Role of Doppler US and elastography prior to biopsy to identify candidates for avoidance of surgery following neoadjuvant chemotherapy for breast cancer
Article information
Abstract
Purpose
This study aimed to evaluate the role of Doppler ultrasound (US) and elastography to identify residual breast cancer for patients showing near complete response following chemotherapy on magnetic resonance imaging (MRI).
Methods
Between September 2016 and January 2018, 40 breast cancer patients who showed near complete response (either tumor size ≤0.5 cm or lesion-to-background parenchymal signal enhancement ratio ≤1.6) on MRI following neoadjuvant chemotherapy were prospectively enrolled. After excluding seven women who did not undergo Doppler US and elastography, 33 women (median age, 49 years; range, 32 to 67 years) were analyzed. On the day of surgery, women underwent Doppler US and elastography for tumor bed prior to US-guided core needle biopsy. Histopathologic results of biopsy and surgery were evaluated. Negative predictive value (NPV) and false negative rate (FNR) of biopsy and the combined Doppler US and elastography were analyzed, respectively.
Results
After surgery, nine women had residual cancers and 24 women had pathologic complete response. The NPV and FNR of biopsy were 92% (24 of 26) and 22% (2 of 9), respectively. The NPV and FNR of combined Doppler US and elastography were 100% (14 of 14) and 0% (0 of 9), respectively. All of nine women with residual cancers had positive vascularity or elasticity. Two women with false-negative biopsy results, having 0.3 cm or 2.5 cm ductal carcinoma in situ at surgery, showed positive vascularity or elasticity.
Conclusion
Tumor bed showing positive vascularity or elasticity indicates residual breast cancer for patients showing near complete response on MRI following chemotherapy.
Key points
Doppler ultrasound and elastography showed positive finding for residual cancer in patients showing near complete response on magnetic resonance imaging. More thorough biopsy or needle localization for surgical excision is needed for patients with positive vascularity or elasticity for tumor bed to reduce false-negative biopsy results.
Introduction
Chemotherapy regimens and targeted therapies have markedly improved in the management of breast cancer. With these improvements, pathologic complete response (pCR) rates after neoadjuvant chemotherapy (NAC) have increased up to 60%–70%, particularly in triple-negative and human epidermal growth factor receptor type 2 (HER2)–positive breast cancers [1,2]. Current standard is to perform surgery of tumor bed, regardless of the degree of tumor response [3,4].
Omitting surgery for exceptional responder to NAC has the potential to decrease unnecessary surgical procedures and related complications and to improve the quality of life [3,4]. Historically, attempts of omitting surgery resulted in unacceptably high rates of loco-regional recurrence [5–7]. However, these initial trials were performed in earlier eras before state-of-the art imaging technology and image-guided biopsy, improved treatments, and our understanding of intrinsic subtypes [8]. The main obstacle for omitting surgery is that the current imaging methods cannot identify pCR accurately [9–13]. Magnetic resonance imaging (MRI) has shown higher accuracy than clinical examination, mammography or ultrasound, but it is not accurate sufficiently to replace surgery [12,13].
As an attempt to identify pCR without surgical intervention, the use of image-guided biopsy of the tumor bed has been evaluated in recent prospective studies [14–16]. The ultimate purpose of these studies is to omit breast surgery when biopsy reveals no tumor. Multiple studies demonstrated that vacuum-assisted biopsy (VAB) allowed prediction of residual cancer with variable performances of negative predictive value (NPV) ranging from 77% to 97.4% [14–17].
We previously performed a prospective feasibility trial to evaluate the diagnostic accuracy of ultrasound (US)-guided biopsy for 40 patients with near complete response based on the MRI selection criteria (either tumor size ≤0.5 cm or lesion-to-background parenchymal signal enhancement ratio [SER] ≤1.6) [16]. In the study, the false-negative rate (FNR) of biopsy was 30.8% (4 of 13) for total participants and 0% for a subgroup with at least five biopsy samples and satisfying more stringent MRI criteria [16].
As secondary analysis of the prospective trial, the purpose of this study was to evaluate the role of Doppler US and elastography to identify residual breast cancer for patients showing near complete response following chemotherapy on MRI.
Materials and Methods
Compliance with Ethical Standards
This was a secondary retrospective study of a prospective trial [16]. The prospective trial obtained approval from the institutional review board (IRB) of Seoul National University Hospital, and the written informed consents were obtained from all participants (IRB No. 1606-122-772). This retrospective study obtained a separate approval from our IRB, and the requirement for written informed consents was waived (IRB No. 1801-096-916).
Patient Enrollment
We prospectively enrolled 40 women with clinical stage II–III breast cancers who completed NAC and satisfied MRI selection criteria (either tumor size ≤0.5 cm or lesion-to-background parenchymal SER ≤1.6) between September 2016 and January 2018 [16]. Only patients with residual unifocal lesion at post-NAC MRI were included in this study. Patients with multi-focal, multi-centric, or bilateral lesions were not included. Patients with diffuse calcifications at post-NAC mammography were not included in this study. There was no specific exclusion criterion on the extent of residual calcifications. Among total 40 patients, four patients had faint calcifications less than 1 cm at tumor bed and remaining 36 patients did not have calcifications at post-NAC mammography. As this study evaluated the role of Doppler US and elastography, we excluded seven women without Doppler US and elastography and then a total of 33 women (median age, 49 years; range, 32 to 67 years) were analyzed in the present study.
During the study period, for NAC regimen, four cycles of adriamycin plus cyclophosphamide followed by four cycles of docetaxel was used for hormone receptor–positive/HER2-negative breast cancer and triple-negative breast cancer. Four cycles of adriamycin plus cyclophosphamide followed by four cycles of docetaxel plus herceptin or six cycles of docetaxel, carboplatin, Herceptin, and pertuzumab was used for HER2-positive breast cancer.
MRI Examination and MRI Selection Criteria
Patients underwent MRI before NAC and after completion of NAC. The MRI protocol using a 3T MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands) consisted of axial dynamic contrast-enhanced (DCE) three-dimensional T1-weighted spoiled gradient-echo sequence (repetition time [TR]/echo time [TE], 4.7/1.7 ms; in-plane resolution, 0.8×0.8 mm; slice thickness, 1.0 mm; field of view [FOV], 320 mm; flip angle, 10°), axial T2-weighted turbo spin-echo sequence with fat suppression (TR/TE, 1,100/125 ms; in-plane resolution, 1.0×1.0 mm; slice thickness, 1.5 mm; FOV, 320 mm), and axial diffusion-weighted sequence using a single-shot echo-planar imaging technique (TR/TE, 7,100/96 ms; in-plane resolution, 1.9×1.9 mm; slice thickness, 3.0 mm; FOV, 370 mm; and b values of 0 and 1,000 s/mm2). For DCE-MRI, one pre-contrast and five post-contrast dynamic images were obtained 90, 180, 270, 360, and 450 seconds after the administration of gadobutrol (Gadovist, Bayer Healthcare, Leverkusen, Germany) at a dose of 0.1 mmol/kg at a rate of 2 mL/s followed by a 20 mL saline flush. MRI interpretation was prospectively and independently performed by one of seven dedicated breast radiologists with 3–15 years of experiences (including S.Y.K., S.H.L., J.M.C., and N.C.). Double reading or retrospective re-review was not performed.
MRI selection criteria were defined as either tumor size ≤0.5 cm or SER ≤1.6 on DCE-MRI after completion of NAC. Tumor size was measured with consideration of all phases of DCE-MRI [18]. SER was measured on the first dynamic phase of DCE-MRI [19]. SER was defined as the signal intensity value of the residual lesion divided by the signal intensity value of the normal parenchyma of the ipsilateral breast, as previously reported [19]. SER ≤1.6 indicates the degree of enhancement of the residual lesion is 1.6 times or less than that of the normal parenchyma.
US Examination and US-Guided Biopsy
Patients underwent US examination and US-guided biopsy at the breast radiology procedure room on the day of surgery. US examination was performed using either EUB-8500 scanner with strain elastography system (Hitachi, Tokyo, Japan) or Aixplorer system with shearwave elastography system (SuperSonic Imagine, Aix en Provence, France) equipped with 6–14 or 4–15 MHz linear-array transducer. US machines were unintentionally chosen according to the availability of US machines at the time. One of seven dedicated breast radiologists with 3–15 years of experiences (including S.Y.K., S.H.L., J.M.C., and N.C.) independently performed US examination and US-guided biopsy.
For US examinations, size of tumor was measured on B-mode US. Doppler US and elastography (either strain or shearwave) were acquired, and vascularity and elasticity were qualitatively interpreted according to the Breast Imaging Reporting and Data System lexicon [20] and a practice guideline [21]. Vascularity was classified as negative (absence of internal vascularity) or positive (presence of internal vascularity). Rim vascularity present at the margin of the lesion was also considered as positive vascularity [20]. Elasticity was classified as soft (score of 1 at strain; dark blue and light blue colors at shearwave), intermediate (scores of 2–3 at strain; green and orange colors at shearwave), and hard (scores of 4–5 at strain; red colors at shearwave) [21]. For data analysis, elasticity was dichotomized as negative (soft) or positive (intermediate or hard).
After US examinations, US-guided biopsy was performed by the same radiologist. Patients were alternatively assigned to undergo either core needle biopsy using 14-gauge automated gun (Acecut, TSK Laboratory, Soja, Japan) or vacuum-assisted biopsy using 10-gauge cable-free VAB device (Mammotome Elite, Devicor Medical Products, Cincinnati, OH, USA). US-guided biopsy was performed for tumor bed. After NAC, the tumor bed was seen as a focal hypoechoic or heterogeneous echoic area at US. We targeted both center and periphery of the tumor bed. We tried to extract a clip, but the clip extraction was not mandatory. Thus, the specimen mammography was not obtained. At least five core specimens were obtained under the local anesthesia. After completion of biopsy, the biopsy cavity was immediately localized for subsequent breast-conserving surgery using a hook wire. Paired biopsy and surgical specimens underwent standard pathologic evaluation for residual disease. pCR was defined as the absence of both invasive and in situ carcinoma in the breast.
Statistical Analysis
Clinicopathologic, MRI, and US characteristics were compared between women with pCR and residual cancer using Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables. NPV of biopsy was defined as women with pCR at the surgical histopathology out of women with no tumors at the biopsy results. FNR of biopsy was defined as women with negative biopsy results out of women with residual disease. Accuracy of biopsy was defined as women with correctly classified by biopsy out of total women. Pathologic and US findings were evaluated for patients with false-negative biopsy results. To evaluate NPV and FNR of combined Doppler US and elastography, patients with positive findings at either Doppler US or elastography were deemed to have residual disease, and those with negative findings at both Doppler US and elastography were deemed to have pCR. The NPV was defined as women with pCR at the surgical histopathology out of women with negative findings at both Doppler US and elastography. The FNR was defined as women with negative findings at both Doppler US and elastography out of women with residual disease. The accuracy was defined as women with correctly classified based on combined Doppler US and elastography out of total women. All statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA), and P-value <0.05 was considered statistically significant.
Results
Patient and Tumor Characteristics
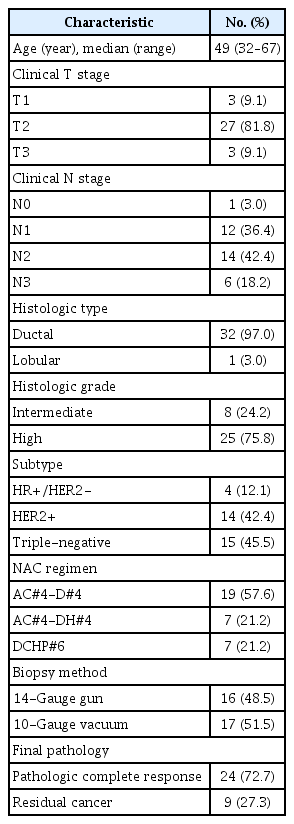
Table 1 summarizes the patient and tumor characteristics of 33 women included in this study. Clinical stage was determined by combination of physical examination and imaging findings (mammography, US, and MRI before NAC). Sixteen women underwent biopsy using 14-gauge gun and 17 women underwent biopsy using 10-gauge vacuum-assisted device. All patients underwent breast-conserving surgery.
Total 24 women (72%) had pCR in the breast defined as complete absence of both invasive and in situ carcinoma at the surgical histopathology. Nine women had residual tumor in the breast as follows: residual ductal carcinoma in situ (DCIS) in two women (0.3 cm and 2.5 cm, respectively), residual invasive ductal carcinomas in six women (median size, 0.7 cm; ranges of 0.01–1.5 cm), and residual invasive lobular carcinoma in one woman (total 6.5 cm but scattered tumor cells with very low cellularity less than 1%).
Clinicopathological and MRI Characteristics Associated with Residual Cancer
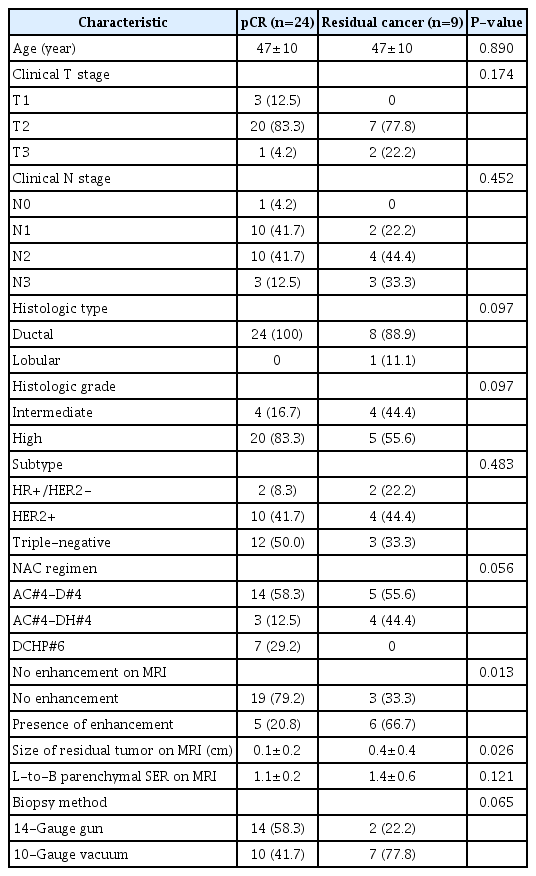
Table 2 compares the clinicopathologic and MRI characteristics between women with pCR and residual cancer. No differences were found in clinicopathological characteristics between the two groups (all P>0.05). Women with pCR more frequently had no residual enhancement in the tumor bed (79.2% [19 of 24] vs. 33.3% [3 of 9], P=0.013), and showed smaller mean tumor size on MRI (0.1±0.2 cm vs. 0.4±0.4 cm, P=0.026) than those with residual cancer.
US-Guided Biopsy in Predicting pCR
Of total 33 women, 26 had no tumor cells at the biopsy specimens and seven had tumor cells at the biopsy specimens. Of the 26 women, 24 were confirmed as pCR but two had residual cancer at the surgical histopathology. Therefore, NPV of biopsy was 92% (24 of 26), FNR of biopsy was 22% (2 of 9), and accuracy of biopsy was 94% (31 of 33). Regarding the details on two women with false-negative biopsy results, no tumor cells were found at the biopsy specimens, but residual DCIS of 0.3 cm (Fig. 1) and 2.5 cm (Fig. 2) were found at the surgical histopathology, respectively. They showed positive findings at either Doppler US (Fig. 1) or elastography (Fig. 2).
A 60-year-old woman with positive Doppler findings and false-negative biopsy results.
She had clinical T2N1 and hormone receptor–negative/human epidermal growth factor receptor type 2–positive breast cancer. After 4 cycles of adriamycin plus cyclophosphamide followed by 4 cycles of docetaxel plus herceptin, she satisfied the magnetic resonance imaging (MRI) selection criteria. She underwent ultrasonography (US)-guided 10-gauge vacuum-assisted biopsy of the tumor bed. She developed bleeding after obtaining three cores, so biopsy was stopped. Biopsy results were negative for malignancy (specifically, stromal fibrosis and foreign body reaction with a few atypical intraductal cells, favor reactive change), but final surgical pathology was ductal carcinoma in situ of 0.3 cm and high grade. A. Axial T1-weighted 5th dynamic contrast-enhanced MR image with subtraction shows 1.3 cm residual enhancement around a clip (arrow). Lesion-to-background parenchymal signal enhancement ratio was 1.2. B. B-mode US shows 1.3 cm isoechoic lesion around a clip (arrow). C. Doppler US shows prominent vessels at the inner margin of the lesion. D. Strain elastography shows even strain across entire lesion, indicating score 1 and negative elasticity.

A 58-year-old women with positive elastography findings and false-negative biopsy results.
She had clinical T2N2 and hormone receptor–negative/human epidermal growth factor receptor type 2–positive breast cancer. After 4 cycles of adriamycin plus cyclophosphamide followed by 4 cycles of docetaxel plus herceptin, she satisfied the magnetic resonance imaging (MRI) selection criteria. She underwent ultrasonography (US)-guided 10-gauge vacuum-assisted biopsy. Biopsy results were negative for malignancy (specifically, stromal fibrosis and degeneration with foreign body reaction), but final surgical pathology was ductal carcinoma in situ of 2.5 cm and intermediate grade. A. Axial T1-weighted 5th dynamic contrast-enhanced MR image shows no residual enhancement around a clip (arrow). Lesion-to-background parenchymal signal enhancement ratio was 0.8. B. B-mode US shows 1.1 cm hypoechoic mass around a clip (arrow). C. Doppler US shows no internal vascularity. D. The maximum elasticity color on the shear wave elastography was green, indicating intermediate elasticity and positive findings.
US Characteristics Associated with Residual Cancer
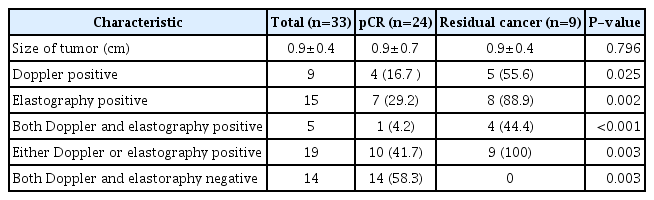
Table 3 compares the US characteristics between women with pCR and residual cancer. Women with residual cancer more frequently showed positive Doppler US (55.6% [5 of 9] vs. 16.7% [4 of 24], P=0.025) and positive elastography (88.9% [8 of 9] vs. 29.2% [7 of 24], P=0.002) than those with pCR. Forty-one point seven percentage (10 of 24) of women with pCR had positive findings on either Doppler or elastography. All of nine women with residual cancer had positive findings on either Doppler US or elastography. Fourteen women had negative findings at both Doppler US and elastography, and all of them had pCR. Nineteen women had positive findings at either Doppler US or elastography. Of the 19 women, 10 had pCR and nine had residual disease. Thus, the NPV, FNR, and accuracy of the combined Doppler US and elastography was 100% (14 of 14), 0% (0 of 9), and 70% (23 of 33), respectively.
US Characteristics According to the MRI Enhancement
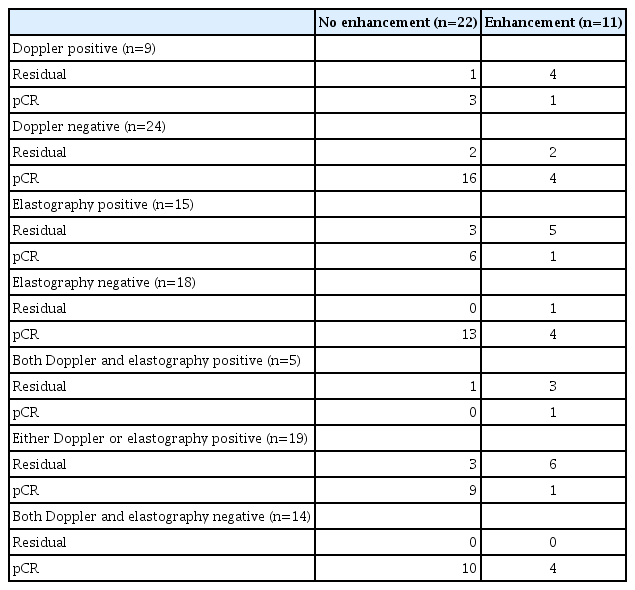
Table 4 demonstrates US characteristics according to the enhancement of MRI. Three women had false-negative MRI (i.e., no enhancement on MRI but residual disease). Of them, two had positive findings at elastography alone, and one had positive findings at both Doppler US and elastography. Five women had false-positive MRI (i.e., enhancement on MRI but pCR). Of them, four had negative findings at both Doppler US and elastography, and one had positive findings at both Doppler US and elastography.
Discussion
We evaluated the role of Doppler US and elastography to identify residual disease for patients showing near complete response on MRI. All patients with final residual disease demonstrated positive findings at either Doppler US or elastography. Furthermore, the two women with false-negative biopsy results showed positive findings at either Doppler US or elastography.
Lesion vascularity and stiffness decrease after NAC [22]. Multiple studies have reported the utility of Doppler US or elastography in differentiating pCR from residual disease [23–25]. Consistent with our study, a recent retrospective study by Nakashima et al. reported the role of Doppler US in reducing false-negative diagnoses of MRI [23]. The false-negative diagnosis of MRI was defined as presence of residual disease on surgical pathology despite absence of enhancement on MRI [23]. Seven of 27 false-negative diagnoses (26%) by MRI were correctly diagnosed as positives by Doppler US [23]. Regarding the utility of elastography, addition of elastography improved the performance of B-mode US alone in predicting pCR, and the combined performances of elastography and B-mode US were comparable with that of MRI [24,25]. Our study corroborates the role of Doppler US and elastography to further classify pCR versus residual disease in patients with near complete response identified on MRI.
Because the current MRI is not accurate enough to differentiate minimally residual cancer and pCR, US- or mammography-guided biopsy on a tumor bed is performed in the clinical trials. The clinical trials recommend to use VAB of 7–10-gauge needle and to sample 6 or more representative cores of a tumor bed [17]. To obtain representative cores, biopsy is performed focusing on the area where a marker clip is inserted, and the most clinical trials recommend to retrieve the marker clip. However, a residual cancer may be present where the clip is not, resulting in a false-negative biopsy. Therefore, it is crucial to accurately detect and target the residual cancer site when performing biopsy.
There were 10 false-positive cases, which mean either Doppler US or elastography positive but pCR. It might be due to granulation tissue containing small vessels and inflammatory cells, fibrosis, or sclerosis even with no tumor cells in the tumor bed [26]. In our study, however, as all tumor beds should have been sampled regardless of US findings, increased sensitivity might be more important than increased specificity.
It needs to be mentioned that our study only included unifocal tumors (lesions) at post-NAC MRI. Multifocal tumors (lesions) at post-NAC MRI were not enrolled in this study. Therefore, our study cannot discuss the appropriate biopsy method or enrollment criteria for multifocal lesions at post-NAC MRI.
Based on our institution’s feasibility trial, we now start a multicenter prospective trial. Women with triple-negative or HER2-positive breast cancers showing near complete response on MRI is enrolled, and 7–10 gauges VAB with at least six cores around the clipped tumor bed are obtained. Women with no evidence of tumor cells on biopsy specimens will omit breast surgery but undergo radiation therapy. During biopsy, radiologists should accurately target the area of residual cancer. Our study demonstrates that the combined Doppler US and elastography are useful for delicately detecting and localizing minimally residual cancer on the area of a tumor bed.
There were limitations in our study. First, the sample size was small. The initial study included 40 women, but seven were excluded due to absence of Doppler US and elastography. Second, patients underwent US-guided biopsy not contrast-enhanced MRI-guided biopsy. Therefore, an enhancing area at post-NAC MRI might not be biopsied during US-guided biopsy. Third, we did not analyze the impact of residual calcifications in identifying residual breast cancer. Further study will be needed to examine whether considering residual calcifications altogether with US findings may be able to better identify residual disease. Forth, we performed qualitative analysis for Doppler US and elastography. We did not analyze quantitative parameters such as number of flow signals, peak flow velocity, strain ratio, maximum or mean stiffness. Qualitative analyses applied in this study are easy and widely available for a variety of US machines but less sensitive to dynamic changes. Quantitative analyses can measure dynamic changes during NAC and are objective but are more complex and lack wide availability [27–29]. The strengths and weaknesses of each method should be considered per each study design, and we selected qualitative analysis given its ease and wide availability for a multicenter study.
In conclusion, our study found the role of Doppler US and elastography to further identify residual disease in patients who exhibited near complete response status on MRI based on our MRI selection criteria. Tumor bed showing positive vascularity or elasticity indicates residual breast cancer for patients showing near complete response on MRI following neoadjuvant chemotherapy.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the National R&D Program for Cancer Control through the National Cancer Center (NCC) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HA22C0098).
Notes
Author Contributions
Conceptualization: Kim SY, Lee HB, Han W, Cho N. Data acquisition: Kim SY, Lee SH, Chang JM, Cho N. Data analysis or interpretation: Cho N. Drafting of the manuscript: Kim SY. Critical revision of the manuscript: Kim SY, Lee HB, Han W, Lee SH, Chang JM, Cho N. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.