evaluation of the fetal hard palate is a feasible method to directly reveal bony defects of the hard palate and communicating amniotic fluid flow through the defect using CDUS. Adding sagittal scans with CDUS as a part of a routine sonographic fetal face evaluation protocol may have additional value in the differential diagnosis of fetal CL with, or without, CP.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI15C1532).