2022 Taiwan clinical multicenter expert consensus and recommendations for thyroid radiofrequency ablation
Article information
Abstract
Radiofrequency ablation (RFA) is a minimally invasive management strategy that has been widely applied for benign and recurrent malignant thyroid lesions as an alternative to surgery in Taiwan. Members of academic societies for specialists in interventional radiology, endocrinology, and endocrine surgery collaborated to develop the first consensus regarding thyroid RFA in Taiwan. The modified Delphi method was used to reach a consensus. Based on a comprehensive review of recent and valuable literature and expert opinions, the recommendations included indications, pre-procedural evaluations, procedural techniques, post-procedural monitoring, efficacy, and safety, providing a comprehensive review of the application of RFA. The consensus effectively consolidates advice regarding thyroid RFA in clinical practice for local experts.
Introduction
Minimally invasive treatments (MITs), including ultrasound (US)-guided radiofrequency ablation (RFA), are widely applied to thyroid lesions. There is a growing body of data that can be used to reach a consensus concerning RFA as an alternative to surgery for the management of thyroid nodules [1-14]. In addition, the application of US-guided RFA in differentiated thyroid cancer (DTC) is also being widely investigated, with increasing interest expressed by physicians and patients in Taiwan [6,11-15]. The Korean Society of Thyroid Radiology (KSThR) guidelines were first developed in 2009 using the modified Delphi method [16] and were subsequently revised in 2012 and 2017. The guidelines were created to comprehensively introduce the evidence-based application of thyroid RFA, and have since been cited worldwide [6]. Meanwhile, the European Thyroid Association (ETA) clinical practice guidelines were issued in 2020, focusing primarily on benign thyroid nodules, with some mention of laser ablation, microwaves, and ethanol ablation (EA) [8]. More recently, an international multidisciplinary consensus statement directed by the American Head and Neck Society was published in 2022, focusing on the treatment of benign and malignant thyroid conditions [11]. Although similar indications, procedure-related evaluations, techniques, and follow-up strategies are noted across these guidelines, consensus differences within categories have also been identified. As countries have differing medical practices, insurance systems, and access to medical resources, it is thus necessary to determine a consensus via comprehensive reviews of evidence-based data, as well as the gathering of local experts’ opinions. To this end, the experts from the Taiwan Academy of Tumor Ablation (TATA), the Endocrine Society of Taiwan, the Taiwan Society of Interventional Radiology (TSIR), and the Taiwan Association of Endocrine Surgeons gathered in order to develop the first consensus statement regarding thyroid RFA in Taiwan. A committee was organized, and a literature review of relevant international and domestic databases was performed. The recommendations contain sections regarding indications, pre-procedural evaluations, procedural techniques, post-procedural monitoring, efficacy, and safety. The goal of these recommendations is to consolidate the advice of local experts, with a particular focus on the most recent and valuable evidence, in order to form a consensus-based guide to direct the application of thyroid RFA in clinical practice.
Methodology
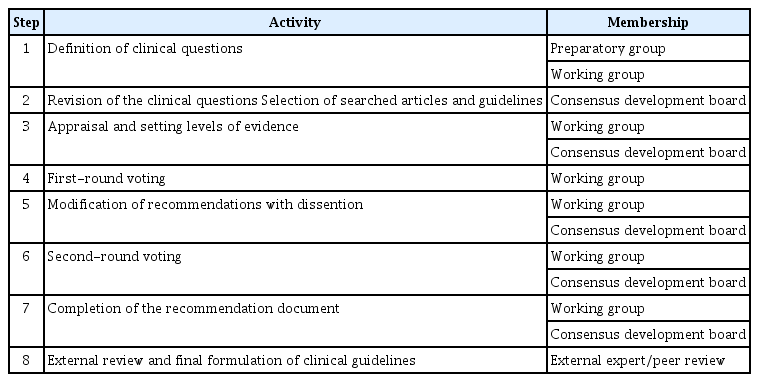
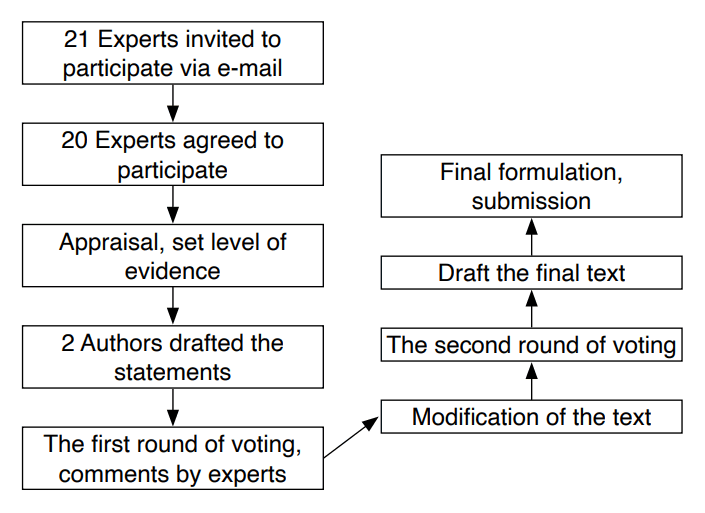
The consensus was developed with input from several groups (Table 1, Fig. 1). As the preparatory group, two authors were responsible for drafting the initial clinical questions. The working group was composed of three authors from the departments of interventional radiology and endocrinology, who were responsible for determining relevant articles to reference when developing the consensus statement and to invite other members to participate. The members of the consensus development board were invited due to their expertise in the fields of endocrinology, interventional radiology, diagnostic radiology, endocrine surgery, and/or otorhinolaryngology. The members included experienced RFA practitioners, some of the most experienced and skilled thyroid RFA experts in Taiwan, local physicians with published articles regarding RFA, and the leaders and/or members of the various academic societies mentioned above. The literature review, assessments, and setting of the recommendations grade and evidence levels were carried out by the board. The level of evidence and the grade of recommendation were based on existing guidelines and grading systems [6,17,18]. The evidence level assessment was primarily related to study design and quality, while the quality of evidence and clinical benefits were the leading factors influencing the recommendation grading [19]. The literature research was performed using various databases, including Medline, EMBASE, the Cochrane Library, PubMed, and the Chinese Electronic Periodical Services (CEPS). Manual searches of the bibliographies of key articles were also performed. The keywords were as follows: "thyroid" AND ["radiofrequency ablation" OR "RF ablation" OR "RFA"] AND ["consensus" OR "recommendation" OR "guideline" "statement"].
The drafted statements were sent to all members of the working group for the first round of voting, together with evidence-based initial reviews and pertinent literature. The modified Delphi method was used to reach a consensus [7,16]. Each suggestion was assessed on a 9-point scale: from 1 (totally disagree) to 9 (totally agree). All results and comments were collated by e-mail. When one statement was supported by ≥80% of the working group (i.e., a rating of 6 to 9 on the 9-point scale) in the initial voting procedure, it was accepted. Statements that did not reach consensus support during the first-round vote were modified. A video conference was held prior to the second round of voting to ensure that the terminologies and meanings were accurately conveyed and the discussion could be comprehensive. The modified statements were discussed, and a second round of anonymous voting was completed by the consensus development board. Each statement was assessed for level of evidence (Table 2). For reference, the evidence levels included: (1) high, reliable evidence and further research is unlikely to change the existing consensus; (2) moderate, further research is likely to influence and may change the recommendation; and (3) low, further research is very likely to have a meaningful impact on the confidence regarding the known effect, and is likely to alter the recommendation. The assessment of randomized controlled trials (RCTs) was performed by applying the 5-point Jadad scale [20]. Generally, an RCT was considered of high quality when the total score was ≥3 points. However, many of our references were related to treatment results, which were retrospective analyses, and thus difficult to be double-blinded. If it was not possible for the study to be double-blinded, it was assessed as high quality with a score of ≥2 points [21].
The modified Delphi method was used to arrive at a consensus. The strength of the recommendations was primarily based on the evidence level, clinical applicability, and net benefits to the patients [19]. The evidence level and the median value from the votes influenced the decision regarding the strength of recommendation for each statement [6,19]. The paragraph presentation format was initiated with a clinical problem, followed by one to several practical suggestions and current evidence. We have sought to avoid overlap or repetition between this consensus and the previous guidelines and various iterations thereof. A statement with a median value of ≥7 with an adequate level of evidence was considered to indicate sufficient evidence to support its benefit in clinical practice. Statements with either insufficient evidence or a lower median rating (between 4 and 6) were defined as weak recommendations. Statements not having reached consensus during the voting procedure, but considered clinically significant, were marked as "not recommended" and still presented to provide a more comprehensive overview of thyroid RFA. Here, the "not recommended" statement decisions were based solely on the voting results of the consensus development board, meaning that evidence was insufficient to determine clinical benefit, not as a reflection of the authors' personal opinions (Table 3). The protocol was supported by the TATA, the Endocrine Society of Taiwan, the TSIR, and the Taiwan Association of Endocrine Surgeons. An online disclosure of financial conflicts of interest within 24 months was obtained from all voting participants, who mentioned no financial disclosure/conflicts of interest.
Indications
Question 1. What Are the Indications of RFA for Benign Thyroid Nodules?
[Statement 1-a] RFA is indicated for patients with symptomatic benign thyroid nodules or with cosmetic concerns. (Strong recommendation, moderate-quality evidence)
Pressure-related symptoms are noted with enlarged thyroid nodules, including neck bulging, pain, foreign body sensation, dysphagia, voice change, and cough. RFA can be beneficial in this regard by reducing nodule size [4], thereby improving compression symptoms [6-8]. Symptoms can be quantified using a scoring system, either by the physician or by patient self-measurement. Cosmetic problems must be considered, and patients can use a visual analog scale (grade 0 to 10) to evaluate their symptoms subjectively [22], while physicians can define the cosmetic score for patients (from 1 to 4) [23]. The severity of cosmetic concerns is related to neck circumference or the location of the thyroid nodule [1,6]. For benign thyroid nodules, there is no definitive indication related to size or volume for RFA. Generally, patients with a smaller neck circumference tend to have cosmetic concerns earlier than those with a larger neck circumference. Considering the nodule location, isthmic nodules more readily contribute to cosmetic problems. A longitudinal observational study involving 215 patients with benign thyroid nodules reported a 67% median volume reduction rate (VRR) at 5 years after single-session RFA. The best response was recorded in small nodules (<10 mL) [24]. A meta-analysis enrolling 12 studies on RFA with 1186 total cases noted a VRR at 2 years of 87%. The size of the nodule appears to correlate with compressive symptoms and the degree of improvement after management [25].
Improvements in compressive symptoms and cosmetic problems by RFA have been confirmed [24,25]. In one study in the United States enrolling 284 patients who were considered potential candidates for RFA prior to surgery, 24.6% of the RFA-eligible group had a confirmed malignancy based on pathology results. Given the rate of occult malignancies, an optimal evaluation of non-dominant nodules prior to RFA and long-term follow-up is essential [26].
[Statement 1-b] Two sessions of US-guided fine-needle aspirations or core needle biopsy are required to confirm the benign status of thyroid nodules prior to RFA. (Strong recommendation, moderate-quality evidence)
Current guidelines worldwide recommend the confirmation of benign cytopathology prior to ablation [6,8,27,28]. The false-negative rate of benign diagnoses based on cytology or histological diagnoses is less than 3%, according to the Bethesda System [29]. However, several studies have noted a higher false-negative rate for large nodules (>3-4 cm) [30,31]. Meanwhile, the malignancy risk of a nodule after two benign cytology results is relatively low [32]. Interpretations of non-diagnostic (ND), and inadequate or indeterminate cytology (e.g., atypia of undetermined significance, follicular lesion of undetermined significance [AUS/FLUS]) may complicate the subsequent decision. Re-aspirating ND nodules can achieve a definitive diagnostic interpretation in up to 60% to 80% of cases [33]. A retrospective analysis involving 514 patients revealed that repeated fine-needle aspiration (FNA) in cases of AUS/FLUS increased detection of follicular adenomas (from 15.2% to 35.9%), but not the detection of malignancy [34]. Repeated sessions of FNA or core needle biopsy (CNB) prior to RFA to confirm the benign status of thyroid nodules are advised. US-guided aspiration is the first choice for symptomatic predominantly cystic (51%-90% fluid component) or cystic (>90% fluid content) thyroid nodules, for both diagnostic and therapeutic purposes [28,35]. US-guided EA is recommended for cases involving a recurrent cyst, and RFA may be considered when local symptoms persist after EA [22,23].
[Statement 1-c] In instances of highly suggested benignity on US prior to RFA (isoechoic spongiform or partially cystic lesions with intranodular comet tail artifact) or low risk of malignancy (EU-TIRADS or ACR-TIRADS ≤3), a single benign diagnosis by FNA or CNB may be sufficient prior to RFA. (Strong recommendation, high-quality evidence)
This clinical question is referenced from Recommendation 1-3 of the 2017 KSThR guideline [6] and discussed by local experts. US-based risk-stratification systems are helpful in assessing the possibility of nodule malignancy. Many international societies have proposed various US-based thyroid imaging, reporting and data systems (TIRADSs) to describe the US characteristics of thyroid nodules [28,36-38]. The TI-RADS published by the American College of Radiology (ACR) is applied worldwide for guidance regarding the management of thyroid nodules based on US appearance [36]. For a single nodule with TI-RADS ≤3 (below the degree of "mildly suspicious"), the cancer risk is no more than 5% [18,27,36]. The European Thyroid Imaging and Reporting Data System (EU-TIRADS) was developed based on a literature review and on the American Association of Clinical Endocrinologists, American Thyroid Association, and Korean guidelines. Similarly, thyroid nodules classified as European Thyroid Imaging and Reporting Data System (EU-TIRADS) 3 present a low risk of malignancy (ranging between 2% and 4%), with a relatively low risk of false-negative results [37]. The Korean TI-RADS (K-TIRADS), revised in 2021, uses a pattern-based system that stratifies the malignancy risk. A pattern-based application involving a combination of echogenicity, composition, and suspicious US features is emphasized. Benign nodules are classified as K-TIRADS 2, while entirely calcified nodules are classified as K-TIRADS 4 [38]. Isoechoic spongiform nodules or predominantly cystic nodules often have a low risk of malignancy, when no other superimposed features of high suspicion (non-oval/round shape, irregular margins, microcalcifications or marked hypoechogenicity in a solid nodule) exist [36,39-41]. In such cases, considering the low malignancy risk, the consensus development board agreed that one benign diagnosis by FNA or CNB may be sufficient.
[Statement 1-d] Radiofrequency ablation is indicated for autonomously functioning thyroid nodules. (Strong recommendation, moderate-quality evidence)
A radioactive iodine uptake scan can distinguish toxic multinodular goiters or autonomously functioning thyroid nodules (AFTNs) from Graves' disease and assess the functionality of nodules that may coexist with Graves' disease. One meta-analysis indicated that approximately 50% (95% confidence interval [CI], 32% to 68%) of patients with AFTNs, demonstrated by thyroid scintigraphy had normal thyrotropin (TSH) levels. Therefore, TSH measurement may not be suitable as a single tool to exclude the possibility of AFTN [42]. In addition, subclinical hyperthyroidism may adversely influence bone loss and fracture risk [43], as well as the cardiovascular system, with examples including atrial fibrillation [44], coronary artery disease, and heart failure, especially for those with a TSH level <0.1 mIU/L [45]. The treatment of associated toxic nodules could avoid the further deterioration of thyroid function [46,47]. As treatment options for AFTN, current guidelines suggest surgery, radioiodine, or prolonged (probably lifelong) thionamide therapy [6,48]. It is worth noting that radioactive iodine (RAI) is contraindicated during pregnancy or breastfeeding [49]. However, the risk of development of hypothyroidism after RFA has been reported to be approximately 10% during a median 5-year follow-up [23]. Hypothyroidism may worsen pre-existing chronic disorders in elderly patients [46].
In instances of AFTNs, RFA has been suggested as a viable alternative treatment [6,7,28,46,47,50,51]. The indications of treatment for patients with AFTNs include cases with symptoms of hyperthyroidism, symptoms of compression, and cosmetic concerns [46]. Clinical success for AFTNs is indicated by a normalization of serum thyroid hormone levels. The efficacy of single-session RFA on AFTN can reach a VRR of 75% with 50% functional remission at 12 months, or reach a status of at least partially cured when surgery and RAI are contraindicated [52]. A recent systematic review and meta-analysis that enrolled 411 AFTNs (391 cases) treated by thermal ablation revealed a 71.2% TSH normalization rate, with a pooled VRR of 69.4% at the 1-year follow-up. Baseline nodule volume was not associated with the rate of TSH normalization [53]. In a prospective trial, patients with large AFTN (>10 mL) treated either with RAI alone or with laser ablation (LA) followed by RAI, demonstrated a higher volume reduction and more improvement in local symptoms from the combined treatment [54]. Furthermore, the combination of LA or RFA and RAI for selected patients presenting with large AFTNs may achieve a more rapid volume reduction [8]. Taken together, the evidence indicates that RFA can be an alternative to surgery or RAI therapy for AFTN treatment, as a way to achieve both volume reduction and hormonal normalization for patients unwilling to undergo surgery or RAI. However, it must be noted that RFA for large AFTNs (volume >20 mL) is less effective than for smaller ones [3,7,47]. Further investigation into such cases should be conducted.
[Statement 1-e] A single benign diagnosis on FNA or CNB is sufficient for confirming a benign nodule in AFTNs. (Not recommended, low-quality evidence)
AFTNs account for 5%-10% of palpable lesions. The association between thyroid carcinoma and hyperthyroidism has primarily been discussed in the form of case studies [55,56]. A literature review of patients with solitary AFTNs managed by thyroid resection revealed an estimated 3.1% (35/1,124) prevalence of malignancy. In a review of 77 malignant hot nodules, only 23 received FNA/CNB prior to surgery; wherein seven (30.4%) cases characterized as benign were false-negative results, and four (17.4%) had ND findings [57]. The reported incidence of malignant hot nodules indicates that the possibility of malignancy cannot be excluded in AFTNs, although they are often considered benign entities in clinical practice [6,18]. Thus, a cautious differential diagnosis is necessary. With further improvements in terms of molecular etiology, detecting oncogenes such as BRAFV600E may be helpful, and could be applied for hyperfunctioning thyroid carcinoma [58,59]. Considering the inconsistencies of the current evidence and expert opinions, a single benign diagnosis on FNA or CNB prior to RFA may be insufficient to confirm the benignity of AFTNs.
Question 2. What Are the Indications of RFA for Locally Recurrent Thyroid Cancers?
[Statement 2-a] RFA is indicated for patients with locally recurrent thyroid cancers or neck lymph node metastases after thyroidectomy, especially for those who present with high surgical risk or refuse surgery. (Strong recommendation, moderate-quality evidence)
Surgery remains the first-line management of locally recurrent thyroid cancers, with subsequent RAI and thyroid hormone suppression therapy suggested worldwide [6,18,52,60]. The decision to perform surgical intervention depends on two divergent considerations: the risk of repeat surgery and the benefits of surgery as compared to other treatment options. The risks associated with anesthesia, and difficulties involving in repeated neck operations with the distortion of normal tissue planes, fibrosis, and scar tissue formation must be considered [61]. An elevated complication rate compared to the first operation has also been noted [62]. Worsening lung function and multiple comorbidities in the elderly also pose surgical challenges. Furthermore, a patient’s refusal of surgery due to personal concerns cannot be ignored. Under these circumstances, the identification of methods to decrease the tumor burden, in order both to control of local recurrence and decrease the risk of subsequent surgery, is crucial. In 2001, Dupuy et al. [63] first reported RFA for locally recurrent thyroid cancer, wherein no recurrence at the treatment site was noted at a median follow-up of 10.3 months in eight patients (mean size, 2.4 cm). In the following years, abundant results have been published regarding MITs, including RFA for locally recurrent thyroid cancer [64-66]. The indications in these studies primarily included patients who had a high risk of surgery or refused surgery, but for whom surgery was indeed the preferred treatment modality. Two meta-analyses have indicated that RFA is an acceptable treatment for locally recurrent thyroid cancer [67,68]. In 2021, the ETA and the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) co-published clinical practice guidelines for the use of MITs for thyroid malignant lesions [12]. MITs may be proposed to achieve local tumor control in advanced thyroid cancers, such as anaplastic or advanced medullary thyroid cancer or with palliative intent [38]. For complete tumor removal, RFA should be applied only if it is judged possible based on a radiological examination. Furthermore, there should be no metastasis beyond the neck. Several studies have reported the effectiveness of RFA for locally recurrent thyroid cancer, with VRRs of approximately 50.9%-98.4% and nearly 0% recurrence during relatively long durations of follow-up [64-67,69,70]. In three Korean studies, which treated locally recurrent thyroid cancers of <2 cm with RFA, the reported VRRs were between 93% and 98.4% [64-66]. Although RFA does not involve performing complete tumor removal, RFA can eliminate the tumor burden, thereby achieving symptom relief or serving as a bridge to other systemic treatment. A systematic review and meta-analysis including 270 patients with 415 thyroid nodules compared the efficacies of RFA and EA. The pooled proportion of lesions that completely disappeared after RFA (68.8%) was indeed higher than that after EA (53.4%; P=0.3384), with fewer procedure sessions required, indicating that both are acceptable treatment modalities to manage locally recurrent thyroid cancer for poor surgical candidates [67]. In a study comparing the efficacy of RFA with repeated surgery for 221 patients with locally recurrent thyroid cancers, both groups had similar recurrence-free survival and complication rates, while the overall complication rate was higher in the repeat surgery group [71].
Locally recurrent thyroid cancer can cause a variety of symptoms, including dysphagia, hoarseness, dyspnea, and cosmetic problems. RFA can be applied when it is judged that the size reduction by RFA could effectively reduce symptoms and improve patients’ quality of life, even if complete removal is not possible [69]. For patients with recurrent malignant lesions who are ineligible for or refuse surgery, the consensus development board suggests that RFA is a safe and effective alternative treatment.
Question 3. What Are the Indications of RFA for Primary Thyroid Cancers, Especially for Papillary Thyroid Microcarcinoma?
[Statement 3-a] RFA may be indicated for patients with papillary thyroid microcarcinoma without lymph node metastasis. (Weak recommendation, moderate-quality evidence)
The incidence of papillary thyroid microcarcinomas (PTMCs) has increased concurrently with advances in examination equipment and improvements in surgical thyroid procedures [72]. Active surveillance (AS) has been proposed as an alternative to immediate surgery for PTMC without clinically evident metastasis or local invasion [18]. For patients deemed at high surgical or anesthesia risk, or for those who wish to receive a more proactive treatment than AS, MITs including RFA, LA, and microwave ablation have emerged as viable alternative options, with effective control of local tumors in year-counted follow-ups [6,14,73-75]. The possibility of distant metastasis should be excluded through a detailed examination, including but not limited to US, as mentioned below in statement 3-b. A prospective study that enrolled 98 patients with PTMCs treated with RFA with a mean 7.8-month follow-up revealed mean VRRs of 0.47±0.27, 0.19±0.16, 0.08±0.11, 0.04±0.10, and 0 at 1, 3, 6, 12, and 18 months after RFA (with 1 indicated the original volume, and 0 as complete elimination), respectively [73]. Meanwhile, a retrospective study involving 198 PTMC patients who were treated using RFA revealed VRRs at the 1- and 2-year follow-ups of 99.6%±1.9% and 99.8%±1.0%, respectively [76]. In addition, a relatively large cohort study of 414 patients with unifocal low-risk PTMC treated by RFA with a mean follow-up time of 42 months revealed a mean VRR of 98.81%±6.41%, without any life-threatening or delayed complications [14]. Furthermore, Zhang et al. [77] investigated RFA versus thyroidectomy while focusing on aspects of efficacy, outcomes, complications, and costs over a 5-year follow-up period. Similar oncological outcomes between the two management strategies were reported, although RFA had shorter average hospitalization times and lower costs [77]. A prospective study in Taiwan, which included 12 patients with 13 PTMCs who underwent RFA as they were ineligible for or refused surgery, reported a median VRR of 100% during the 12-month follow-up period, with eight tumors (61.54%) having completely disappeared [13]. When weighing the options of surgery, radioiodine therapy, and MIT, a multidisciplinary team involving members with expertise in interventional radiology is crucial [12]. AS and US-guided MITs, including RFA, are suggested as alternatives to thyroidectomy for selected patients with PTMC [78,79]. In summary, the consensus development board had a weak recommendation regarding RFA for patients with PTMC without lymph node (LN) metastasis. Careful patient enrollment with a detailed pre-procedural image evaluation and a shared decision-making process with the patient are essential [14,76].
[Statement 3-b] Thorough pre-RFA neck LN imaging studies (often including US, computed tomography, or positron emission tomography) should be performed prior to RFA for thyroid cancer, to confirm the existence of LN or distant metastasis. (Strong recommendation, moderate-quality evidence)
Pre-RFA US and/or computed tomography are widely accepted methods for confirming the existence of LN metastasis [18,52,80]. Metastatic LNs on US examination are hypoechoic and calcified, usually with an aspect ratio >1 and strong vascularity. Pre- and post-contrast computed tomography (CT) with a dedicated protocol is preferred to diagnose thyroid cancer [38]. The scan range should extend to the superior mediastinum to evaluate upper mediastinal LNs and anatomic variations such as the aberrant right subclavian artery [81]. CT images indicate that metastatic LN presents as swollen, low-density, and calcified lesions with hypervascularity [82]. The accuracy of US alone in diagnosing LN metastasis is superior to that of CT alone (P<0.05), while their combination is superior to either US or CT alone (P<0.05). The sensitivity, specificity, and accuracy of combined examinations have been reported to be 84.15%, 85.71%, and 89.85%, respectively [82]. According to a meta-analysis enrolling 5,656 thyroid cancer patients and comparing the diagnostic performance between US and CT, CT had a higher sensitivity in the assessment of whole cervical LN metastasis (0.664 vs. 0.593, P=0.002), while US had a higher specificity (0.834 vs. 0.911, P<0.001) [80]. Prior to the RFA procedure, we suggest performing a US and/or CT examination to confirm the LN status and record the characteristics of suspicious metastatic lesions. Multidisciplinary team discussion is constructive when weighing different therapeutic managements [12].
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is non-invasive, and a nodule with a maximum standardized uptake value (SUVmax) ≥5 is highly indicative of malignancy, with a sensitivity of 76% and specificity of 85% [83]. There is increasing evidence noting the differentiation between Hürthle cell carcinoma and follicular adenomas on PET [84,85]. In a study enrolling 46 patients with indeterminate results on FNA, which surveyed the efficacy of PET/CT for detecting malignant lesions, the sensitivity, specificity, and positive predictive and negative predictive values were 94%, 62%, 59%, and 95%, respectively [86]. Pre-ablative 18FDG-PET is helpful for the further assessment of malignant FDG-avid LNs in patients with PTMC or papillary thyroid carcinoma (PTC) [86]. For patients who have low-SUV follicular neoplasms (SUVmax <5) and are unsuitable or unwilling to undergo surgery, RFA could be a viable alternative [85]. Appropriate application considering the local healthcare system and costs should be discussed with the patient.
Question 4. What Are the Indications of RFA for Follicular Neoplasms?
[Statement 4-a] RFA for follicular neoplasms has a lack of evidence for treatment benefits. (Strong recommendation, low-quality evidence)
The differences between follicular carcinoma and adenoma include the presence of capsular, vascular, or extrathyroidal invasion, as well as existing nodal or distal metastasis [87]. RFA for follicular neoplasms (FNs) was first recommended by the KSThR in 2012 [88], although much controversy remains. Achieving pathological confirmation prior to surgical treatment for FN is challenging [89,90]. FNA has a false-positive rate between 22.2% and 35% in FN cases [87,89,91]. The association between malignancy and FN size remains debatable [92]. A recent 5-year follow-up study demonstrated that RFA can be safe and effective in treating patients with FNs <2 cm in size, without recurrence during a 66-month follow-up [93]. Due to inconsistencies in the literature and a lack of high-quality evidence, RFA should not be recommended as a first-line therapy for FN. The possibility of minimally-invasive follicular cancer with delayed surgery in cases of malignancy is a consideration [94]. The consensus development board agreed that there is currently a lack of evidence regarding RFA for FN. The discovery of the high incidence rate in PTC of the oncogenic BRAFV600E mutation (50%-70%) and the subsequent development of BRAF-targeted therapies have changed the landscape of thyroid cancer treatments. In addition, the ongoing evolution of molecular marker panels may help to determine the degree of benignity for FN [95].
Pre-procedural Evaluation
Question 5. What Are the Appropriate Evaluation Procedures (Laboratory and Imaging) for Patients prior to RFA?
[Statement 5-a] Prior to RFA, a comprehensive survey using a pre-procedural checklist should be performed. (Strong recommendation, moderate-quality evidence)
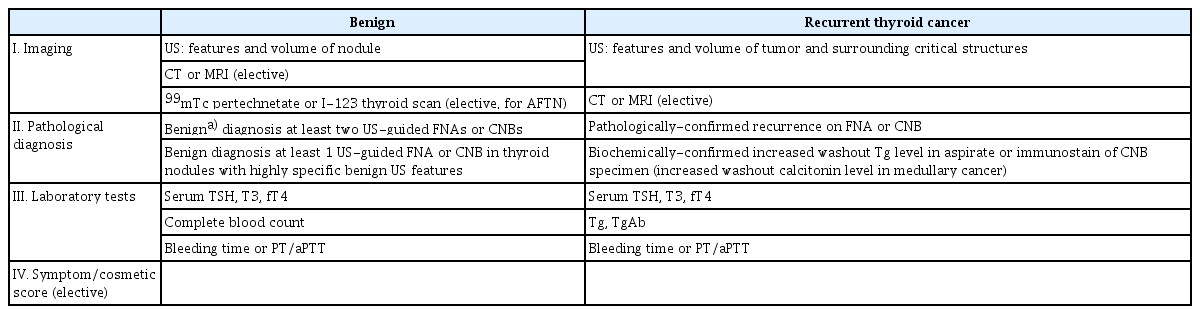
A pre-RFA checklist is presented in Table 4. Combining pathological results and US features to evaluate benignity is essential. The size, characteristics, composition, and intranodular vascularity of the nodule(s) on US should be recorded [2,6,7]. Subjective symptom and cosmetic scores recorded prior to RFA have revealed improvements in quality of life and cosmetic scores when compared with follow-up data post-RFA [96,97]. Pre-RFA laboratory tests should include a complete blood count, bleeding tendency, and thyroid function tests [5,6,8,98]. If there is a concern about discontinuation of anti-platelet or antithrombotic medications, consultation with a specialist is recommended (as mentioned in statement 6-a below). In locally recurrent thyroid cancer, in addition to serum TSH, T3, and fT4 level tests, laboratory tests should also include thyroglobulin (Tg), and anti-Tg antibody (anti-Tg Ab) tests [18,66,80].
A technetium 99mTc pertechnetate or I-131/I-123 thyroid scan is helpful in determining AFTNs [47]. However, debate continues regarding routine checks of thyroid antibodies prior to RFA of a benign thyroid nodule l [64,99]. For patients with a positive antibody finding, the possibility of hypothyroidism associated with both RFA and the natural course of thyroiditis should be considered. For large thyroid nodules with intrathoracic extension or locally recurrent thyroid cancer, CT or magnetic resonance imaging may benefit the evaluation procedure [6]. It should be noted that RFA is not currently covered by the National Health Insurance System in Taiwan; therefore, the related costs must be detailed to the patient prior to the RFA procedure. The consent form should contain the following points, and be clearly explained to the patient prior to the procedure:
– A detailed history of thyroid surgery/management and of currently prescribed drugs, including anti-platelet, anticoagulant, and antithyroid drug (ATD) use or thyroid hormone supplementation.
– The number of expected treatment sessions
– Potential RFA complications, and the possibility of regrowth for treated nodules requiring additional treatment
– Need for follow-up visits
– Potential requirement for further medication or admission after the RFA procedure, dependent on individual circumstances
Question 6. What Are the Recommendations for Adjusting the Use of Anticoagulants or Anti-platelet Drugs prior to RFA?
[Statement 6-a] The benefits and potential harm related to the interruption of antiplatelets and/or anticoagulants prior to RFA should be evaluated. A specialist consultation and blood test with prothrombin time and activated partial thromboplastin time may be required prior to RFA, according to individual circumstances. (Strong recommendation, low-quality evidence)
Balancing the risk of thromboembolism with the risk of bleeding is crucial. The 2017 Korean guidelines include a recommendation for medication withdrawal based on a study performed using gastrointestinal endoscopy [100], but no current guidelines have such a recommendation specifically for thyroid RFA. A study retrospectively reviewed patients with dysplastic Barrett's esophagus receiving RFA. No post-RFA bleeding was noted by withholding anticoagulant/anti-platelet agents 5 days prior to RFA, and by maintaining anticoagulation at an international normalized ratio <1.5 [101]. The efficacy and half-life of various drugs, including aspirin, other anti-platelet drugs, anticoagulants such as warfarin, and non-vitamin K antagonist oral anticoagulants are varied [102,103]. Furthermore, the decisions regarding drug discontinuation are varied and based on individual circumstances, including the specific planned procedure, bleeding risk factors, and personal preferences [103,104]. Thus, it may be necessary to consult a specialist, such as a cardiologist, prior to RFA.
Standard Techniques
Question 7. What Is the Appropriate Technique of RFA for Benign Thyroid Nodules/Thyroid Cancer?
[Statement 7-a] Perithyroidal/perilesional lidocaine injection is recommended for local anesthesia, instead of general anesthesia or deep sedation. (Strong recommendation, moderate-quality evidence)
[Statement 7-b] The trans-isthmic approach and moving-shot technique are recommended as standard procedures for thyroid RFA. (Strong recommendation, moderate-quality evidence)
Perithyroidal lidocaine injection as local anesthesia is indicated for pain relief and enhanced stability during the procedure [105]. Injection of 1%-2% lidocaine (2-10 mL) into the puncture site along the thyroid capsule is recommended, as general anesthesia or sedation may delay the detection of complications such as hoarseness or skin burn [106]. Compared with general anesthesia, perithyroidal anesthesia can decrease both the total opioid dose and level of patient sedation, without significantly affecting patient tolerance [107].
The trans-isthmic approach and the moving-shot technique are both recommended in the 2017 KSThR guideline [6]. The trans-isthmic approach involves an RF electrode inserted via the isthmus, in the midline-to-lateral direction, to approach the target [98]. The moving-shot technique involves ablation unit-by-unit, from the deepest to the most superficial portion of the nodule. By ablating at a fixed point, then slowly pulling back the electrode tip and moving to the next fixed point, using the "pull-back" technique to complete ablation of all dimensions of the target nodule under real-time monitoring is achieved [108]. In addition, hydrodissection with 5% glucose water between the nodule and dangerous triangle or other vital organs is necessary, as is decreased ablation energy when bordering the recurrent laryngeal nerve or vagus nerve [4,47,48]. It is also helpful to test the patient's voice intermittently during the procedure to check for possible voice change.
Post-RFA Care
Question 8. What Are the Appropriate Clinical, Laboratory, and Imaging Evaluations and Frequency of Post-RFA Follow-up Visits?
[Statement 8-a] After RFA, clinical and laboratory findings, including thyroid function tests and imaging, should be evaluated. (Strong recommendation, moderate-quality evidence)
The RFA procedure is complete upon noting a transient hyperechoic zone on US. Clinical symptoms, including the length and degree of pain (i.e., via the visual analog scale, hoarseness, dysphasia, foreign body sensation, cough), and the corresponding treatments should be recorded. The VRR is calculated as [initial volume–final volume]×100/initial volume. Changes in size, volume, intranodular vascularity, and echogenicity on US examination should be recorded at each follow-up [6-8]. The ideal timing of US follow-up visits includes 1, 3, 6, 12, and 18 months after RFA, depending on the post-RFA status, regional medical facilities, and individuals' ability to access care. It is recommended to educate each patient regarding post-RFA self-care, including mild compression at the treatment site for 20-30 minutes, avoidance of aggressive neck movements or labored coughs, and self-monitoring for potential complications.
Thyroid function should be monitored, including levels of TSH, T3, and fT4, usually at 6 months after RFA [6,26,109]. The therapeutic response of AFTNs to RFA can be classified into three categories: remission (euthyroid state after ATD withdrawal), improvement (reduction of medication), and no response (unchanged medication dose) [46]. For patients with hyperthyroidism or toxic nodular goiters, the ATD dose can be adjusted or ceased according to TSH changes. Patients with autoantibodies (antithyroid peroxidase antibody and anti-Tg Ab) should be monitored for the development of subclinical or overt hypothyroidism after RFA [6,110]. For patients with elevated serum TSH receptor antibody levels, the coexistence of Graves’ disease should be considered. Our consensus development board considers that although RFA can achieve biochemical improvement related to AFTNs, a slower dose reduction of ATD after RFA may be necessary. For AFTN, subsequent drug treatment or additional RFA session(s) may be required to improve clinical success. The mean number of RFA sessions for AFTN has been reported to be 1.8-2.2 (1-6 sessions) [46]. The decision should be based on the patient’s biochemical status in terms of the serum TSH level, rather than on scanning or the under-ablated portion on US. Any additional RFA procedure can be considered at 3 to 6 months after the initial RFA, since volume reduction is often most prominent during the first 3 months [46]. Table 5 includes the post-procedural checklist.
Question 9. What Are the Specific Considerations Regarding Post-RFA Care for Primary/Locally Recurrent Thyroid Cancer, as Different from Benign Nodules?
[Statement 9-a] For patients with thyroid cancer, laboratory tests, including serum Tg and anti-Tg antibody levels, should be added to post-RFA evaluations. (Strong recommendation, low-quality evidence)
After the ablation of a recurrent malignant lesion, follow-up US examinations should be performed at 1, 3, 6, and 12 months, and every 6 to 12 months thereafter, according to the status of the treated tumor [6,18]. The above time intervals may be adjusted according to individual circumstances. The residual tumor diameter, volume, vascularity, and the development of any new metastatic tumors should be evaluated [6]. After a successful ablation procedure, intense enhancement of the locally recurrent cancer disappears completely on CT; meanwhile, on US, a hyperechoic lesion without a Doppler signal is noted [82].
The Tg level often decreases to below 5-10 ng/mL approximately 25 days after thyroidectomy [111]. The anti-Tg Ab level may rise transiently after an operation or RAI [19], and qualitatively interfere with Tg immunometric assay assessments, potentially leading to a false-negative serum Tg level reading [18,112]. Washout Tg measurement in FNA content (FNA-Tg) is used to evaluate suspicious recurrent or metastatic LNs and lesions. A systematic review and a meta-analysis estimated the diagnostic accuracy of this method, reporting a pooled sensitivity of 91%-95%, and specificity of 94%-94.5% [113,114]. The cutoff value of 1 ng/mL had the highest sensitivity, while the setting of 40 ng/mL had the highest specificity. Considering this obvious gap, internal validity must be established for each facility [115]. The post-RFA checklist is presented in Table 5.
Question 10. Is a Single Session of RFA Sufficient for Patients with Non-functioning Thyroid Nodules?
[Statement 10-a] Additional treatment may be considered with marginal regrowth or unsolved symptomatic problems after RFA. (Strong recommendation, moderate-quality evidence)
A 50% volume reduction at 1 year is often considered as technically successful [116]. Most nodules present a favorable volume reduction at 6 months after the initial RFA procedure [4,6,8,25,117-119]. Indications for repeat RFA are observed in 24.1%-57.9% of patients in a longer follow-up period [2,118]. The initial solidity and volume affect the efficacy of RFA; as such, larger nodules require more treatment sessions than smaller nodules [46,118,120]. In one retrospective study evaluating 186 patients with benign nodules treated with RFA, the VRR of large nodules (>30 mL) was significantly greater than that of smaller nodules at the 1-month follow-up. At the 6-month follow-up, a similar therapeutic efficacy was noted for nodules of all sizes [121]. A systematic review compared the volume changes between RFA and LA at a 6-month follow-up. Two sessions of RFA were more effective than a single-session treatment, especially for nodules with an initial volume >20 mL [122]. Although opinions vary widely regarding the proper timing of additional treatment, there is a general consensus supporting additional treatment when symptoms are persistent after the initial treatment, or upon detection of tumor regrowth, defined as a nodule volume increase of ≥50% compared to the minimum recorded volume measured during follow-up [2,47,75,119,123].
During a 5-year follow-up, RFA was associated with a lower risk of regrowth and of requiring retreatment, as compared to LA [124]. The regrowth risk was associated with low-energy delivery, younger age, and larger nodules. In the study the ablated nodules were divided into two parts: the ablated area (hypoechoic) and the residual viable area (isoechoic, surrounding ablation area). The volume of the viable area specifically increased during regrowth; thus, follow-up for this area is helpful to detect regrowth and determine the timing of additional ablation procedures [46]. The residual vital ratio (RVR) is defined as the ratio of residual vital volume to the total volume, as calculated by contrast-enhanced US (CEUS) at the first follow-up. A retrospective study evaluated 206 benign nodules having undergone RFA. With an RVR >44.5%, the nodule tended to show regrowth at the first follow-up, indicating that RVR could be an early quantitative predictor for regrowth [125]. Considering the limited sensitivity of color-Doppler US for the detection of smaller vessels with slower blood flow [126,127], CEUS can be a useful diagnostic tool to detect residual vital tissue after RFA [123,127]. For non-functioning thyroid nodules, the timing of repeated RFA can be considered as 6 months after the initial RFA.
Question 11. Is RFA an Effective Management Modality for Locally Recurrent Thyroid Cancer or Primary Thyroid Cancer?
[Statement 11-a] RFA is an effective treatment option for patients with primary thyroid cancer above T1bN0M0 who refuse or are ineligible for surgery. (Not recommended, low-quality evidence)
The applications of RFA for locally recurrent thyroid cancer or PTMC are discussed in the previous section (statements 2-a and 3-a). RFA as a procedure for PTC at stages above T1bN0M0 (tumor size >1 cm) remains in the developmental stage, with its effectiveness currently disputed. Surgery has an advantage in the detection and management of microscopic metastasis in central LNs as compared to thermal ablation [128]. There have been reported mean VRRs of between 47.8% and 100% for RFA in primary thyroid DTC [129]. Obtaining confirmation of the thyroid epithelial origin of the lesion prior to RFA is crucial. In a retrospective study enrolling 182 patients, the efficacy of RFA and surgery on local tumor progression for T1bN0M0 PTC was compared, revealing no significant difference in local tumor progression [130]. A longer-term follow-up study (mean 48 months) of six patients with PTC reported a mean VRR of 98.5%±3.3%, without local tumor recurrence during the 3-year follow-up period [131]. The ablation of sufficient margins surrounding the cancer tissue is essential [74,132]. However, the detection and treatment of residual primary lesions and LN metastasis remain challenging [132,133]. Due to the current lack of high-quality evidence and the discrepant expert opinions, the local consensus is that RFA for patients with primary thyroid cancer above T1bN0M0 is not recommended. Patients with primary thyroid cancer who seek treatment with RFA should be limited to those with low-risk thyroid cancer, with local-regional disease only, and involve comprehensive pre-RFA imaging evaluations [7,48,129,130].
Question 12. What Are the Complications of RFA and the Corresponding Management Strategies?
[Statement 12-a] RFA is safe and well-tolerated, and is associated with a low incidence of complications when performed by experienced operators. (Strong recommendation, high-quality evidence)
[Statement 12-b] Some unexpected complications, including bleeding, hematoma, voice change, vocal cord palsy, and goiter rupture, should be considered. (Strong recommendation, moderate-quality evidence)
Many guidelines and meta-analyses conducted worldwide have confirmed the low incidence of complications and relatively good tolerability of thyroid RFA procedures [4-8,134,135]. A systematic review and meta-analysis enrolling 2,421 patients having received RFA revealed an overall RFA complication rate of 2.38% (95% CI, 1.42% to 3.34%), and a major RFA complication rate of 1.35% (95% CI, 0.89% to 1.81%) [136]. A recent multicenter study including 762 cases of benign nodules that underwent RFA in Taiwan reported a complication rate of 4.8%, a majority of which were voice change (2.7%) [137]. The overall and major complication rates were significantly higher for malignant thyroid nodules than for benign nodules [136]. Currently, there have been no reports of life-threatening complications [5,6]. A comprehensive understanding of US-based neck anatomy is necessary. Major complications are regarded as unexpected events that lead to substantial morbidity and disability, usually involving nerve injuries (recurrent laryngeal nerve, cervical sympathetic ganglion, brachial plexus, and spinal accessory nerve), nodule rupture, and permanent hypothyroidism for RFA. Minor complications include pain, skin burn, hematoma, transient thyrotoxicosis or hypothyroidism. Pain is the most common complaint, with pain in the head, ears, shoulders, back, and/or teeth having been reported. Most patients are tolerant of the pain under local anesthesia during the procedure. A few studies have reported patients suffering from severe pain, thereby requiring additional analgesics after RFA [134,138]. Voice change caused by injury to the recurrent laryngeal nerve or vagus nerve is the most common major complication, the incidence rate of which has been reported to be 1.45%, with a permanent voice change rate of 0.17%. Of note, the incidence of voice change is higher for locally recurrent thyroid cancers (7.95%) than for benign thyroid nodules [136]. Possible mechanisms include direct thermal injury to the nerve, stretching of the nerve over the thyroid swelling, or hematoma on the nerve. Variations in the location of the vagus nerve may increase the risk of nerve damage during the procedure. A bulging thyroid nodule may also alter the relative location of the vagus nerve, a situation which should be carefully noted by the physician [134-136].
To avoid complications, continuous monitoring of the needle tip under US is mandatory during the procedure. Hydrodissection is useful for preventing thermal injury in areas of critical structures. Horner syndrome may be caused by thermal damage to the middle cervical sympathetic ganglion (mCSG), with symptoms of ptosis, miosis, and unilateral anhidrosis of the face. Conjunctiva congestion may be an initial symptom of mCSG injury. The location of the mCSG is usually posterior to the carotid sheath and anterior to the longus colli muscle; although variations adherent to the carotid sheath have also been noted [139]. On US, mCSG presents as a spindle-shaped hypoechoic structure around the common carotid artery [140].
Nodule rupture is relatively uncommon, but is considered a major complication of RFA, with a rate of approximately 0.2%-2.5% [141-143]. Sudden neck bulging and pain are the most common symptoms, with the anterior type being the most common [144]. The mechanism could be related to, but not limited to, delayed hemorrhage or tearing of the thyroid capsule at a weak point under post-procedural massaging or movement of the neck. Most patients can be managed conservatively, but invasive treatment, including incision and drainage or surgery, may be required in cases of abscess formation [141]. A retrospective study that enrolled a total of 26 patients indicated that a ruptured nodule with an initial maximum diameter of >4.5 cm may require invasive management [145].
Hematoma is caused by mechanical or thermal injury to the vessels, which is revealed by a gradual enlargement of the hyperechoic area in or around the ablated nodules on US. The locations may include perithyroidal, subcapsular, and intranodular spaces [146]. Hematoma is usually managed effectively with direct compression of the neck for a period of 30 minutes to 2 hours [6,8], and most hematomas disappear within 1 to 2 weeks. Careful evaluation of the peri-thyroidal vessels prior to insertion and after removal of the electrode is necessary. Active bleeding during needle puncture is visible as a rapidly expanding hypoechoic or anechoic signal, and can be blocked by direct ablation at the visible bleeding point [135,146,147]. Unlike bipolar electrodes, the use of monopolar electrodes is not suggested for pregnant women or patients with electrical devices such as a pacemaker, based on considerations of insufficient safety evidence [148]. RFA is a safe modality with a low complication rate; nevertheless, physicians should be knowledgeable and vigilant of potential complications (Table 6), with detailed records taken at every follow-up.
The above statements are listed in Table 7.
Conclusion
US-guided thyroid RFA is safe and effective for patients with benign thyroid nodules and recurrent malignant lesions. The application of RFA for primary thyroid cancer remains in the developmental stage and is currently controversial; in addition, longer follow-up to evaluate the efficacy and the presence of residual/missing metastasis is required. Thyroid RFA should be performed at institutions with trained experts and the appropriate facilities, in close accordance with the comprehensive pre- and post-RFA checklist. Serial follow-up to evaluate the degree of volume reduction and subjective improvements, including the cosmetic score, and to monitor for potential complications is essential. We anticipate providing physicians with further references and advice regarding RFA in clinical practice through these guidelines, and will continue to search for the latest published articles to regularly revise the consensus.
Notes
AUTHOR CONTRIBUTION
Conceptualization: Lin WC, Chen WC, Chen HS, Wang WH. Data acquisition: Chen WC, Wang PW, Chou CK, Chou FF. Data analysis or interpretation: Wang PW, Chiang PL, Chang YH, Liu FH, Huang SC, Luo SD, Wu MS. Drafting of the manuscript: Chen WC, Chi SY, Chan YC, Chen WC. Critical revision of the manuscript: Lin WC, Cheng KL, Tseng FY, Chen ST, Wang CY. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We are grateful to our colleagues in the Division of Metabolism and Diagnostic Radiology at Kaohsiung Chang Gung Memorial Hospital for constructive discussions of the content.
References
Article information Continued
Notes
Key point
Radiofrequency ablation (RFA) is minimally invasive and effective for patients with benign and recurrent malignant thyroid lesions who are unsuitable for or refuse surgery. Thorough implementation of a pre- and post-RFA checklist accompanied by serial follow-up is crucial for treatment efficacy and patient satisfaction. The expert consensus provides critical, evidence-based, and practical recommendations regarding the application of thyroid RFA in Taiwan.