Biomechanical changes of the common carotid artery and internal jugular vein in patients with multiple sclerosis
Article information
Abstract
Purpose
Investigations of the hemodynamic changes of the venous system in patients with multiple sclerosis (MS) have shown contradictory results. Herein, the biomechanical parameters of the internal jugular vein (IJV) and common carotid artery (CCA) of MS patients were extracted and compared to healthy individuals.
Methods
B-mode and Doppler sequential ultrasound images of 64 IJVs and CCAs of women including 22 healthy individuals, 22 relapsing-remitting multiple sclerosis (RRMS) patients, and 20 primary-progressive multiple sclerosis (PPMS) patients were recorded and processed. The biomechanical parameters of the IJV and the CCA walls during three cardiac cycles were calculated.
Results
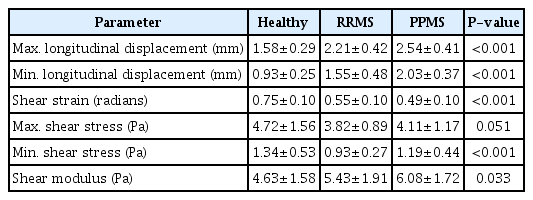
The IJV maximum and minimum pressures were higher in the MS patients than in the healthy subjects, by 31% and 19% in RRMS patients and 39% and 24% in PPMS patients. The venous wall thicknesses in RRMS and PPMS patients were 51% and 60% higher than in healthy subjects, respectively. IJV distensibility in RRMS and PPMS patients was 70% and 75% lower, and compliance was 40% and 59% lower than in healthy subjects. The maximum intima-media thicknesses of the CCAs were 38% and 24%, and the minimum intima-media thicknesses were 27% and 23% higher in RRMS and PPMS patients than in healthy individuals, respectively. The shear modulus of CCA walls in RRMS and PPMS patients was 17% and 31%, and the radial elastic moduli were 47% and 9% higher than in healthy individuals.
Conclusion
Some physical and biomechanical parameters of the CCA and IJV showed significant differences between MS patients and healthy individuals.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and autoimmune disease that affects the central nervous system [1]. Chronic inflammation caused by MS can activate the immune system and lead to atherosclerosis and arterial-venous diseases [2]. Inflammation has destructive effects on the walls of blood vessels that cause lymphocytes to enter the brain tissue and destroy myelin [3]. Chronic cerebrospinal venous insufficiency (CCSVI) is a disorder involving obstruction in the venous blood flow pathways out of the brain. Studies have investigated the possible link between CCSVI and some diseases of the nervous system, such as MS [4], through analyses of the hemodynamic properties of the internal jugular vein (IJV) in patients with MS [5-9]. Some of these studies have reported changes in the venous system of people with MS compared to those in the healthy group [4-6]. However, other studies have found opposite results, reporting that there were no significant differences in any parameters according to the Zamboni criteria between healthy individuals and MS patients [5,7].
One of the reasons for the discrepancy between the results obtained from different studies is the qualitative evaluation of images of the vascular system with the Doppler technique. Ultrasound imaging techniques are used to extract the physical parameters of the vessel wall [8], the volume and velocity of blood flow, the presence of venous blood reflux, and vascular obstruction [4,5]. There are two hypotheses regarding the potential relationship between MS and the vasculature. Due to chronic inflammation caused by MS, there may be changes in the physical and biomechanical parameters of the IJV, vertebral vein (VV), and deep cerebral vein (DCV) [4-7]. If biomechanical changes are observed in the IJV, examining whether the common carotid artery (CCA), as another component of the vascular system, can show the possible changes in its biomechanical parameters caused by MS.
MS is classified into four categories [9]: According to the manifestation of the disease, its process, clinical tests, and Expanded Disability Status Scale (EDSS) score, MS is classified into four categories [10]: (1) relapsing-remitting multiple sclerosis (RRMS): in 85% of patients, the disease is accompanied by relapsing-remitting periods; this period has stages of relapse and recovery, and the symptoms of the disease disappear during the recovery period; (2) secondary progressive multiple sclerosis: with the passage of time and the progress of the disease, many patients enter a new secondary progressive period, which is with or without recovery periods; (3) primary-progressive multiple sclerosis (PPMS): in this category, which includes 10% of patients, the disease is progressive from the beginning, with worsening symptoms and without recovery periods; (4) progressive-Relapsing multiple sclerosis: in this very rare form that includes only 5% of patients, periods of relapse and simultaneous progression of the disease occur without recovery periods.
The present study used ultrasound images to evaluate possible changes in the vascular system of people with MS. The aim of this study was to process Doppler ultrasound images and B-mode images from the CCA and the IJV to extract the physical and biomechanical parameters of vessels in RRMS and PPMS patients and compare them with healthy vessels.
Materials and Methods
Compliance with Ethical Standards
The study was approved by the Ethics Review Board of Tarbiat Modares University (IR.MODARES.REC.1397.035), and all procedures were performed in accordance with the Declaration of Helsinki. The study was performed from August 2020 to September 2021 at a single center, and all the patients filled out and signed an informed consent form.
Study Population
In this study, 22 healthy subjects, 22 subjects with RRMS, and 20 subjects with PPMS were evaluated. Healthy subjects were volunteers who did not have any diseases related to the nervous system (e.g., Alzheimer's or Parkinson's diseases) and were age-appropriate and matched with the groups of MS patients. Since women comprise the highest frequency of MS patients, and to eliminate the effect of sex on vascular parameters, in this study, the vascular parameters of women aged 20-40 years were evaluated. MS was diagnosed by a physician using the McDonald [9] criteria. The patients were selected based on disease status and EDSS score [10], after ensuring complete health in terms of other diseases and neurological abnormalities and that the patients did not take hormonal drugs.
The clinical symptoms of MS are the result of plaques that have been created due to the destruction of myelin sheaths in the central nervous system, and these symptoms are defined according to the neuro-anatomical location of the plaques. These symptoms include sensory disorders, vision disorders, diplopia, bladder dysfunction, cognitive impairment, muscle weakness, lack of muscle coordination, fatigue, and intestinal problems, all of which cause a decrease in the quality of a person's life [1]. The subjects were selected from patients who were treated under the supervision of a neurologist at the Iranian Center of Neurological Research, Imam Khomeini Hospital, based on the inclusion criteria. Their disease was confirmed by magnetic resonance imaging (MRI). All female patients with MS diagnosed by the MRI-based McDonald criteria were consecutively included in this study. The criteria for exclusion from the study included other neurological diseases, cardiovascular problems, diabetes, involvement in professional athletics, and smoking. Informed consent was obtained from all subjects for the examination.
Ultrasonography
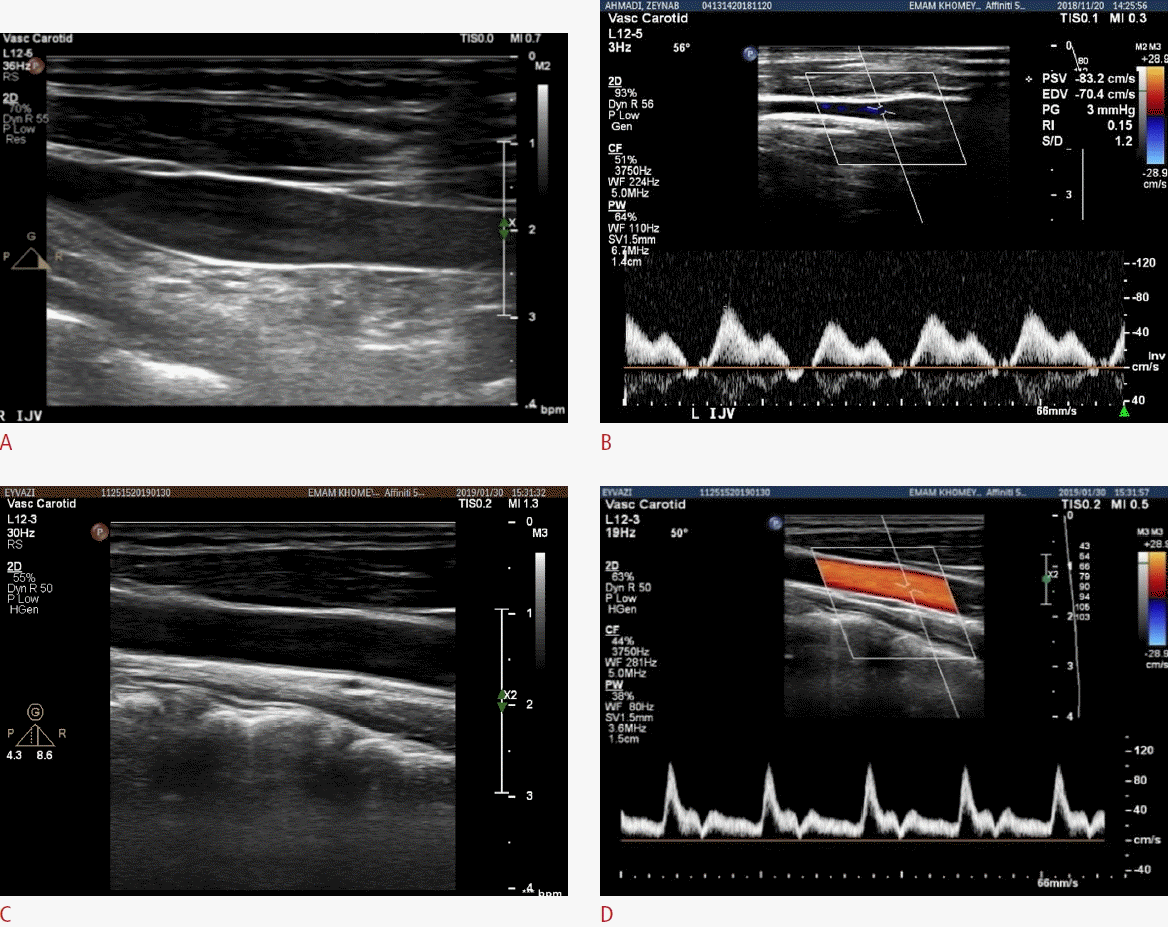
The images were acquired using a Philips ultrasound machine (San Diego, CA, USA), equipped with a linear array probe in a frequency range of 6-12 MHz, and the possibility of two-dimensional B-mode, M-mode, and Doppler imaging. B-mode and Doppler images of the IJV and the CCA in the supine position are shown in Fig. 1. The ultrasound examinations were performed with a depth of 4 cm and an image size of 660×840 pixels. The imaging was performed by a sonologist at the imaging center of Imam Khomeini Hospital.
Motion Detection Algorithms
The duration of venous blood reflux, the intima-media thickness of the CCA wall, the intima-medial thickness of the IJV wall, the cross-sectional area (CSA) of the IJV, and systolic and diastolic blood velocities were measured using ImageJ software (developed by Wayne Rasband at the National Institutes of Health). The intima-medial thickness of the CCA was obtained in the diastolic phase and systolic phase, but due to the thinness of the intima-media layer in the IJV, it was measured only at the diastolic phase.
The maximum gradient algorithm was used to measure the momentary radial changes of the vessel wall [11]. The block matching algorithm was used to measure the momentary longitudinal displacement of the vessel wall. These programs have been validated in the authors’ previous study [12]. They were extracted for three cardiac cycles with a temporal resolution of 33 ms.
The biomechanical parameters of the CCA and IJV were obtained by existing equations. The systolic and diastolic blood pressures were measured from the radial arteries of the subjects by a calibrated sphygmomanometer, assuming that they remain nearly constant throughout the arterial tree [8]. Instantaneous changes in the diameter of the IJV in the supine position were extracted using the maximum gradient algorithm. The pressure-diameter relationship of the IJV was extracted by fitting a quadratic curve to the results of the Donahue et al.'s study [13], and using this relationship, the instantaneous changes in the IJV pressure were estimated. The results were reported as the mean±standard deviation for each group.
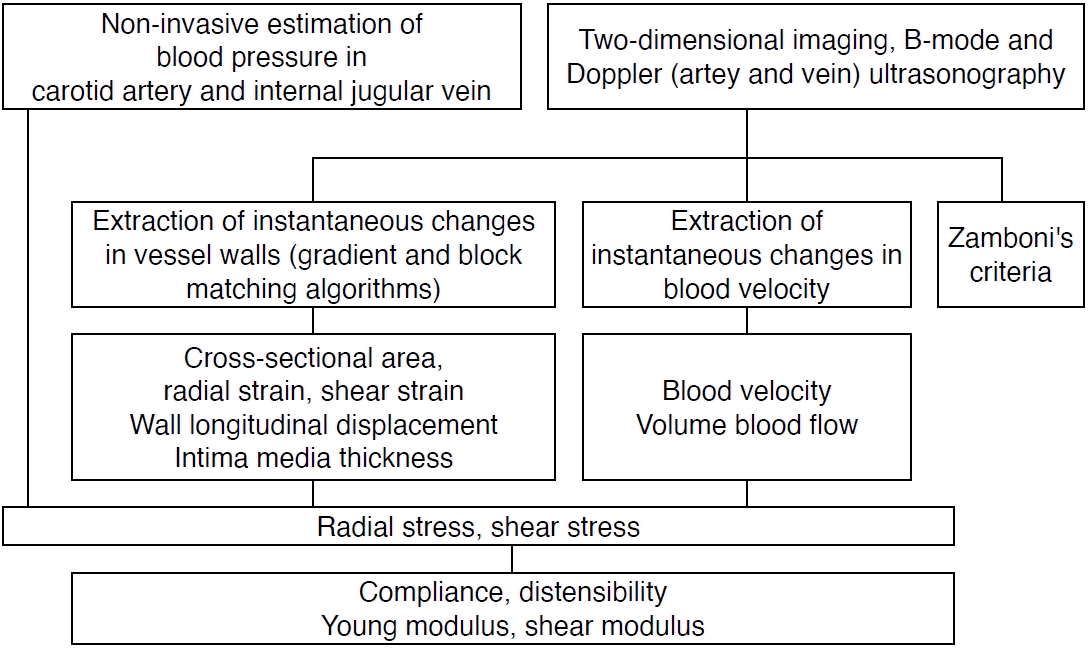
Biomechanical Parameters of Vessels
Volume blood flow (Q) in a vessel with the CSA (A) is obtained from the following equation:
Distensibility is defined as the ratio of volume changes to the initial volume versus pressure changes and is obtained by dividing compliance by volume. Distensibility is calculated as in a previous study [14], where V is the initial volume:
The radial strain (εr) is defined as:
D and D0 are the systolic and diastolic diameters of the vessel. The radial stress (δ) is estimated from the following equation [15]:
P, D, and IMT are the blood pressure, the diameter of the vessel, and the intima-media thickness, respectively. The Young modulus (E) is estimated to be the difference between the maximum stress (δmax) and the minimum stress (δmin) divided by the radial strain (εr):
Shear stress (δs) causes longitudinal displacement of the vessel wall. It is calculated using the following equation [16]:
Shear stress causes an angular change referred to as shear strain (εs) [17]:
Lmax and Lmin are the maximum and minimum longitudinal displacements of the vessel wall, respectively. The shear modulus (Es) is obtained from the difference between the maximum shear stress (δ s,max) and the minimum shear stress (δs,min) divided by the shear strain (εs) according to the following equation [17]:
The pressure-diameter relationship of the IJV was extracted by curve-fitting to the results of Donahue et al.'s study [13] with a correlation coefficient of 82%:
Based on the momentary changes of the IJV diameter (mm), pressure changes (Pa) in the IJV were estimated.
In order to investigate the presence of CCSVI, the four parameters presented by Zamboni et al. [5] were evaluated. Reflux measurement in the DCV, as the fifth parameter of Zamboni et al. [5], was not performed due to a lack of access to the facilities required for imaging of cerebral vessels. The evaluated parameters were as follows:
1. Reflux in IJV and/or the VV in sitting and supine postures that lasts more than 0.88 seconds.
2.Measurement of the CSA of the IJV, with stenosis defined as a CSA ≤0.3 cm2.
3. Blood flow is not Doppler-detectable in the IJV and/or VV.
4. The difference between the CSA (∆CSA) of the IJV in both sitting and supine positions, which is positive in normal individuals.
A flow chart of the methods used to evaluate physical and biomechanical properties is shown in Fig. 2.
Statistical Analysis
The statistical analysis of variables was performed descriptively and the results were shown as mean and standard deviation for each group. The normality of data distribution within groups was investigated using the Kolmogorov-Smirnov test. Mean values were compared between the study groups using one-way analysis of variance (ANOVA) with a confidence level of 95% (P<0.05). In this study, three groups (including healthy subjects, patients with RRMS, and patients with PPMS), were examined, so the number of samples required for ANOVA was estimated [18]. The least significant difference analysis was used to investigate the differences between multiple groups. Discriminant analysis [19] was used to examine which of the parameters extracted had the highest sensitivity, specificity, and percentage of correct classifications in diagnosing healthy individuals and patients.
Results
The clinical characteristics of healthy individuals and patients with RRMS and PPMS are given in Table 1.
The systolic blood pressure and the diastolic blood pressure in the brachial artery of the three groups were measured using a pressure gauge (Table 1). There were no significant differences in the systolic and diastolic brachial artery blood pressures, age, and boy mass index between the three groups (P>0.05).
Fig. 3 shows an example of momentary changes in the diameter of the IJV during three cardiac cycles. It is observed that the diameter of the IJV in PPMS patients (dashed line) and RRMS patients (round dots) is larger than that in the healthy group (solid line). This difference was also noted between the two patient groups. The results of the physical and biomechanical parameters of the IJV in the three examined groups are shown in Table 2.
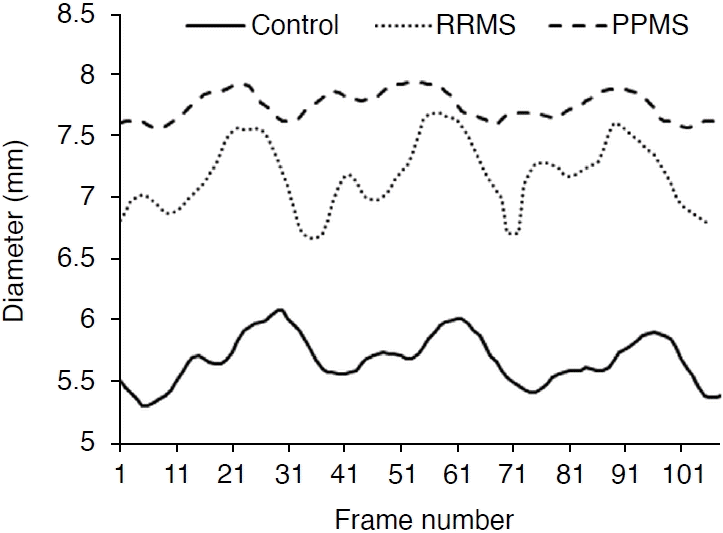
Diameter changes of the internal jugular vein in the healthy group, patients with relapsing-remitting multiple sclerosis (RRMS), and patients with primary-progressive multiple sclerosis (PPMS).
As shown in Table 2, the maximum and minimum diameters of the IJV in patients with RRMS were 28% and 42% higher, respectively, than in healthy individuals. The maximum and minimum diameters of the IJV in patients with PPMS were 17% and 31% higher, respectively, than in the healthy group. The wall thickness of IJV in individuals with RRMS and PPMS was higher by 51% and 60%, respectively, than in healthy individuals. The peak systole velocity in patients with RRMS and PPMS was lower by 39% and 31% respectively, in comparison to the healthy group, while the end-diastole velocity in RRMS and PPMS patients was negligibly higher than in the healthy group (2% and 4%, respectively). The maximum blood flow does not show a significant difference among the three groups (P=0.519), but the minimum blood flow in RRMS and PPMS patients was 100% and 81% higher than in the healthy group. Compliance in patients with MS was significantly lower than in healthy individuals (P<0.001). Furthermore, distensibility in RRMS and PPMS patients compared to healthy individuals was also significantly lower, by 70% and 75%, respectively (P<0.001).
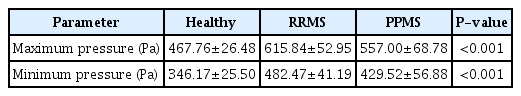
The maximum IJV pressure in patients with RRMS and PPMS was higher by 31% and 19%, and the minimum IJV pressure was 39% and 24% higher in RRMS and PPMS patients respectively, than in healthy individuals. The venous pressure in RRMS patients was significantly higher than in PPMS patients (Table 3).
Using the maximum gradient algorithm [11] in MATLAB, radial displacement changes of CCA were measured in the three groups during three cycles (Fig. 4). In patients with PPMS (dashed line) and RRMS (round dots), the diameter of the CCA was higher than in the healthy group (solid line). The diameter increase was more evident in PPMS patients.
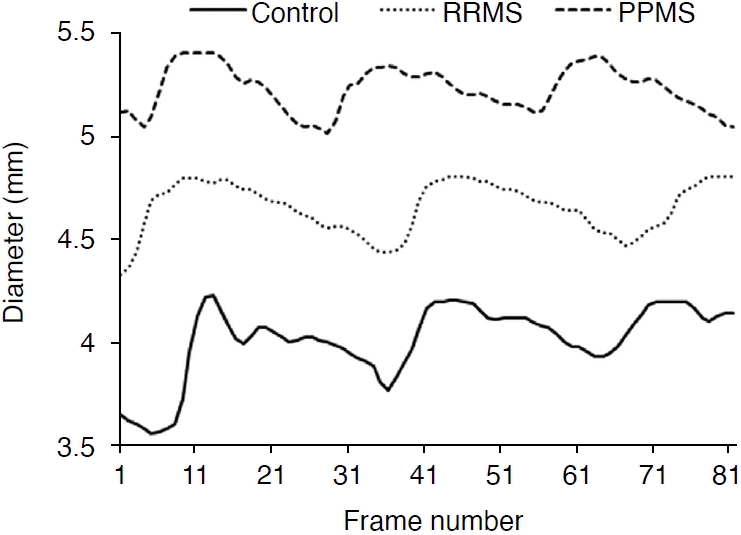
Diameter changes of the common carotid artery in the healthy group, patients with relapsing-remitting multiple sclerosis (RRMS), and patients with primary-progressive multiple sclerosis (PPMS).
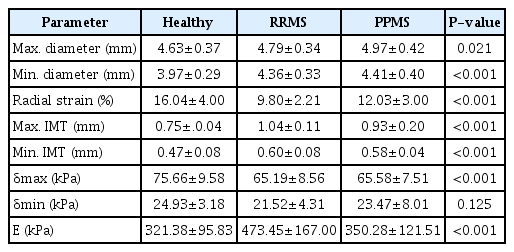
The physical and biomechanical parameters of the CCA in the healthy, RRMS, and PPMS groups are shown in Table 4.
The maximum and minimum diameters of the CCA in patients with RRMS and PPMS, compared to healthy individuals, were 4%, 7%, 10%, and 11% higher, respectively. A significant difference was found between the peak systolic diameter of the CCA in the healthy and PPMS groups (P=0.021). However, there was no significant difference in the peak systolic diameter of the CCA between the two groups of patients and also between the healthy group and RRMS group (P>0.001). The radial strain of CCA in individuals with RRMS and PPMS was lower by 38% and 25%, respectively, compared to the healthy group. Regarding the maximum IMT and minimum IMT, in RRMS and PPMS patients, higher values (by 38% and 24%, 27%, and 23%, respectively) than in healthy subjects were observed. There was a significant difference in the maximum stress between the healthy group and both groups of patients (P<0.001), but no significant difference in the minimum stress between the two groups of patients (P=0.125). The Young modulus showed a significant difference among the three groups (P<0.05). The Young modulus was significantly higher, by 47% and 9%, in people with RRMS and PPMS than in healthy individuals, respectively (P<0.001).
The results of the hemodynamic parameters of the CCA in the healthy, RRMS, and PPMS groups are shown in Table 5.

Peak systole velocity, end-diastole velocity, maximum blood flow, and minimum blood flow of the CCA in the healthy, RRMS, and PPMS groups
As shown in Table 5, no significant difference was observed in peak systole velocity among the three groups (P=0.065). A significant difference was observed between RRMS and PPMS patients in end-diastole velocity (P=0.005). The end-diastole velocity in PPMS patients was 28% higher than in patients with RRMS. A significant difference was observed in the minimum blood flow among the three groups (P=0.011), but there was no significant difference in the maximum blood flow (P=0.119). There was a significant difference in the minimum blood flow values between the healthy group and PPMS group, as well as between the two groups of patients (P<0.05).
Fig. 5 shows momentary changes in the longitudinal displacement of the CCA in the healthy group, RRMS patients, and PPMS patients during three cardiac cycles. The longitudinal displacement of the CCA in the healthy group (solid line) was significantly less than in the RRMS patients (round dots) and PPMS patients (dashed line) (P<0.001). The longitudinal displacement of the CCA was greater in PPMS patients than in RRMS patients.
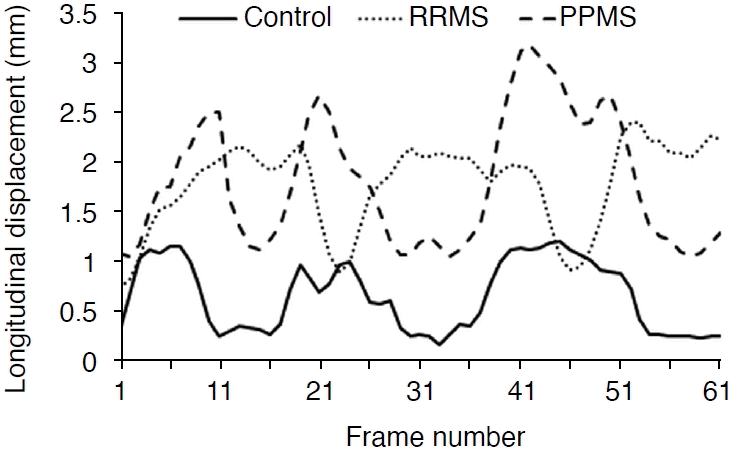
The longitudinal displacement of the common carotid artery during three cardiac cycles in the healthy group, patients with relapsing-remitting multiple sclerosis (RRMS), and patients with primary-progressive multiple sclerosis (PPMS).
The results of the physical and biomechanical parameters of the CCA in the longitudinal direction for the three groups are shown in Table 6.
The maximum and minimum longitudinal displacements of the CCA in patients with RRMS and PPMS were 39%, 60%, 66%, and 100% higher, respectively, than in healthy subjects. There were significant differences in the maximum and minimum longitudinal displacements of the CCA wall between patients with RRMS and PPMS (P<0.001). The shear strain was lower by 26% and 34% in RRMS and PPMS patients than in healthy individuals, respectively, but it did not show a significant difference between the RRMS and PPMS patient groups. For the values of minimum shear stress (Pa), a significant difference was observed among the three groups (P<0.001). However, there was no significant difference among the three groups for maximum shear stress values (P=0.051), although the healthy subjects and RRMS patients showed a significant difference in the maximum shear stress (P<0.001).
Shear modulus values differed significantly among the three groups (P=0.033). However, there was no significant difference in shear modulus between patients with RRMS and PPMS (P>0.05). The shear modulus was 17% and 31% higher in RRMS and PPMS patients than in healthy individuals, respectively.
Based on the study of Zamboni et al. [5], blood reflux lasting more than 0.88 seconds was not observed in the IJV in any of the healthy individuals or patients with MS. A CSA ≤0.3 cm2 was observed in 54% of the healthy individuals and in 15% of the patients with PPMS. In the RRMS patients, the CSA was not less than 0.3 cm2. No undetectable blood flow by the Doppler technique was observed in any group. Negative values for ∆CSA were not observed in any of the three groups.
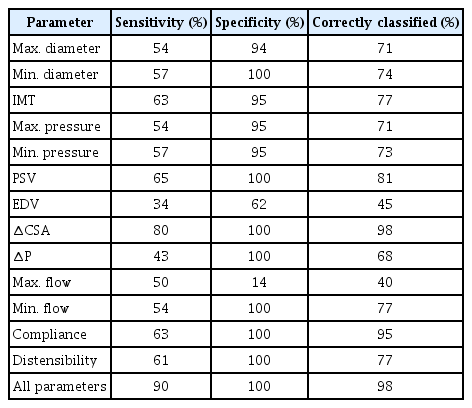
The results of the discriminant analysis of the physical parameters of the IJV and CCA are shown in Tables 7 and 8.
The ∆CSA parameter had the highest sensitivity (80%), specificity (100%), and correctly classified percentage (98%).
Among the parameters extracted from the CCA, maximum longitudinal displacement had the highest sensitivity (59%), specificity (91%), and correctly classified percentage (72%). The results for all CCA parameters are shown in a bold row (72% sensitivity, 100% specificity and 89% correctly classified percentage). Therefore, evaluating all the extracted physical and biomechanical parameters maximized the accuracy of the diagnosis.
Discussion
MS is not just an inflammatory disease, with its destruction of myelin sheaths and consequent effects on the white matter. This disease, due to the inflammation associated with it, also causes dysfunction of the endothelial layer of arteries [3], which can lead to cardiovascular problems, including atherosclerosis [20]. The existence of contradictory results in studies that examined the possible changes in MS patients' vascular systems might be due to the lack of attention to the biomechanical parameters of the jugular vein wall, as well as ignoring the inherent differences in the venous system between different people. It is possible to study the radial motion, distensibility, strain, and elasticity of the vessel wall using B-mode ultrasound images [8]. In addition, using vascular Doppler images, the volume and velocity of blood flow, the presence of venous blood reflux, and obstruction in the vascular pathway [4,5] can be examined. In the present study, the biomechanical parameters of the IJV and the CCA were evaluated using ultrasound images. A study by Coen et al. [21] found that changes in the frequency and accumulation of a particular type of collagen in the structure of the jugular vein wall were more common in people with MS than in healthy people. This process makes the jugular vein wall thicker in certain areas. In the present study, the thickness of the IJV's intima-media layer was 51% higher in patients with RRMS and 60% higher in patients with PPMS compared to healthy individuals. The diameter changes of the IJV in the supine position also were significantly higher in the RRMS and PPMS patients than in healthy individuals. An increased CSA of the sagittal sinus in people with MS was reported by Bateman et al. [22]. Grazzini et al. [23] observed that developmental venous anomalies in patients with MS were higher than in healthy individuals. In the current study, the compliance and distensibility of the IJV were higher in the healthy group, and significantly higher IJV pressure was observed in patients with MS compared to healthy individuals. Increased venous pressure in the superior sagittal sinus in MS patients has also been reported by Bateman et al. [24,25]. In this study, the radial strain of the CCA wall in patients with RRMS and PPMS was 38% and 25% lower, respectively, and the elastic modulus of patients with RRMS and PPMS was 47% and 9% higher, respectively, than in healthy individuals. These findings indicate a decrease in the elasticity of the CCA wall. Karageorgos et al. [26] reported reduced CCA wall stiffness in patients with atherosclerosis, while another study [27], observed an increase in the elastic modulus in people with cardiovascular disease. In this study, it was observed that with the progression of the disease, the elastic modulus of the artery increased. The thickness of the intima-media layer of the carotid artery is an important parameter in the early diagnosis of atherosclerosis [12,20].
An increase in carotid artery intima-media thickness could predict early preclinical cerebrovascular disease [28]. In our study, the intima-media layer of the CCA in patients with MS was significantly higher than in the healthy group (P<0.05). The same result in people with atherosclerosis was also observed in retrospective studies [29]. Yuksel et al. [20] showed the thickness of the intima-media layer of the right carotid artery in patients with MS was significantly higher than in the control group.
Based on the study of Zamboni et al. [5], reflux in blood flow (>0.88 seconds) and narrowing (<0.3 cm2) of the IJV were observed in 71% and 37% of patients with MS, respectively. The presence of undetectable currents was extracted by the Doppler technique in 52% of patients and in 3% of healthy subjects. A negative numerical value for the difference between the CSA of the IJV in the supine and sitting positions was noted in 55% of the patients and 11% of the healthy individuals. In our study, the reflux time was less than 0.88 seconds. A CSA less than 0.3 cm2 in the supine position was observed in 15% of patients with PPMS, 58% of healthy individuals, and no patients with RRMS. Torres et al. [30] showed bilateral narrowing of the IJV in most subjects. No significant difference in the presence of jugular vein narrowing in healthy individuals and patients with MS was also observed by Martin et al. [31]. In the present study, there was no undetected flow in any of the patients with MS and healthy individuals. Furthermore, a negative value in the ∆CSA parameter was not observed in any subjects, regardless of group. Therefore, it seems that the Zamboni parameters cannot be correct criteria for detecting MS.
CCSVI is confirmed by the presence of two or more of Zamboni's criteria. In this study, DCV imaging was not performed due to a lack of appropriate equipment to examine intracerebral arteries. Therefore, the fifth criterion (the presence of reflux in the DCV) was not examined, which is one of the limitations of the present study. Sex is also associated with arterial stiffness and vascular problems; therefore, in the present study, only the female sex was examined. Medications used by patients with MS can also affect the vascular system, which was not considered in the study.
This study aimed to evaluate the vascular parameters with the perspective that MS can affect the arterial tree in addition to affecting the hemodynamic parameters of the jugular vein, which have been the subject of contradictory studies. The present study proposed and investigated some biomechanical parameters for investigating the differentiation of MS patients. These parameters can be used as criteria for examining cardiovascular lesions.
By examining the physical and biomechanical parameters of the CCA and IJV in patients with MS, a significant difference in some physical and biomechanical parameters was observed, confirming the hypothesis of changes in the vascular system of patients with MS compared with healthy individuals. Due to the lack of patients with two or more of the Zamboni criteria, it can be concluded that the Zamboni criteria are not appropriate indicators of changes in the vascular system of people with MS. In conclusion, the parameters of ∆CSA and compliance in the IJV can help differentiate between MS patients and healthy individuals. In the case of the CCA, the maximum longitudinal displacement can be used to distinguish healthy and MS patient groups.
Notes
Author Contributions
Conceptualization: Rastegari K, Mokhtari-Dizaji M. Data acquisition: Rastegari K, Mokhtari-Dizaji M, Harirchian MH, Hashemi H, Yazdi NY, Saberi H. Data analysis or interpretation: Rastegari K, Mokhtari- Dizaji M. Drafting of the manuscript: Rastegari K, Mokhtari-Dizaji M, Harirchian MH, Hashemi H, Yazdi NY, Saberi H. Critical revision of the manuscript: Rastegari K, Mokhtari-Dizaji M. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was approved by the Faculty of Medical Sciences, Tarbiat Modares University. This work was supported in part by the Iran National Science Foundation (INSF).
References
Article information Continued
Notes
Key point
The biomechanical parameters of the internal jugular vein and the common carotid artery of patients with multiple sclerosis (MS) were extracted using image processing. The Zamboni criteria were not found to be accurate indicators of changes in the vascular system of people with MS.