Ultrasound-guided ethanol ablation for cystic thyroid nodules: effectiveness of small amounts of ethanol in a single session
Article information
Abstract
Purpose
The aim of this study was to evaluate the efficacy of ethanol ablation (EA) in the treatment of cystic thyroid nodules using low-dose ethanol regardless of the initial volume of the nodule or properties of the aspirate.
Methods
Sixty-one nodules in 60 patients were treated with EA from October 2013 to January 2020. In each patient, EA was performed only once, using less than 5 mL of ethanol (99.5%) instilled and removed completely after a few minutes of retention. Nodule volume, the symptom score, the cosmetic score, and complications were evaluated before and after treatment. The therapeutic success rate (TSR) and volume reduction rate (VRR) according to nodule volume and properties of the aspirate were evaluated. Therapeutic success was defined as the absence of any residual fluid or sufficient volume reduction (≥50%) with improvement of nodule-related symptoms.
Results
The 61 nodules comprised 38 pure cysts and 23 predominantly cystic nodules. The initial nodule volume was 21.9±15.2 mL (range, 4.4 to 77.2 mL). The TSR was 88.5% (100% in pure cysts and 69.6% in predominantly cystic nodules, P<0.001). The TSR of pure cysts was 100% regardless of nodule volume and properties of the aspirate. In predominantly cystic nodules, the TSR and VRR gradually decreased as volume increased. One patient experienced arrhythmia during the procedure, but completely recovered without sequelae.
Conclusion
Single-session EA using low-dose ethanol might be effective for the treatment of symptomatic cystic thyroid nodules regardless of the initial cyst volume and properties of the aspirate, especially in pure cysts.
Introduction
Most thyroid cystic nodules are benign and asymptomatic; however, some cystic nodules grow over time and may induce symptoms or cosmetic problems [1]. In these cases, simple aspiration can be considered as a first-line option for symptom control. However, the recurrence rate after simple aspiration is as high as 50%-80% [2]. If the symptoms are refractory to repetitive aspirations, ultrasound-guided ethanol ablation (EA) can be an alternative to surgery. EA has been widely used since Rozman et al. applied it for the first time to cystic thyroid nodules in 1989 [3].
In general, two sclerotic procedures have been described in previous studies: (1) using simple ethanol injection after aspiration of the internal cystic contents of thyroid nodules; (2) evacuating ethanol completely from a cystic nodule after few minutes of retention following the former method. No difference has been found between these two methods in the complication and success rates [4]. Therefore, the choice of whether to perform ethanol injection with aspiration or retention may depend on the operator’s preference. The reported independent predictors of EA include initial volume and vascularity in predominantly cystic nodules, but no factor is known to be independently related to efficacy in pure cysts [5]. In et al. [6] reported that complete aspiration of cystic components was associated with the efficacy of EA in benign thyroid cystic nodules.
However, a sufficient consensus has not yet been established regarding the number of sessions and the total amount of ethanol to use for instillation [7]. Most studies reported success rates without clearly distinguishing between single- and multiple-session therapy, but single-session therapy seemed to work well enough (success rates of 64%-100%) according to some studies [3,4,8-10]. Moreover, the amount of ethanol injected was decided mostly by certain empirical proportions (10%-150%) relative to the volume of nodule or the aspirate, and these proportions have varied across studies [5,11-14]. The possibility of using low-dose ethanol in EA was presented by Zingrillo et al. [15,16], who achieved high efficacy by injecting 2-4 mL of ethanol depending on the nodule volume without removal [8]. In their studies, additional multiple EA sessions were performed with ethanol injections that increased to up to 10 mL per session if the volume reduction was less than 50% of the initial nodule volume. To the best of our knowledge, studies focusing on minimal usage of ethanol (low-dose and single-session) in EA have not been published. We aimed to evaluate the efficacy of single-session EA using low-dose (≤5 mL) ethanol regardless of the initial volume of the nodule or properties of the aspirate.
Materials and Methods
The national public Institutional Review Board approved this retrospective study (P01-202005-21-001) with a waiver for consent forms. However, all patients provided written informed consent prior to fine-needle aspiration cytology (FNAC) and EA.
Patient Selection
From October 2013 to January 2020, a total 83 pure cysts or predominantly cystic thyroid nodules in 75 patients (54 women and 21 men; mean age, 44.2 years; range, 24 to 76 years) were treated with EA at Withsim Clinic. Most nodules were confirmed as benign by ultrasound-guided FNAC. In a few cases of pure cysts, the cytologic results were nondiagnostic or unsatisfactory, even with repeated fine-needle aspiration. EA was performed to treat recurrent pure cysts, regardless of nondiagnostic results [7]. All patients treated with EA had nodule-related pressure symptoms or cosmetic problems. The exclusion criteria were as follows: (1) patients who had undergone any previous chemical or thermal ablative procedures for the target lesion; (2) patients who did not receive any follow-up after treatment.
Fig. 1 shows the process of study selection. Among the 83 nodules, 13 were excluded because of missing follow-up data after EA. Another nine nodules treated with other ablative procedures before EA were excluded from the study. Among those nine nodules, three nodules had been treated with radiofrequency ablation (RFA) and six nodules had previously received EA. Finally, 61 nodules in 60 patients (42 women and 18 men; mean age, 43.1 years; range, 24 to 76 years) were included in the current study.
Pre-procedure Evaluation
All patients underwent ultrasonography and ultrasound-guided FNAC using an 8 to 17 MHz linear probe (E-CUBE 15 EX, Alpinion Medical Systems Co., Ltd., Anyang, Korea), performed by one of two physicians (Cho W and Sim JS) with more than 10 years of experience. Nodule size, proportion of the cystic component, vascularity, and properties of the internal fluid content were assessed on the ultrasound examination and during FNAC. The volume (V) of each nodule was calculated as: V=π×w×d×l/6, where w is the width, d is the depth, and l is the length. Vascularity was graded into four categories (0, no intranodular vascularity; 1, perinodular vascularity only; 2, intranodular vascularity <50%; and 3, intranodular vascularity >50%) [5,17]. The viscosity of the internal fluid content was classified as watery, mucoserous, or sticky (colloid). The color of the aspirate was also classified as pale yellowish, brownish, dark bloody, and red bloody [6]. The laboratory tests usually included a thyroid function test (measurements of thyrotropin, triiodothyronine, and free thyroxine), while a blood coagulation battery (bleeding time, prothrombin time, and activated partial thromboplastin time) was considered based on the patient’s medical history or risk for hemorrhage. A symptom score was estimated using a 10-cm visual analogue scale (graded from 0 to 10) by patients. The cosmetic score was measured by a physician (1, no palpable mass; 2, no cosmetic problem but a palpable mass; 3, a cosmetic problem on swallowing only; and 4, a readily detected cosmetic problem) [7].
EA Procedure
All EA procedures were performed with ultrasound guidance by one head and neck surgeon (Cho W) having more than 10 years of experience in ultrasound-related practice, including ultrasound, FNAC, and chemical and thermal ablation for thyroid nodules. Cho W has been qualified for thyroid, head, and neck ultrasound examinations by the Korean Society of Ultrasound in Medicine since 2012.
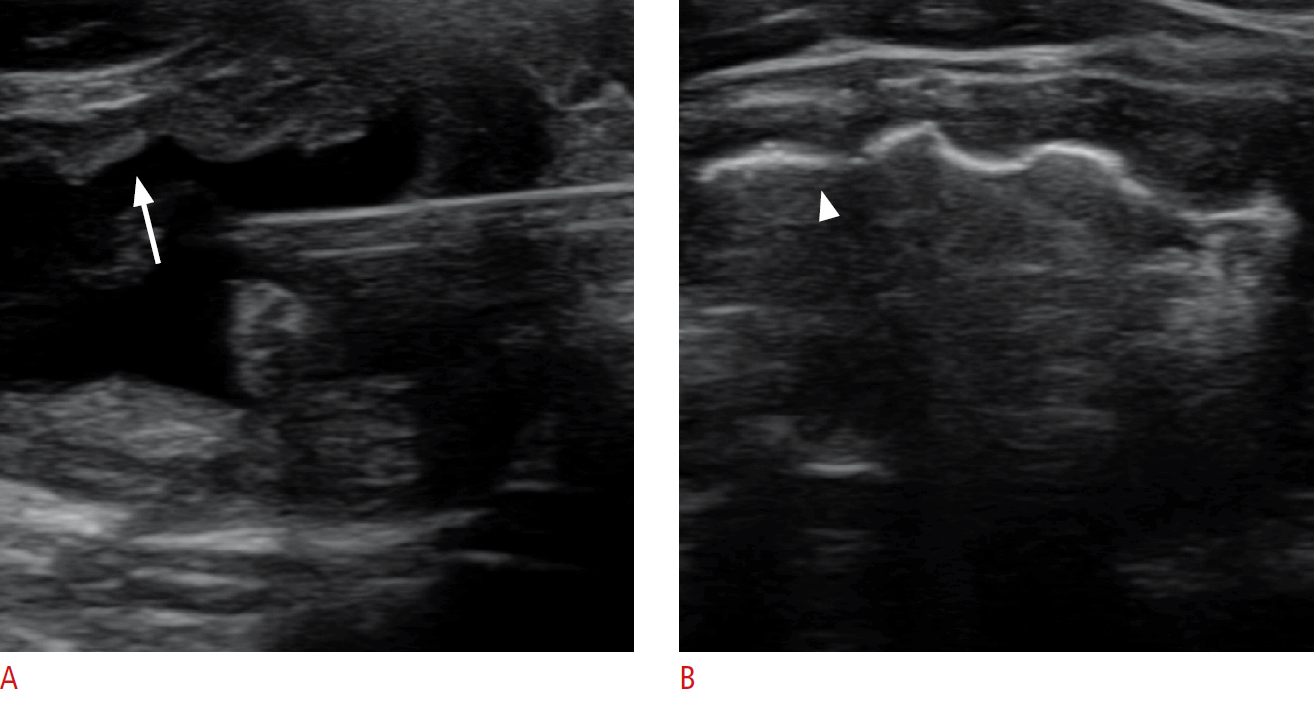
EA was performed in an outpatient clinic. Patients were placed in a supine position with mild neck extension. In a sterile field, 2% lidocaine was injected at the skin puncture site and thyroid pericapsular space for pain control. A 17-gauge disposable needle (coaxial introducer needle, PT17094, M.D.L., Delebio, Sondrio, Italy) was inserted into the nodule under ultrasound guidance via the isthmic or para-isthmic area to prevent a change of needle position and ethanol leakage while swallowing during the procedure. To avoid injury of the vessels along the needle tract, color Doppler was applied before needle insertion. All procedures were done with a single puncture using a three-way connector. The internal contents were removed with the utmost care to prevent ex-vacuum hemorrhage [18]. Residual debris or colloids were removed as much as possible. For cases with high-viscosity colloid contents, repetitive room-temperature saline irrigation was used for dilution. At the end of the extraction procedure, a small amount of saline was injected and maintained for few seconds to verify the absence of leakage before ethanol instillation. If no leakage was observed, 99.5% sterile ethanol (Daihan Dehydrated Alcohol Inj., Dai Han Pharm. Co., Ltd., Seoul, Korea) was slowly instilled after total removal of the mixed saline. When the ethanol was instilled, the inner surface of the cyst changed to a hyperechoic line on ultrasonography (Fig. 2). Regardless of the volume of cystic nodule or aspirate, less than 5 mL of ethanol was injected, and the pattern of distribution was monitored until the entire inner surface showed echogenic change. After a few minutes, all the instilled ethanol was re-aspirated completely. At any point, if the patient complained of any atypical symptoms, such acute severe cervical pain or altered vital signs, which could be a sign of ethanol leakage, the ethanol was removed right away and the procedure was stopped. After removing the ethanol completely, the needle was withdrawn slowly from the neck with minimal negative pressure of the syringe to prevent leakage of the ethanol remaining inside the needle [5]. Gentle pressure was applied to the puncture site with sterile gauze, and the patient then remained under observation for an additional 30 minutes. During and after the procedure, any discomfort or complications were documented.
Posttreatment Assessment
The patients were followed up at 1, 3, 6, and 12 months after treatment. After 1 year, ultrasonography examinations were performed annually. Follow-up evaluations were conducted in the same manner as the preprocedural assessment using ultrasonography and a physical examination. The success of treatment was defined as the absence of any residual fluid or a sufficient volume reduction rate (VRR) (≥50%) at the last follow-up with regression of nodule-related symptoms [5,8,9,12,13,19,20]. Any adverse events during the follow-up period were addressed.
Data Analysis
All statistical analyses were conducted in SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The Student t test and Mann-Whitney U test were used to evaluate the differences between pure cysts and predominantly cystic nodules, except for the variables of viscosity, vascularity, transient burning sense (TBS), and therapeutic success rate (TSR), which were analyzed using the chi-square test. The Wilcoxon signed-rank test was used compare changes in nodule volume, symptom scores, and cosmetic scores from the initial status to the last follow-up after EA. Multiple linear regression analysis was performed to identify independent predictors of VRR, including initial volume, viscosity of the internal contents, color of the aspirate, vascularity, amount of ethanol, ethanol retention time, and presence of TBS during EA. All statistical tests were two-tailed, and P-values <0.05 were considered to indicate statistical significance.
Results
Baseline Characteristics
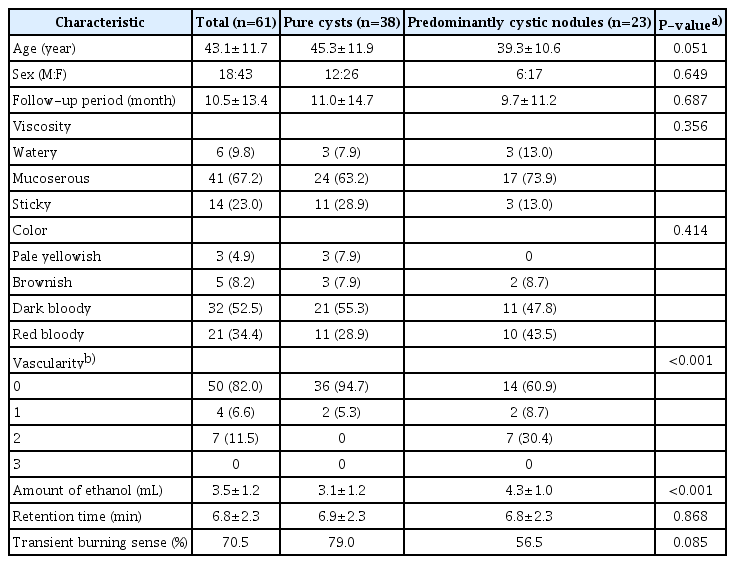
A summary of the baseline characteristics of the study population is shown in Table 1. The 61 nodules comprised 38 pure cysts and 23 predominantly cystic nodules in 60 patients. One female patient underwent EA for bilateral symptomatic cystic nodules on different days. The mean initial volume was 21.9±15.2 mL (range, 4.4 to 77.2 mL). The mean follow-up period was 10.5±13.4 months (range, 1 to 66 months). In predominantly cystic nodules, the proportion of cystic composition was 80.0%±13.8% (range, 40% to 90%). The internal fluid contents were watery in six cysts, mucoserous in 41 cysts, and sticky (colloid) in 14 cysts. The color of the aspirate was pale yellowish in three cysts, brownish in five cysts, dark bloody in 32 cysts, and red bloody in 21 cysts. Most of the nodules (n=50, 82.0%) showed no vascularity. Predominantly cystic nodules had a significantly higher proportion of vascular lesions than pure cysts (P<0.001). Viscosity (P=0.356) and color (P=0.414) showed no significant differences between the two subgroups.
Usage of Ethanol during EA
The mean amount of ethanol was 3.5±1.2 mL (range, 1 to 5 mL) and the mean retention time was 6.8±2.3 minutes (range, 0.9 to 13.0 minutes). The amount of ethanol according to the size of the cystic nodule was 2.5±0.9 mL (range, 2 to 5 mL) in 13 nodules with a volume below 10 mL, 3.2±0.9 mL (range, 2 to 5 mL) in 19 nodules with a volume of 10-20 mL, 3.8±1.3 mL (range, 1 to 5 mL) in 18 nodules with a volume of 20-30 mL, and 4.9±0.3 mL (range, 4 to 5 mL) in 11 nodules with a volume over 30 mL. The amount of ethanol used was greater in predominantly cystic nodules (4.3±1.0 mL) than in pure cysts (3.1±1.2 mL, P<0.001). In predominantly cystic nodules, the volume of ethanol used was 3±0 mL in two nodules with a volume below 10 mL, 3.9±1.1 mL (range, 3 to 5 mL) in seven nodules with a volume of 10-20 mL, 4.5±0.9 mL (range, 3 to 5 mL) in eight nodules with a volume of 20-30 mL, and 4.8±0.4 mL (range, 4 to 5 mL) in six nodules with a volume over 30 mL. There was no significant difference in retention time between the two subgroups (P=0.868).
Treatment Outcomes
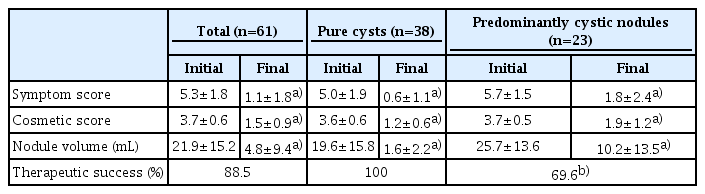
The results of EA are summarized in Table 2. The overall TSR was 88.5%. In pure cysts, a TSR of 100% was noted, which was significantly different from the TSR of 69.6% in predominantly cystic nodules (P<0.001). The nodule volume decreased significantly from 21.9±15.2 mL (range, 4.4 to 77.2 mL) to 4.8±9.4 mL (range, 0 to 44.6 mL). The mean symptom score (P<0.001) and cosmetic score (P<0.001) also showed significant improvements at the last follow-up.
The TSR was 100% in pure cysts regardless of the initial nodule volume, and tended to decrease in predominantly cystic nodules as the volume of the nodule increased (Fig. 3). The TSR of predominantly cystic nodules was 71.4% in seven nodules with a volume of 10-20 mL, 75.0% in eight nodules with a volume of 20-30 mL, and 50.0% in six nodules with a volume over 30 mL. In predominantly cystic nodules, successful treatment was achieved in 10 of 12 nodules (83.3%) with a cystic portion of 80%-90%, five of five nodules (100%) with a cystic portion of 70%-80%, zero of two nodules (0%) with a cystic portion of 60%-70% and one of four nodules (25.0%) with a cystic portion below 60%.
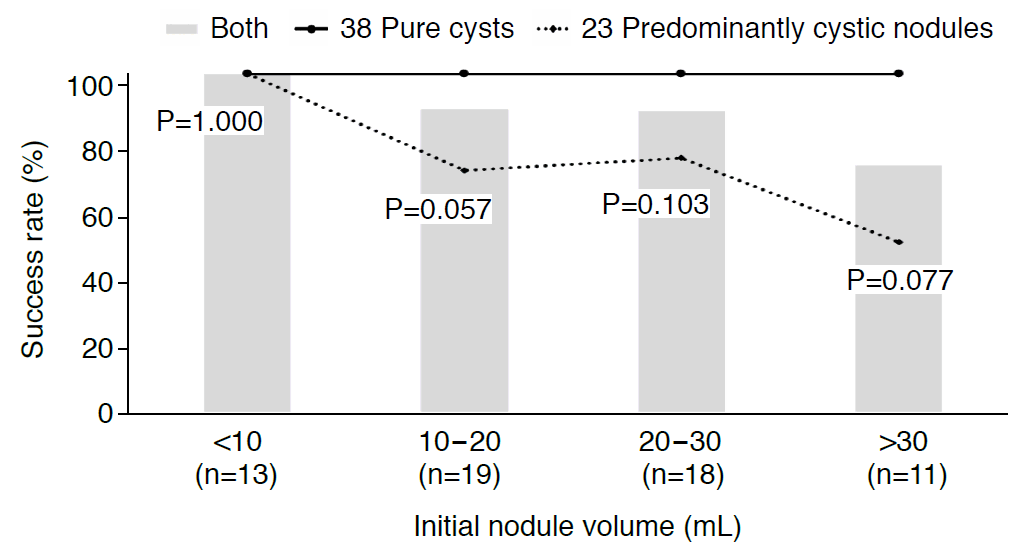
Success rates of ethanol ablation by initial nodule volume (overall and subgroups).
Success was defined as the disappearance of the cystic component, or a nodule volume reduction greater than 50% at the end of follow-up with improvement of nodule-related symptoms. Regardless of the target nodule volume, the success rate reached 100% in all pure cysts. In predominantly cystic nodules, the success rate showed a tendency to decrease as the target nodule volume increased. In each volume fraction, a comparison between subgroups showed no statistically significant differences (P-values calculated by the Mann-Whitney U test).
The overall TSR was 100% in 13 nodules smaller than 10 mL, and then tended to decrease as the initial nodule volume increased: 89.5% in 19 nodules with a volume of 10-20 mL, 88.9% in 18 nodules with a volume of 20-30 mL, and 72.7% in 11 nodules with a volume of 30 mL. However, within each volume group, there was no statistically significant difference between pure cysts and predominantly cystic nodules (P>0.05).
VRR and VRR-Related Factors
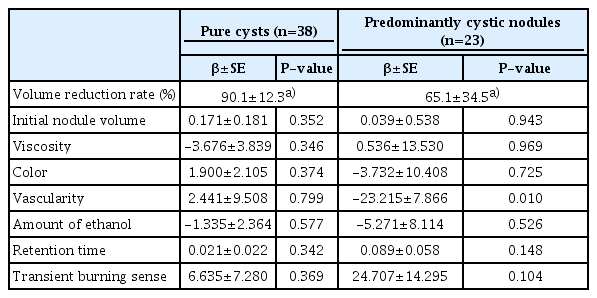
The overall mean VRR was 80.7%±26.1% (range, 4.1% to 100%) at the last follow-up. The mean VRR was 90.1%±12.3% (range, 53.8% to 100%) in pure cysts and 65.1%±34.5% (range, 4.1% to 99.0%) in predominantly cystic nodules, which was a statistically significant difference (P<0.001). Multiple linear regression analysis showed that vascularity (P=0.010) was the only independent predictor of VRR in predominantly cystic nodules, whereas no factor was significantly associated with VRR in pure cysts (Table 3).
Nodules Unresponsive to EA
Seven predominantly cystic nodules (11.5%) were classified as showing treatment failure because they did not meet the criterion for success due to a low VRR (17.3%±11.6%). The decision to perform an additional procedure was made at least 3 months after treatment. If a patient complained of nodule-related discomfort, the additional treatment was conducted as soon as possible after the decision was made. If not, in consideration of the fact that the treated cystic nodules were benign, whether to perform additional treatment and the timing of the treatment were decided in consultation with the patient.
For six of these seven nodules, additional ablative procedures were performed (additional EA in three nodules, RFA in two nodules, and combined additional EA and RFA in one nodule), while one patient refused further treatment. Five of the additional procedures were performed slightly more than 3 months after the initial treatment. One additional EA procedure was done at the point of 29 months of follow-up.
In principle, RFA was performed as an additional procedure in unresponsive nodules that contained a solid component of more than 20% [7]. However, one hemorrhagic nodule showing bloody aspirate had been treated with additional EA prior to RFA for the purpose of bleeding control inside the nodule [21]. All additional EA procedures were performed in the same manner using low-dose ethanol.
Complications
Complications and side effects were defined according to the quality improvement guidelines of the Society of Interventional Radiology [22]. In the current study, complications developed in five (8.2%) patients, but none of the complications were permanent.
Minor Complications
Intra-cystic hemorrhage was observed in four cases (one pure cyst and three predominantly cystic nodules) during the procedure, but recovered within a week. During EA, 43 patients (70.5%) experienced TBS within a few seconds after the injection of ethanol, which needs to be differentiated from acute severe pain that could be a sign of leakage. TBS mostly subsided within 10 minutes. A higher proportion of TBS was noted in the pure cystic group (79.0%) than in the predominantly cystic group (56.5%), but there was no statistically significant difference (P=0.085) (Table 1). Furthermore, TBS was not significantly related to VRR in either subgroup (P=0.335 in the pure cystic group and P=0.075 in the predominantly cystic group) (Table 3).
Major Complications
There were no serious complications such as voice change, skin burns, or subcutaneous abscess formation, except for one patient with cardiac arrhythmia. The patient started to complain of palpitation and dizziness about 30 seconds after the injection of 1 mL of ethanol. The heart rate increased to 150 beats/min without altered mental status. We discontinued the procedure by evacuating the ethanol immediately after recognizing the changes in the patient’s status. The retention time was 55 seconds (the shortest among the study participants). The patient was transferred to the emergency department and hospitalized for 1 day of observation, and then completely recovered without sequelae.
Discussion
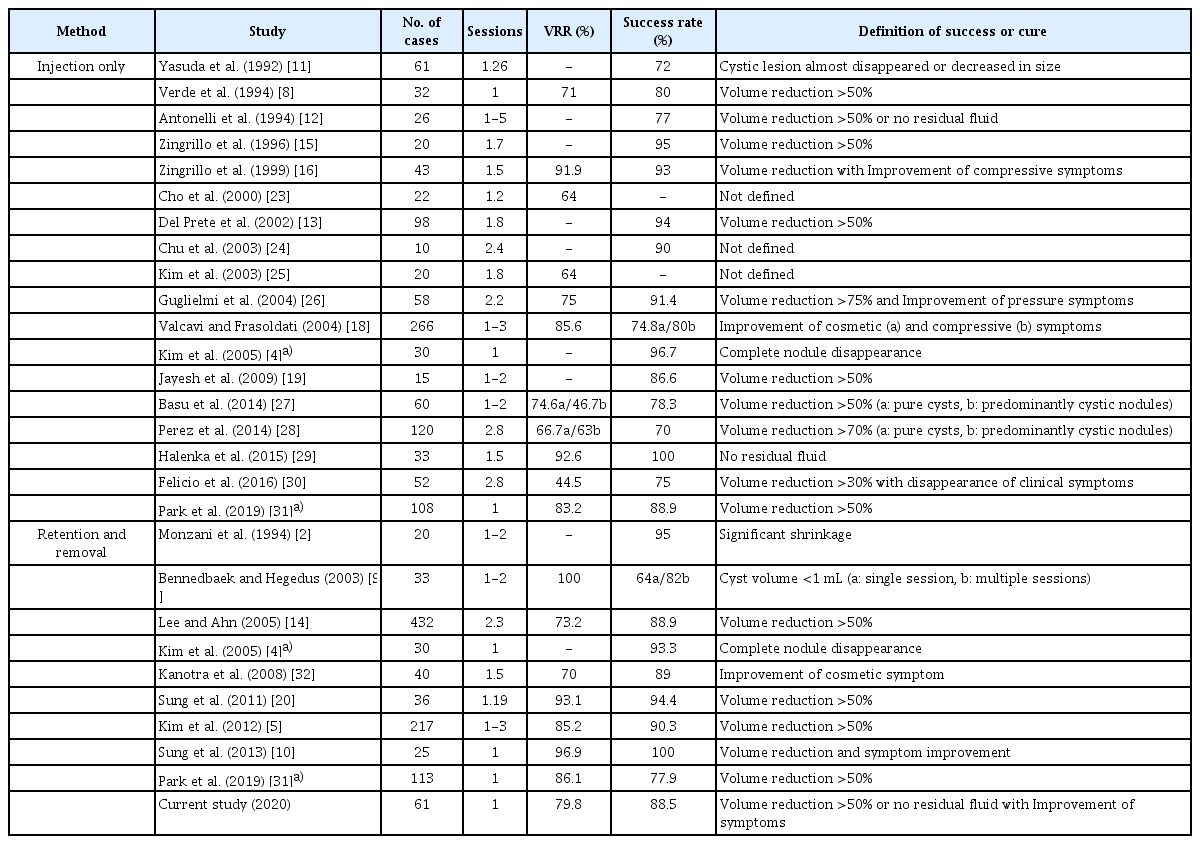
Many studies have reported that EA is effective and safe for treating symptomatic thyroid cystic nodules (Table 4). The reported TSR is 70%-100%, the reported VRR is 44.5%-100%, and the reported complication rate is 9%-34.6% [2,4,5,8-16,18-21,23-32]. In the current study using low-dose ethanol in a single session, the TSR (88.5%) was similar to the reported results and the complication rate (8.2%) was lower. Regardless of the nodule volume and properties of the aspirate, low dose single-session EA showed high efficacy, especially for pure cysts, in which the TSR reached 100%.
Judgments of success (percutaneous ethanol sclerotherapy for cystic thyroid nodules) in the literature
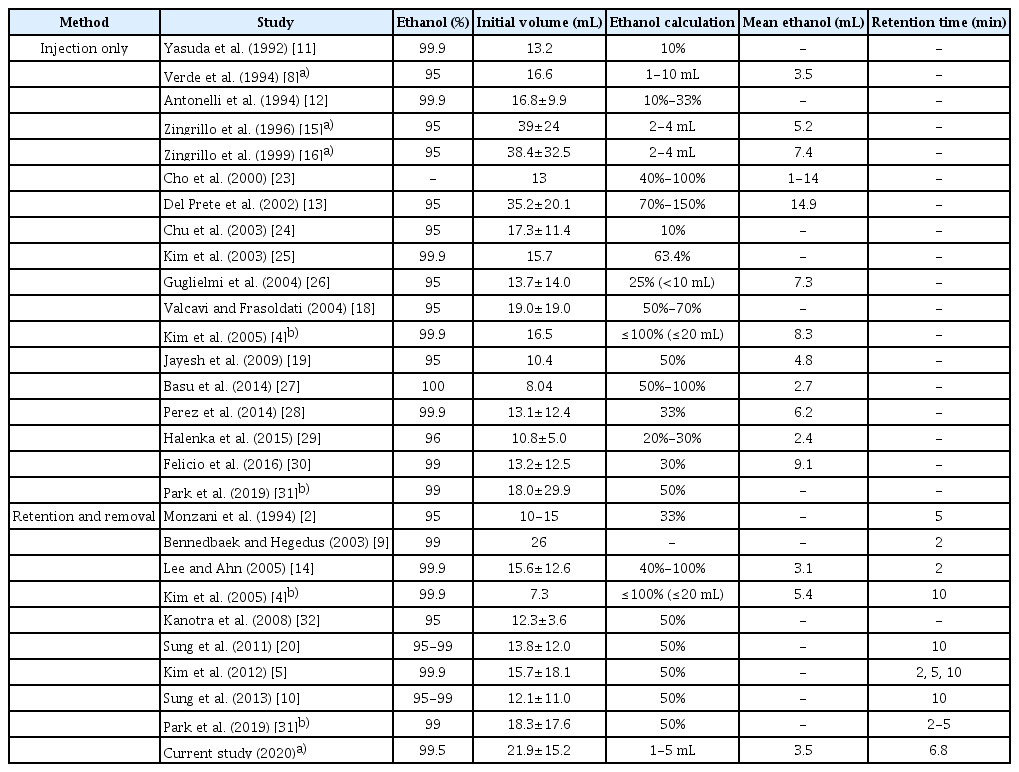
Many factors related to the standardization of the procedure remain unclear, with considerable variation in the literature (e.g., ethanol concentration, amount of ethanol injection, ethanol retention time, whether the injected ethanol is removed, and the definition of therapeutic success) (Table 5). The amount of ethanol instilled has been mostly chosen based on certain proportions to the amount of aspirate or nodule volume, with ranges of 10%-150% and 1-20 mL, respectively. Zingrillo et al. [15,16] achieved a high TSR (95%) using a small amount of ethanol. They determined the amount of ethanol to be injected according to the nodule volume: less than 2 mL of ethanol for cystic nodules with a volume less than 20 mL; 3 mL for cystic nodules with a volume of 20-30 mL; and 4 mL for cystic nodules with a volume over 30 mL. The mean amount of ethanol used was 5.2-7.4 mL because their treatments were performed in multiple sessions [15,16]. Lee and Ahn [14] investigated 432 thyroid complex cysts, and found no relationship between the amount of injected ethanol and the reduction in nodule volume. In addition, many studies injecting ethanol at a small proportion (10%-33%) of the aspirate volume showed good results for EA, even though they did not use a fixed amount of ethanol [2,11,12,24,26,28-30]. The present study also used a small amount of ethanol (mean, 3.5 mL; range, 1 to 5 mL) for cystic thyroid nodules and showed similar efficacy. Therefore, it may be suggested that a small amount of ethanol is enough to treat cystic thyroid nodules.
However, most existing studies using small amounts of ethanol reported outcomes including the effects of multiple treatment sessions. Therefore, it has been challenging to determine whether low-dose ethanol really works, especially in single-session EA. In the literature, the research that best matches the concept of low-dose single-session EA was performed by Verde et al. [8], who achieved a TSR of 80% by using less than 10 mL of ethanol once (mean, 3.5 mL) for cystic thyroid nodules (mean volume, 16.6 mL). That technique is most similar to the technique presented in the current study, in which less than 5 mL of ethanol was used once, and the main difference between the techniques is whether the instilled ethanol was removed. Previous studies revealed that efficacy was not affected by the choice of technique in this regard [4,31]. However, retained ethanol, especially when a double puncture is used, can cause extrathyroidal ethanol leakage, resulting in pain, voice change, a feeling of intoxication, and periglandular fibrosis [2,4,18,31,33,34]. Furthermore, a longer retention time may increase the risks of complications related to manipulating the inserted needle, such as leakage of injected ethanol [5,20]. Hence, the optimal ways to achieve the effect of treatment while minimizing complications are (1) using a small amount of ethanol; (2) making a single-puncture using a three-way connector; and (3) evacuating the instilled ethanol after retention (at least 2 minutes).
The diversity of the reported success rate for EA in the literature might be related not only to the heterogeneous nature of cystic thyroid nodules, but also to differences in evaluating the outcomes [30]. The major criteria for judging the success of treatment, or whether a cure has been achieved, have been a VRR greater than 50%, the improvement of nodule-related pressure or cosmetic symptoms, and the absence of a residual cystic component (Table 4). On the one hand, some studies used relatively strict criteria to judge success (e.g., complete nodule disappearance or near total absence of a cystic component [<1 mL]), but on the other hand, some researchers have unclearly defined success using ambiguous terms (e.g., a "significant shrinkage" or a "decrease in size") [2,4,9,11]. Considering that the nodule-related symptoms of cystic thyroid nodules mainly stem from an increasing cystic component, it would be more reasonable to use both changes of nodule volume and disappearance of fluid content, along with improvements of clinical symptoms, when evaluating the outcomes of EA.
Previous studies have suggested that factors related to the success of EA include the proportion of the solid portion, the initial volume, vascularity, the retention time of ethanol and the sufficient evacuation of internal content before ethanol instillation [7]. In most existing studies (Table 5), the mean volumes of the nodules treated were under 20 mL, except for a few studies by Zingrillo et al. [15,16] (38.4-39 mL), Del Prete et al. [13] (35.2 mL), Bennedbaek and Hegedus [9] (26 mL), and Suh et al. [35] (20.1 mL). The threshold at which volume starts to influence the effectiveness of EA has been considered to be 10 mL in all types of cystic nodules and 20 mL in predominantly cystic nodules [5,35]. In the current study, the mean volume was 21.9 mL and 11 nodules (18.0%) were over 30 mL. Similar to other studies, the efficacy of single-session low-dose EA was lower in the nodules containing a solid component (predominantly cystic nodules). The increased vascularity of solid components may cause ethanol drainage, thus limiting the success of EA [7,21]. We obtained an equivalent result, finding that vascularity was an independent predictor of VRR. The differences between the current study and others are as follows: (1) in pure cysts, the efficacy remained high regardless of the target lesion volume; (2) in predominantly cystic nodules, although the changes were not statistically significant, the effectiveness started to decrease from 10 mL and remained unchanged until 10-30 mL, but plummeted at an initial volume of over 30 mL; and (3) initial volume, retention time, and amount of ethanol were not significantly related to efficacy. Of particular note, even though less than 5 mL of ethanol was used in each case, we used more ethanol in predominantly cystic nodules than in pure cysts. Nonetheless, the latter showed better outcomes.
Localized pain during EA is the most common complication and can be a sign of ethanol leakage into the subcutaneous tissue [7,14]. However, acute pain needs to be differentiated from tolerable TBS radiating to the ear or neck, which might be observed in up to 71% of patients [9,13,16]. In the current study, 70.5% of patients experienced TBS. If patients had TBS, we assumed that the time when they stopped complaining of it was the end point of retention. However, TBS per se was not correlated with VRR. TBS needs to be investigated more precisely in future research to determine how this finding can be understood and applied in clinical practice.
One of our patients experienced arrythmia immediately after the instillation of ethanol, a previously unreported complication of thyroid EA, although a similar situation (bradycardia and sinus arrest) has been observed in the ethanol treatment of hepatocellular carcinoma. Ethanol-induced arrythmia is presumed to be related to the direct entry of ethanol into the blood vessels inside the cystic nodule. However, the mechanisms leading to arrhythmia remain obscure [36]. In our experience, even though the treatment seemed to be insufficient due to discontinuation of the procedure (1 mL of ethanol with 55-second retention), the final volume at 46 months of follow-up was 0.52 mL (vs. 25.58 mL initially). Paradoxically, we were able to see the possible efficacy of low-dose single-session EA due to this case of a complication.
The current study has several limitations. First, the retrospective single-center study design might have led to selection bias. Although we have found evidence for the possibility of using a small amount of ethanol in a single session, to support this approach, prospective studies evaluating the efficacy of consistent low-dose usage compared to higher doses need to be performed in the future. In addition, the included population was relatively small and the follow-up duration was short, which might limit the generalizability of our study results.
In conclusion, single-session EA using low-dose ethanol (≤5 mL), regardless of the volume of target nodule or aspirate, was effective in the treatment of symptomatic cystic thyroid nodules. The efficacy was superior for pure cysts. When the existence of a solid component is noted, nodules showing lower vascularity are better candidates. These results may be useful in the process of preprocedural selection and planning for patients complaining of discomfort related to cystic thyroid nodules.
Notes
Author Contributions
Conceptualization: Cho W, Sim JS, Jung SL. Data acquisition: Cho W. Data analysis or interpretation: Cho W, Jung SL. Drafting of the manuscript: Cho W, Jung SL. Critical revision of the manuscript: Cho W, Sim JS, Jung SL. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We would like to acknowledge Dr. Yangsean Choi for assistance.