Ultrasound findings of subpial hemorrhage in neonates
Article information
Abstract
Purpose
Subpial hemorrhage (SPH) is a subtype of intracranial hemorrhage characterized by damage to the adjacent brain parenchyma. The aim of this study was to describe the sonographic features of SPH in neonates.
Methods
The cranial ultrasound (US) findings of neonates with SPH confirmed by brain magnetic resonance imaging (MRI) were analyzed retrospectively. Initial and follow-up US and MRI scans were reviewed by two pediatric radiologists who were blinded to both clinical history and outcomes. The US features were compared with the MRI findings.
Results
Sixteen patients were included (median gestational age, 38 weeks; range, 26 to 40 weeks; 69% term). SPH was detected most often in the temporal lobe (63%), and multiple SPHs were found in seven of 16 neonates, based on MRI. Acute SPH with an underlying venous infarct (UVI) was detected on US in 15 of 16 patients: small or large fan-shaped hyperechoic lesions (n=7 and 4, respectively) and gyriform hyperechoic lesions (n=4). The sonographic yin-yang sign was observed in three of the four large fan-shaped SPH cases. The accompanying findings on US were intraventricular hemorrhage (four out of six MRI-confirmed cases), and concurrent periventricular venous infarcts (five out of nine MRI-confirmed cases). In five patients, subpial cysts were observed on follow-up US or MRI (n=4 and n=4, respectively).
Conclusion
Acute SPH with UVI can appear as a peripheral fan-shaped or gyriform hyperechoic lesion on cranial US. SPH can be detected and suspected based on the US features of SPH with the accompanying findings.
Introduction
Subpial hemorrhage (SPH) is a subtype of intracranial hemorrhage that was not widely recognized until recently and remains incompletely understood [1-6]. SPH is not uncommon in neonates and infants [1-6], and it was estimated to account for approximately 15% of perinatal intracranial hemorrhages in a postmortem study [1]. The subpial space, which constitutes a tight anatomic space composed of collagen, is a potential space between the pia mater and the glia limitans at the outermost layer of the cortex [1,5]. Therefore, SPH occurs along the cerebral sulci in a manner similar to subarachnoid hemorrhage (SAH), but SPH tends to be thicker and more confined than SAH [2-6].
SPH is characterized by damage to the adjacent brain parenchyma [2-6]. Recent studies using magnetic resonance imaging (MRI) have reported underlying cerebral infarcts that appear to be diffusion restrictions or signal changes in the adjacent brain parenchyma of all SPHs [3-9]. Assis et al. [4] reported that SPH with underlying cerebral infarct showed a distinct MRI pattern resembling the yin-yang symbol, in which the underlying infarct had a typical lobulated configuration.
Fetal distress, birth trauma, and coagulopathy are commonly suggested risk factors of SPH in neonates and infants [1-6]. Friede assumed that severe hypoxia might be a risk factor for SPH [1]. Huang and Robertson [2] postulated that local trauma with a contusion or venous compression or occlusion might cause SPH in term neonates. In recent studies, Cain et al. [3] and Assis et al. [4] reported that SPH often had concomitant hemorrhage and the medullary vein was often engorged on brain MRI. Cain et al. [3] also hypothesized that the MRI findings of SPH with underlying parenchymal ischemia suggest a venous stroke. Khalatbari et al. [6] postulated that deep medullary vein engorgement and superficial medullary vein engorgement might share similar multifactorial risk factors. Deep medullary vein engorgement may result in periventricular hemorrhagic venous infarct and intraventricular hemorrhage (IVH) [6,7], and superficial medullary vein engorgement might result in SPH [3,6]. Although the coexistence of deep and superficial medullary vein engorgement has been reported in the same infant [3,6], the coexistence of SPH and periventricular venous infarct has not been reported.
Recent studies have reported the brain MRI findings of SPH, whereas limited research has examined the sonographic features of SPH [4,5]. It is also essential to be familiar with the ultrasound (US) features of SPH in neonates because US is a primary imaging modality for intracranial pathologic conditions in neonates. Therefore, this study aimed to describe the US findings of SPH as confirmed by MRI, including its initial features and evolution, as well as to identify the accompanying findings of SPH and determine whether these findings could be seen on US.
Materials and Methods
Compliance with Ethical Standards
This was a two-center retrospective study with institutional review board of the Inje University Haeundae Paik Hospital (2022-06-005), SMG-SNU Borame Medical Center (10-2022-68) approval and a waiver of informed consent from each participating institution.
Study Population
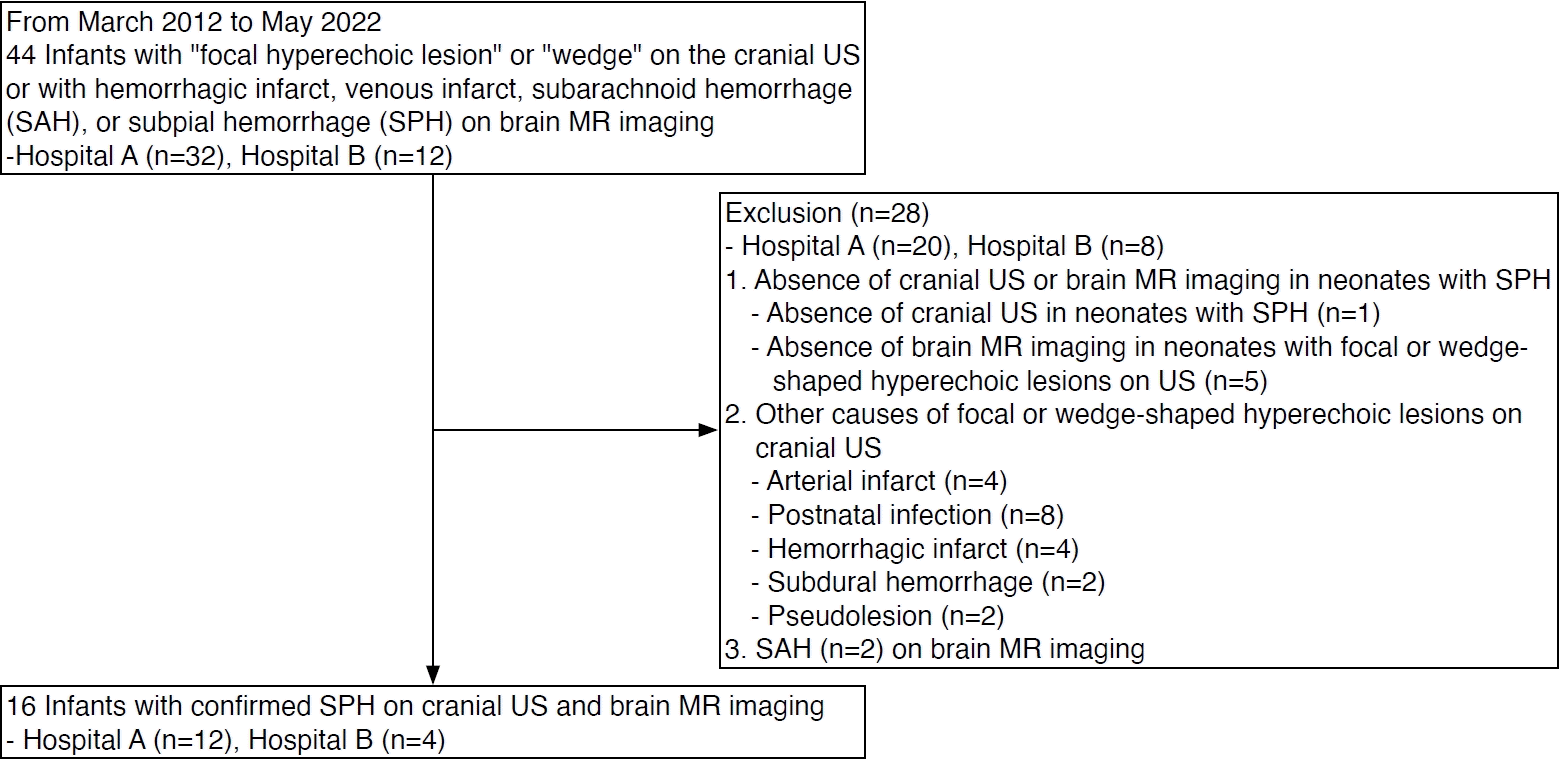
A retrospective keyword search of the institutional radiological report search system from March 2012 to May 2022 was performed. The search keywords for cranial US were "focal hyperechoic lesion" and "wedge." The search keywords for brain MRI were "hemorrhagic infarct," "venous infarct," "subarachnoid hemorrhage," "SAH," and "subpial hemorrhage." After a broad keyword search, two pediatric radiologists reviewed MRI scans to confirm the presence of SPH, defined as (1) hemorrhage closely opposing the underlying sulci, and (2) a hyperintense lesion of the cerebral cortex adjacent to the hemorrhage on T2-weighted imaging (T2WI) with or without diffusion-weighted imaging (DWI), as well as displacing or flattening the underlying parenchyma [3,8]. The T2 hyperintensity of the underlying cerebral parenchyma in close contact with SPH was defined as an underlying venous infarct (UVI). SPH was also considered to be present if a subpial cyst with hemorrhage along the cerebral sulci and signal change in the adjacent brain parenchyma were recognized. This study included neonates in whom SPH was confirmed by MRI and for whom cranial US was available. A neonate was defined as an infant less than 1 month of corrected age. The following information was collected using the standardized data sheet: patient demographics (gestational age [GA], sex, birth weight, mode of delivery), clinical presentation (initial presentation, such as clinical hypoxic-ischemic encephalopathy [HIE], clinical seizure, electrographic seizures, and apnea; Apgar score; complications of pregnancy; fetal distress; birth trauma; placenta pathology; coagulation abnormality; sepsis) and clinical outcomes (survival, neurologic deficit, developmental delay). Clinical HIE was defined as abnormal neurologic behavior following perinatal hypoxia-ischemia, including a decreased level of consciousness or tone, weak or loss of normal reflexes, and seizure. Clinical seizures are defined as the occurrence of sudden, paroxysmal, abnormal motor or autonomic behaviors.
Image Acquisition
MRI scans of the brain were obtained using different clinical scanners at two hospitals. The specific scanning parameters varied, but the same essential sequences were performed, including three-dimensional T1-weighted imaging (or sagittal and axial T1-weighted imaging in some cases), axial fast spin echo T2WI, and axial gradient echo (GRE) or susceptibility-weighted imaging (SWI) sequences. Axial DWI was performed in most of the initial scans.
Cranial US was performed using different machines at two medical centers, including the following representative models: LOGIQ S8 XDclear 2.0 (GE Healthcare, Seongnam, Korea) using 4-12 MHz microconvex and 4-15 MHz linear transducers, and Aplio i800 (Canon Medical Systems, Otawara, Japan) using 3.5-11.5 MHz sector and 2-9.5 MHz linear transducers. Standard oblique coronal and sagittal images were obtained through the anterior fontanelle in all cases. To examine the cerebellum, a supplemental view through the mastoid fontanelle was additionally used.
Image Analysis
All images were reviewed by two pediatric radiologists (13 years and 15 years of experience after the pediatric radiology fellowship, respectively) who were blinded to clinical history and outcomes to describe the US features of SPH confirmed by MRI. The accompanying findings on MRI were also evaluated, including medullary vein engorgement, IVH, germinal matrix hemorrhage (GMH), subdural hemorrhage (SDH), cerebellar hemorrhage, concurrent periventricular venous infarct (CPVI), and cerebral sinovenous thrombosis. CPVI on MRI was defined as a focal or diffuse signal change with or without diffusion restriction in the periventricular and deep white matter. CPVI is typically a hemorrhagic venous infarct with hypointensity on SWI or GRE.
The US findings were analyzed and correlated with the MRI findings by two readers who were aware of the MRI findings. The location, shape, size, and echogenicity of SPHs were evaluated on US. In coronal sonographic images, SPH that involved almost all of one lobe or a greater extent was defined as large. The accompanying findings including IVH, GMH, SDH, cerebellar hemorrhage, and CPVI were also evaluated. CPVI was considered to be detected on US if a focal or diffuse periventricular hyperechoic lesion was found in the corresponding area where it was observed on MRI. The echogenicity was considered hyperechoic when it was equal to or greater than that of the choroid plexus. Even if the echogenicity was equivocal, it was considered hyperechoic if it was focal, asymmetrical, or heterogeneous. All available follow-up US and MRI scans were also reviewed.
Results
Clinical Features
Patients’ demographics, clinical presentations, and outcomes are summarized in Table 1. A flowchart of patient selection is shown in Fig. 1. Sixteen neonates were included in this study (median GA, 38 weeks; range, 26 to 40 weeks). Most were male (n=11, 69%) and born at term gestation (n=11, 69%). Of the 16 neonates, eight were delivered vaginally.
The common presenting signs were clinical seizure (38%), clinical HIE (31%), and apnea (25%). In six neonates (38%), SPH was detected incidentally on cranial US without clinical signs. In 10 symptomatic neonates, symptoms were seen within the first day of life. In two of 16 neonates, the initial US did not show SPH, and SPH was detected on follow-up US at 5 and 77 days of age, respectively.
All neonates survived; four patients were referred to another hospital, and the clinical outcomes could therefore not be evaluated. In the other 12 patients, five showed mild neurologic defects, and one showed developmental delay at a median of 13 months (range, 4 to 93 months).
Brain MRI
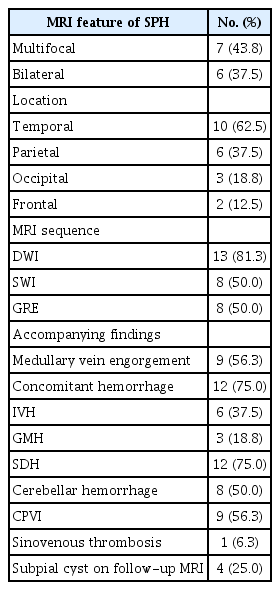
Table 2 summarizes the MRI features of SPH. Multiple SPHs were found on MRI in seven of 16 neonates (44%). SPHs were bilateral in six neonates (38%). Only one neonate had a midline shift with uncal herniation (Supplementary Fig. 1). SPH was located most often in the temporal lobe (63%). The signal change in UVI adjacent to SPH included cytotoxic edema with or without intra-axial hemorrhage (Fig. 2).
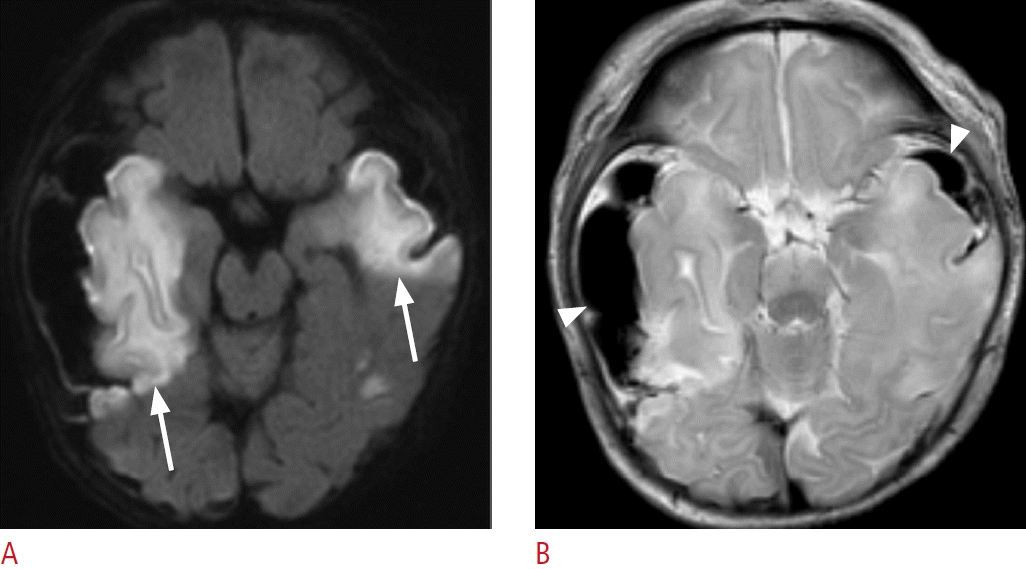
Magnetic resonance imaging of a 5-day-old female term neonate (gestational age, 40+4 weeks) with signs of birth trauma.
A. Axial diffusion-weighted imaging shows diffusion restrictions (arrows) in the bilateral temporal lobes. B. Axial T2-weighted imaging demonstrates subpial hemorrhage (arrowheads) in the bilateral temporal convexity and an underlying venous infarct without intra-axial hemorrhage in the adjacent temporal lobe.
Axial DWI was performed in 13 neonates, and the cerebral cortex adjacent to SPH showed restricted diffusion in 12 of 13 neonates. In one of these 13 neonates, SPH was initially identified as a subpial cyst. SWI was performed in eight of 16 neonates, and GRE was obtained in the remaining eight neonates. Medullary vein engorgement was observed in seven of eight neonates with SWI and in two of eight neonates with GRE. Concomitant hemorrhage was observed in 12 neonates (75%) on I: IVH (n=6), GMH (n=3), SDH (n=12), and cerebellar hemorrhage (n=8). All six neonates with IVH had small amounts of IVH (GMH-IVH grade II). GMHs were found in the ipsilateral (n=2) and contralateral (n=1) caudothalamic grooves. Most SDHs were scant or small amounts (n=11), and they were most commonly found in the posterior fossa (n=11), followed by the ipsilateral (n=8) and contralateral (n=1) cerebral convexity.
In nine of the 16 neonates, one (n=2) or multiple (n=7) CPVIs were present in the periventricular white matter, with six showing a hemorrhagic infarct and three showing a non-hemorrhagic infarct. The nine neonates with CPVI had medullary vein engorgement (n=6), IVH (n=2), and cerebral sinovenous thrombosis (n=1).
Initial Cranial US
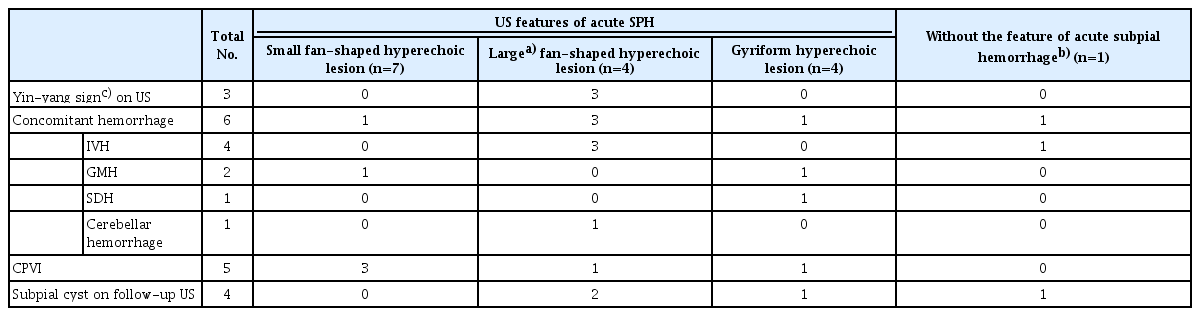
Table 3 summarizes the US imaging features of SPH. All neonates underwent cranial US before brain MRI. The initial US was performed between 0 and 5 days of life. The average time interval between US and MRI was 6.5 days (range, 0 to 41 days). In three of the 16 neonates, MRI was performed on days 15, 25, and 41, respectively, after the lesion was detected by US because they were clinically unstable.
Acute SPH was detected on US in 15 of the 16 neonates: small fan-shaped hyperechoic lesions (n=7), large fan-shaped hyperechoic lesions (n=4), and gyriform hyperechoic lesions (n=4). In the one remaining neonate, SPH was found as a subpial cyst on the eighth follow-up US at 77 days of age. Small SPHs with UVI were seen on US as small fan-shaped lesions with no distinction between intra-and extra-axial lesions (Fig. 3). In three of four patients with large fan-shaped hyperechoic lesions, hypoechoic extra-axial fluid was also observed on US (Fig. 4), thus showing a yin-yang sign [4]. When correlated with brain MRI, the hypoechoic extra-axial lesions corresponded to SPH, and the fan-shaped hyperechoic lesions corresponded to UVI with or without hemorrhage. In four neonates with gyriform hyperechoic lesions on cranial US, the SPHs were multifocal and relatively thin on MRI (Fig. 5). In the correlation with brain MRI, scant SPH was present in gyriform hyperechoic lesions, most of which were UVIs.
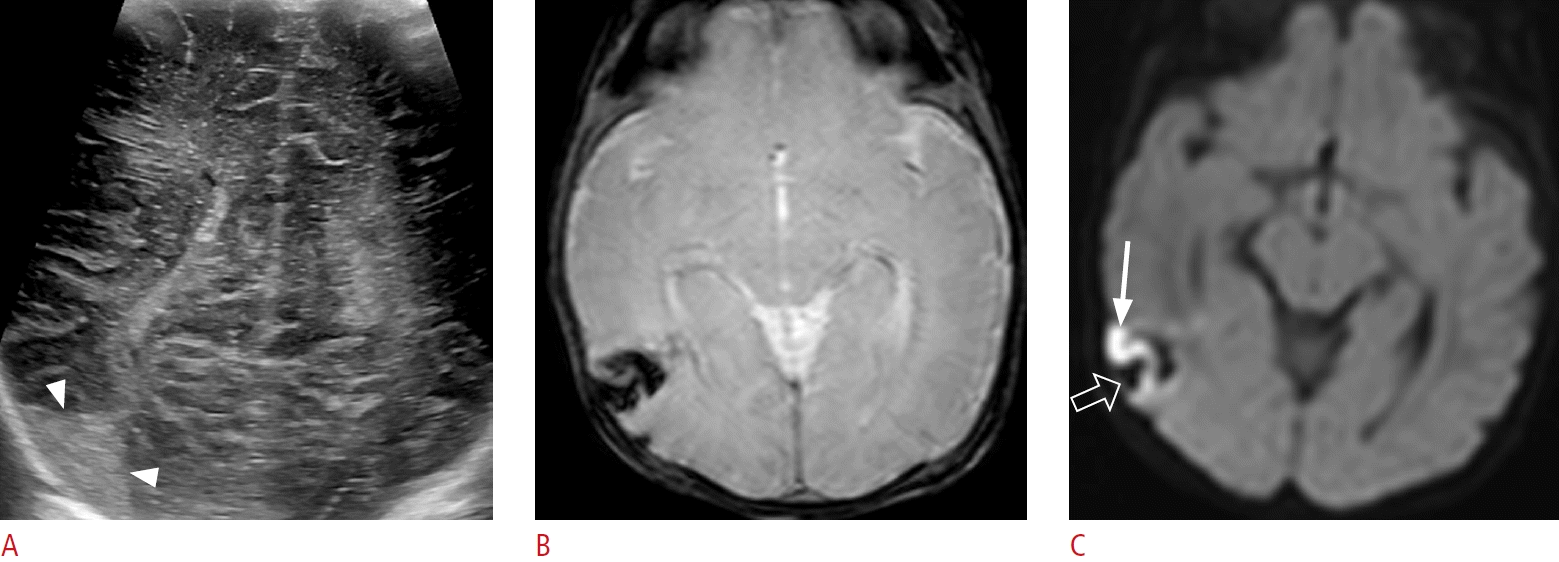
Small fan-shaped hyperechoic lesion in a 3-day-old male term infant (gestational age, 40+1 weeks; birth weight, 3,870 g; Apgar score, 8/9) without clinical signs.
A. An oblique coronal sonographic image with a high-frequency linear transducer shows a small fan-shaped hyperechoic lesion (arrowheads) in the right temporal lobe. B. An axial gradient echo sequence taken the next day shows a small fan-shaped dark signal lesion in the right temporal lobe, which is the corresponding area observed on ultrasonography. This is a combination of subpial hemorrhage and underlying hemorrhagic venous infarct. C. Axial diffusion-weighted imaging shows a small diffusion restriction in the right temporal cortex (arrow) in contact with subpial hemorrhage (open arrow), suggesting a yin-yang sign.
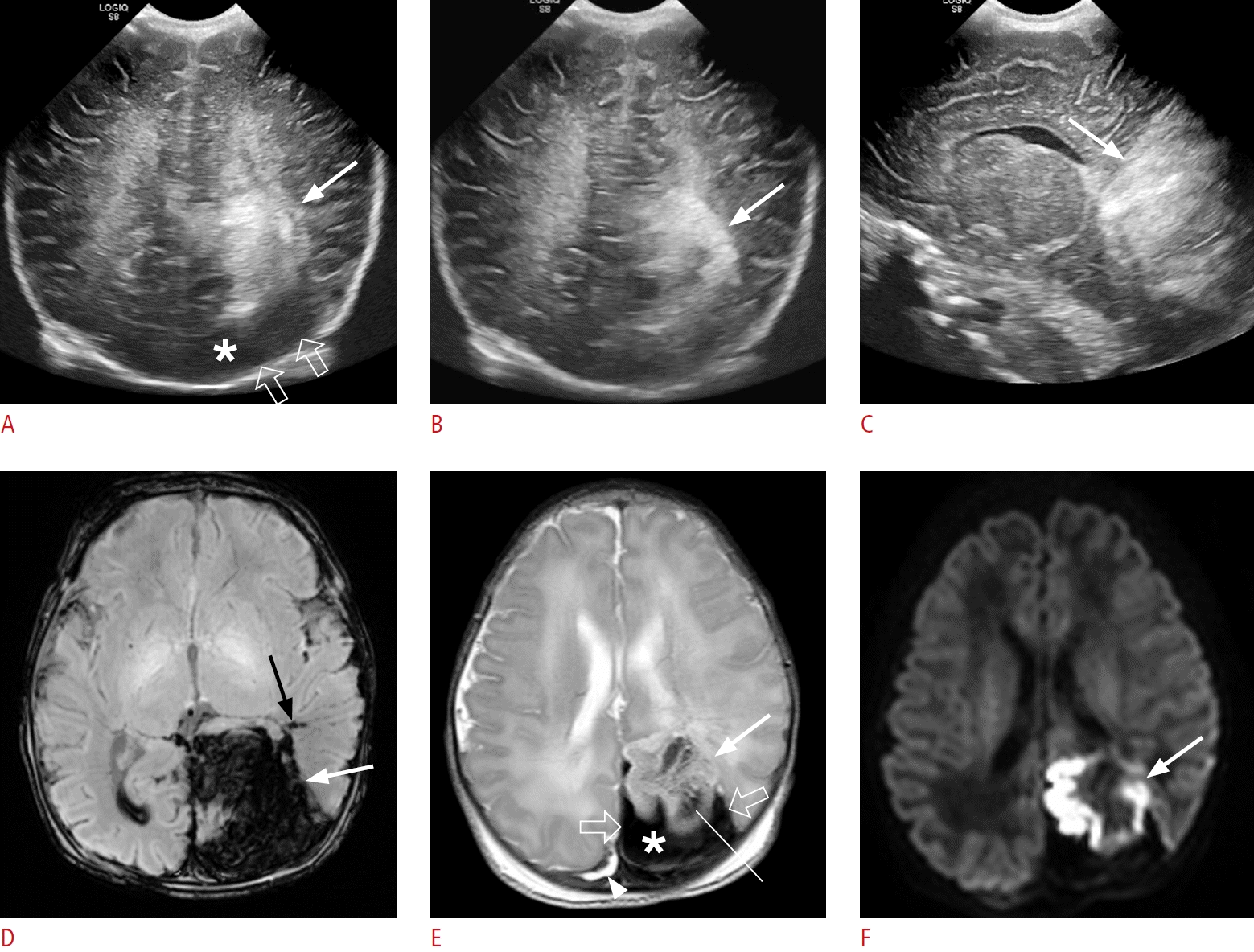
A large fan-shaped hyperechoic lesion in a 1-day-old male term infant (gestational age, 38+6 weeks; birth weight, 3,300 g; Apgar score, 8/9), presenting with apnea and preterm labor.
A-C. Oblique coronal (A, B) and sagittal (C) sonographic images show a large fan-shaped hyperechoic lesion (arrow) in the left parietooccipital lobe. Hypoechoic extra-axial fluid (open arrows and asterisk in A) is observed compared to the hyperechoic underlying venous infarct, and some echoic extra-axial fluid (B) is also observed. The sonographic yin-yang sign is considered to be present. Because subpial hemorrhage is present between the pia mater and the underlying venous infarct, hyperechoic pia mater is displaced and cerebral sulci are not visible on ultrasonography. D-F. On the same day, susceptibility-weighted imaging (D), T2-weighted imaging (E), and diffusionweighted imaging (F) show a large fan-shaped dark signal lesion in the left parietooccipital lobe (white arrow in D), which is a combination of subpial hemorrhage (open arrows and asterisk in E) appearing thicker and more confined, and a large amount of intra-axial hemorrhage in the adjacent parenchyma (arrow in E and F). Deep medullary vein engorgement is observed in the left temporal lobe (black arrow in D), and superficial medullary vein engorgement is also observed in the left parietal lobe (thin line in E). A small amount of intraventricular hemorrhage is present in the right lateral ventricle (D), and multiple small periventricular venous infarcts are present in the left temporal lobe (not shown). A small amount of subdural hemorrhage is observed along the right parietal convexity (arrowhead in E).
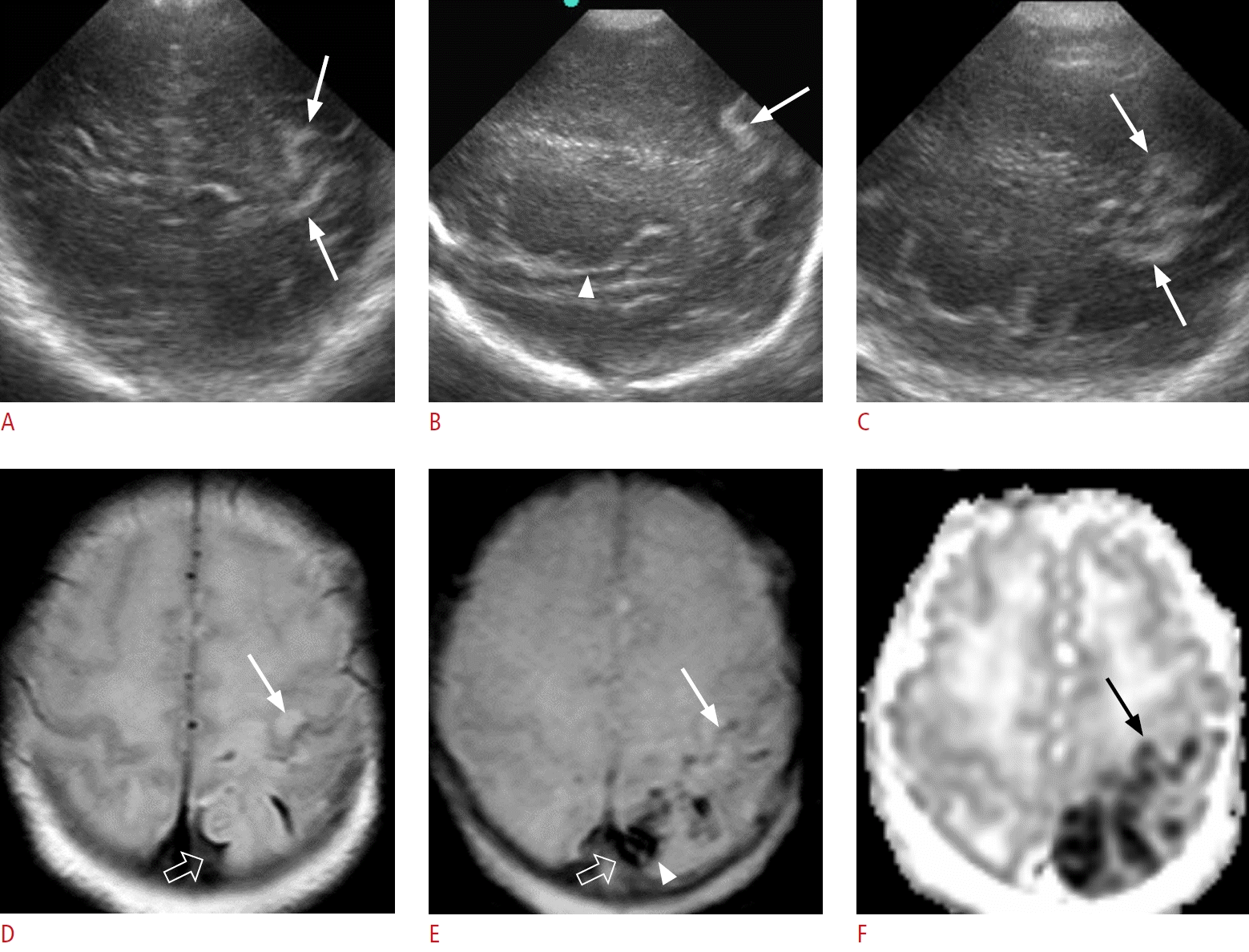
A gyriform hyperechoic lesion in a 1-day-old male term infant (gestational age, 34+6 weeks; birth weight, 2,240 g; Apgar score, 7/8), without clinical signs.
A-C. Oblique coronal (A) and left parasagittal (B, C) sonographic images show gyriform hyperechoic lesions (arrows) along the left parietal cortex, posterior to the posterior extension of the Sylvian fissure (arrowhead in B). D-F. The next day, axial T2-weighted imaging (T2WI) (D) demonstrates hyperintensity in the left parietal cortex (arrow) with multifocal thin dark signal lesions along the left parietal sulci. An axial gradient echo (GRE) sequence (E) demonstrates thin dark signals along the left central sulcus (arrow). These are scant amounts of subpial hemorrhages because an apparent diffusion coefficient (ADC) map (F) shows cortical restricted diffusion along the left precentral (black arrow) and postcentral gyri (not shown). T2WI (D) and GRE (E) sequences demonstrate another confined extra-axial hemorrhage along the left high parietal sulci (open arrow), with intra-axial hemorrhage in the adjacent parietal cortex (arrowhead in E), which suggests subpial hemorrhage with underlying hemorrhagic venous infarct. An ADC map (F) shows multifocal restricted diffusion in the left parietal cortex in close contact with the subpial hemorrhage, which can be differentiated from subarachnoid hemorrhage in terms of the presence of adjacent cortical diffusion restriction.
Concomitant hemorrhage was observed in six neonates on US: IVH (n=4), GMH (n=2), SDH (n=1), and cerebellar hemorrhage (n=1). Of the nine patients with CPVI observed on MRI, five were seen as periventricular hyperechoic lesions on US, and four of them had hemorrhage on MRI (Supplementary Fig. 2).
Follow-up US and MRI
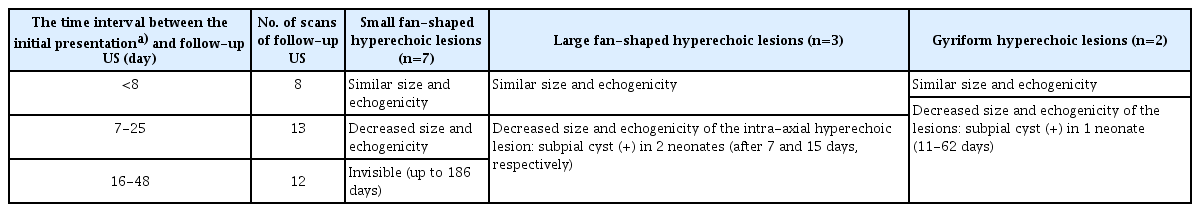
The evolution of SPH in the 12 neonates with follow-up cranial US is summarized in Table 4. In seven neonates with small fan-shaped hyperechoic lesions, there were no significant changes in the lesions on follow-up US until 8 days of age. On follow-up US after 9 days of age, the size and echogenicity of the lesions decreased, and the lesions became invisible on US after 16 days of age. In the three neonates with large fan-shaped hyperechoic lesions, the size and echogenicity of the intra-axial hyperechoic lesions decreased on follow-up US after 7 days of age, and a subpial cyst was detected in two neonates at 7 days of age (Supplementary Fig. 1C, G) and 15 days of age, respectively. There was no recurrent SPH on follow-up US or MRI.
Of the 16 neonates, two had two sessions of follow-up MRI by 15 months of age. In one neonate with a large SPH, the subpial cyst, which was observed on brain MRI at 51 days of age, disappeared with tissue loss at 14 months of age. In another neonate with a small SPH, the lesion was nearly invisible on T2WI at 15 months of age, and the extent of dark signal intensity decreased on SWI.
In five patients, subpial cysts were observed on follow-up US or MRI (median, 10.5 days; range, 7 to 15 days—except for one neonate with SPH, which was initially found as a subpial cyst). Three of them were large fan-shaped hyperechoic lesions on US.
Discussion
In this study, all SPHs observed on MRI were detected in the corresponding area on US. Acute SPH appeared as a peripheral hyperechoic lesion with cortical involvement on US. Acute hemorrhage, infarction, and even acute infection may appear as hyperechoic lesions on US [10-13]. These hyperechoic lesions can be differentiated by the affected site or shape along with the accompanying findings. Depending on the size and multifocality of SPH and the degree of hemorrhagic infarct in the adjacent brain parenchyma, it appeared as a small or large fan-shaped hyperechoic lesion or as a gyriform hyperechoic lesion. When the amount of SPH was small, it seemed to present as small fan-shaped or gyriform lesions on US. Gyriform SPH may be difficult to differentiate from SAH or acute meningitis with gyral infarct or cerebritis on US [3,6]. SAH appears as an extra-axial hyperechoic lesion on US, and it is challenging to detect a small amount of sulcal SAH [14-16]. However, SPH is characterized by adjacent brain damage [3-5], and it can appear as a peripheral intra-axial hyperechoic lesion; thus, even a small lesion can be seen on US. Additionally, a fan-shaped lesion of SPH on US can be seen as a yin-yang appearance when the lesion is large, with the extra-axial hypoechoic lesion and the intra-axial hyperechoic lesion being distinguished [4], and if the lesion is small, it can be seen as an intra-axial lesion. If there are risk factors for SPHs, such as birth trauma, if an infection is not clinically suspected, or if the lesion is located in the temporal lobe or is associated with CPVI, then the possibility of SPH should be considered for gyriform or small fan-shaped peripheral hyperechoic lesions on US. In addition, since SPH observed on US may not change until about 1 week, US should be performed within 1 week of age to detect SPH in cases in which there are risk factors for SPH. Furthermore, large fan-shaped lesions, which have more clinical significance, can be detected as subpial cysts on US even after 1 month of age.
SPH is associated with superficial and deep medullary vein engorgement or thrombosis [3,6]. The deep medullary veins have a fan-shaped branching configuration that converges into the ventricles [7,17,18]. For this reason, deep medullary vein engorgement may result in a fan-shaped periventricular hemorrhagic venous infarct [6,7,19]. Similarly, the centripetal drainage of the deep medullary veins and centrifugal drainage of the superficial intracerebral veins can explain the fan-shaped configuration of SPH with UVI on US. In addition, the fact that superficial and deep medullary vein engorgement can coexist might suggest the potential coexistence of SPH, CPVI, and concomitant hemorrhage [3,6,7]. In this study, SPH was frequently accompanied by CPVI, which had not been reported in previous studies of SPH.
Our study included a relatively large number of small SPHs that were clinically asymptomatic, but both detected on US and confirmed by MRI. Although the long-term prognosis of SPH is unknown, since it directly affects the cerebral cortex, SPH may be associated with acquired focal cortical dysplasia, local vascular disorganization, or a poor long-term prognosis [5]. Therefore, it would also be meaningful to detect small SPHs and monitor the clinical prognosis.
Previous studies have reported a high frequency of SDH in asymptomatic neonates [20-22], and a recent study has reported that SDH did not affect neurodevelopmental outcomes at 2 years [23]. Although concomitant hemorrhages were common in neonates with SPH, some cases of SDH can be coincidental and have low clinical significance. In this study, CPVI, rather than concomitant SDH, was more frequently detected on US, and these findings can be used to help diagnose SPH.
The present study has several limitations. First, the small sample size places limitations on recognizing the clinical and imaging features of SPH. Second, since this was a retrospective and multicenter study, the timing of the initial US and MRI, the imaging modality, and time interval for follow-up observation, and the MRI sequence were all inconsistent. In particular, it is possible that medullary vein engorgement was underestimated in neonates who underwent GRE rather than SWI. Of note, medullary vein engorgement was more commonly observed in neonates who underwent SWI. Third, due to the short follow-up period, a developmental assessment tool could not be used, and there were limitations in evaluating patients’ long-term prognoses. Further long-term follow-up studies will help determine the long-term clinical outcomes of neonates with SPH.
In conclusion, cranial US may be an excellent screening tool for detecting SPH in neonates and monitoring its evolution. Acute SPH with UVI can appear as a peripheral hyperechoic lesion on US—specifically as small or large fan-shaped hyperechoic lesions and gyriform hyperechoic lesions. Moreover, SPH is frequently accompanied by CPVI and IVH, which can be detected on US. It occurs most commonly in the temporal lobe, and multifocal cases are frequent. Knowledge of the US features of SPH in neonates would help radiologists in detecting lesions and making differential diagnoses.
Notes
Author Contributions
Conceptualization: Lim YJ, Chung ML, Cho YJ. Data acquisition: Lim YJ, Shin SM, Kim H, Chung ML, Hahn S, Cho YJ. Data analysis or interpretation: Lim YJ, Shin SM, Kim H. Drafting of the manuscript: Lim YJ, Kim H. Critical revision of the manuscript: Shin SM, Chung ML, Hahn S, Cho YJ. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Supplementary Material
Supplementary Fig. 1.
A 1-day-old male term infant (gestational age, 38+6 weeks; birth weight, 3,210 g; Apgar score, 2/5) with signs of asphyxia. He was treated with therapeutic hypothermia for clinical hypoxic-ischemic encephalopathy (Sarnat stage III). He was additionally diagnosed with acute coagulopathy and sepsis. (https://doi.org/10.14366/usg.22199).
Supplementary Fig. 2.
A 1-day-old male term infant (gestational age, 38+1 weeks; birth weight, 3,380 g) with signs of fetal distress. (https://doi.org/10.14366/usg.22199).
References
Article information Continued
Notes
Key point
Subpial hemorrhage (SPH) can occur along the cerebral sulci, but SPH tends to be thicker and more confined than subarachnoid hemorrhage, and it is characterized by damage to the adjacent brain parenchyma. It is known to be associated with medullary vein engorgement or thrombosis. Acute SPH with an underlying venous infarct can appear as a small or large fan-shaped hyperechoic lesion or as a gyriform hyperechoic lesion on ultrasonography (US). SPH is frequently accompanied by a concurrent periventricular venous infarct and concomitant hemorrhage, which can be detected on US. Furthermore, short-term follow-up neuroimaging can demonstrate subpial cysts in neonates with SPH, mainly in large lesions. Knowledge of US features of SPH in neonates would help radiologists detect lesions and make differential diagnoses.