Ablation therapy for patients with colorectal liver metastases with and without extrahepatic metastases: evaluation of long-term outcomes and prognostic factors
Article information
Abstract
Purpose
Ablation is a valuable treatment alternative to surgery for colorectal liver metastases. This study reports the long-term clinical outcomes in patients treated with ablation for colorectal liver metastases with or without extrahepatic metastases.
Methods
Patients with colorectal liver metastases treated with ultrasound-guided ablation at Herlev Hospital, Denmark were included in this retrospective study.
Results
This study included 284 patients with 582 metastases. Complete ablation was obtained in 258 patients (91%) evaluated within 6 weeks. During follow-up, 94 patients (33%) developed local recurrence. The median survival for all patients was 31 months, with 1-, 3-, and 5-year survival rates of 82%, 45%, and 21%, respectively. The median survival for patients with extrahepatic metastases (n=49, 17%) was 24 months compared with 33 months for patients without (P=0.142). Propensity score-adjusted Cox regression showed that extrahepatic metastases were associated with increased mortality, with a hazard ratio (HR) of 1.45 (95% confidence interval [CI], 1.02 to 2.05; P=0.039). In multivariate Cox regression analysis for all patients, increased mortality risk was found for a diameter ≥2.6 cm (HR, 1.59; 95% CI, 1.23 to 2.05), >1 metastasis (HR, 1.66; 95% CI, 1.28 to 2.16), and extrahepatic metastases (HR, 1.45; 95% CI, 1.04 to 2.03). Male sex (HR, 0.75; 95% CI, 0.58 to 0.98) and receiving chemotherapy (HR, 0.69; 95% CI, 0.52 to 0.92) were associated with decreased mortality.
Conclusion
Ablation for colorectal liver metastases offers acceptable survival rates, including for patients with extrahepatic metastases. In addition, chemotherapy was associated with improved survival for both patients with and without extrahepatic metastases.
Introduction
Worldwide, colorectal cancer is the third most common cancer, and it is the second most common cause of cancer-related mortality in both sexes [1]. In addition, up to 50% of patients develop liver metastases, leading to a poorer prognosis [2,3].
Hepatic resection of liver metastases from colorectal cancer is the gold-standard treatment for technically resectable tumors in operable patients with sufficient liver function and volume of the future liver remnant [4]. Some patients may not be amenable to surgical resection due to the patient being non-operable or the tumor being non-resectable. In addition, a minimally invasive approach is favorable in patients who may undergo subsequent procedures for recurrence. Ablation, especially microwave and radiofrequency ablation, has proven valuable as an alternative to surgery for colorectal liver metastases [5-12]. The CLOCC study, a phase II, randomized trial with nearly 10 years of follow-up, showed improved overall survival and progression-free survival with radiofrequency ablation combined with chemotherapy in patients with non-resectable colorectal liver metastases compared with chemotherapy alone [13]. Ablation has been described as a safe treatment with a significantly lower overall complication rate and shorter hospital stay compared with surgical resection [14]. The procedure itself is less invasive than resection when performed percutaneously or laparoscopically. However, the evidence regarding prognostic factors in patients with extrahepatic metastases is limited.
The aim of this study was to examine prognostic factors and report long-term clinical outcomes in patients treated with ablation for colorectal liver metastases with or without extrahepatic metastases.
Materials and Methods
Compliance with Ethical Standards
The study was approved by the Danish Health Authority (3-3013- 1311/1) and Danish Data Protection Agency (2012-58-0004). No approval was needed from the Danish National Committee on Health Research Ethics (j. nr. 15011945). The requirement for informed consent was waived since data from medical records were obtained retrospectively. The study was reported according to the checklist of Strengthening the Reporting of Observational Studies in Epidemiology [15].
Study Population
This study included all patients treated with microwave or radiofrequency ablation for colorectal liver metastases from 1995 through 2014 at Herlev Hospital, Denmark. A retrospective review of information from medical records was collected by J.K. Patients were excluded if medical records were missing or if there was no information regarding the treatment of the primary tumor or ablation therapy.
Patients were included after multidisciplinary team conference decisions. In patients with no evidence of extrahepatic spread, the procedure was intended as a curative approach with or without additional treatment such as concomitant systemic chemotherapy, hepatic arterial infusion, or external radiation. Location of the metastases in the liver or patient-related factors such as poor performance or multiple previous operations led to ablation being considered the preferred treatment instead of liver resection. The size of the largest lesions was not considered a contraindication for a curative approach. However, multiple ablations or cluster needle ablations were used in some cases. Patients could receive chemotherapy in relation to treatment of the primary tumor and/or in relation to the first ablation procedure either as neoadjuvant or adjuvant treatment, a small group of patients did not receive chemotherapy at all. Further details regarding treatment regimens and the duration of chemotherapy were not available in the medical records. For patients with extrahepatic metastases, the ablation procedure was part of a debulking strategy, and additional treatment of the extrahepatic metastases depended on the outcome of the ablation therapy. In this palliative group, ablation was indicated if it was considered to provide symptom relief or if debulking was considered clinically beneficial. The TNM classification at the time of diagnosis of the primary tumor was defined based on the medical records, operative records, and histopathological results.
Ablation Technique
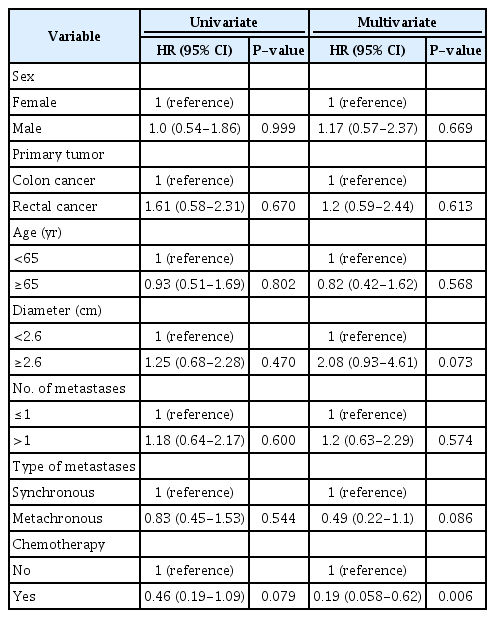
Ablation procedures were performed under general anesthesia under ultrasound guidance and performed percutaneously, laparoscopically, by open surgery, or in combination with hepatic resection. All patients included in this study were treated in a single session. The patients were admitted to the hospital the day before ablation and the procedures were performed by one of three ultrasound experts. During the procedure, microbubbles were visualized on ultrasound, which confirmed that heat had been applied and within 10 minutes after ablation, most of the microbubbles had disappeared. In addition, during radiofrequency procedures, radiofrequency noise caused image-disturbing artifacts on both B-mode and contrast-enhanced ultrasound, making it impossible to monitor the procedure while ablation was ongoing. Therefore, after the termination of active ablation, contrast-enhanced ultrasound was performed to ensure avascularity of the ablated area and, if necessary, to provide guidance for the immediate retreatment of visualized non-ablated areas with the patient still under full anesthesia (Fig. 1). In the authors’ experience, residual viable colorectal liver metastases investigated by contrastenhanced ultrasound shows enhancement and wash-out patterns. If this was seen during follow-up imaging, or if there was any doubt, an ultrasound-guided biopsy was done. Further technical details have previously been published [6]. Informed consent was obtained from all patients before the procedure. Technical effectiveness was defined as the achievement of complete ablation visualized on contrast-enhanced ultrasound 4-6 weeks after the procedure (Fig. 2). Local recurrence was defined as the appearance of new tumor foci at the ablative margin after complete ablation visualized on imaging follow-up. All patients followed the same standard 5-year follow-up regimen, which included contrast-enhanced ultrasound and a blood sample for carcinoembryonic antigen after 6 weeks. In the first 2 years after ablation, a computed tomography (CT) scan with contrast was performed every third month and followed by a test for carcinoembryonic antigen (CEA) in a blood sample, ultrasound, and contrast-enhanced ultrasound. The following 3 years, a CT scan with contrast was performed every 6 months and followed by CEA testing, ultrasound, and contrast-enhanced ultrasound.
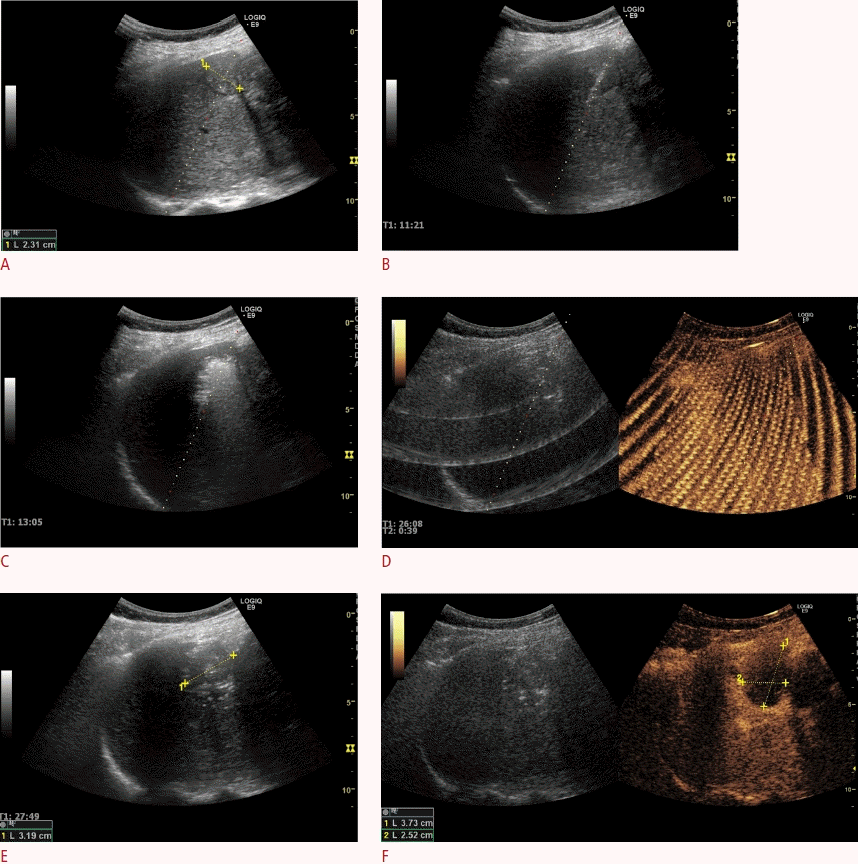
Radiofrequency ablation of a solitary colorectal liver metastasis and an examination immediately after the procedure.
A. Tumor is visualized with ultrasound before the procedure. B. Radiofrequency probe is positioned correctly under B-mode ultrasound guidance. C. During the procedure: The radiofrequency causes tissue evaporation with air-bubble artifacts. D. During the procedure: Radiofrequency noise causes image-disturbing artifacts on both B mode and contrast-enhanced ultrasound, making it impossible to monitor the procedure while ablation is ongoing. E. B-mode ultrasound immediately after the procedure cannot sufficiently evaluate viability. F. Examination of the area immediately after the procedure: With contrast-enhanced ultrasound mode, tissue vascularization and thus viability can be evaluated, thereby making it possible to judge the ablation and identify potential untreated areas, which in this case were not present.
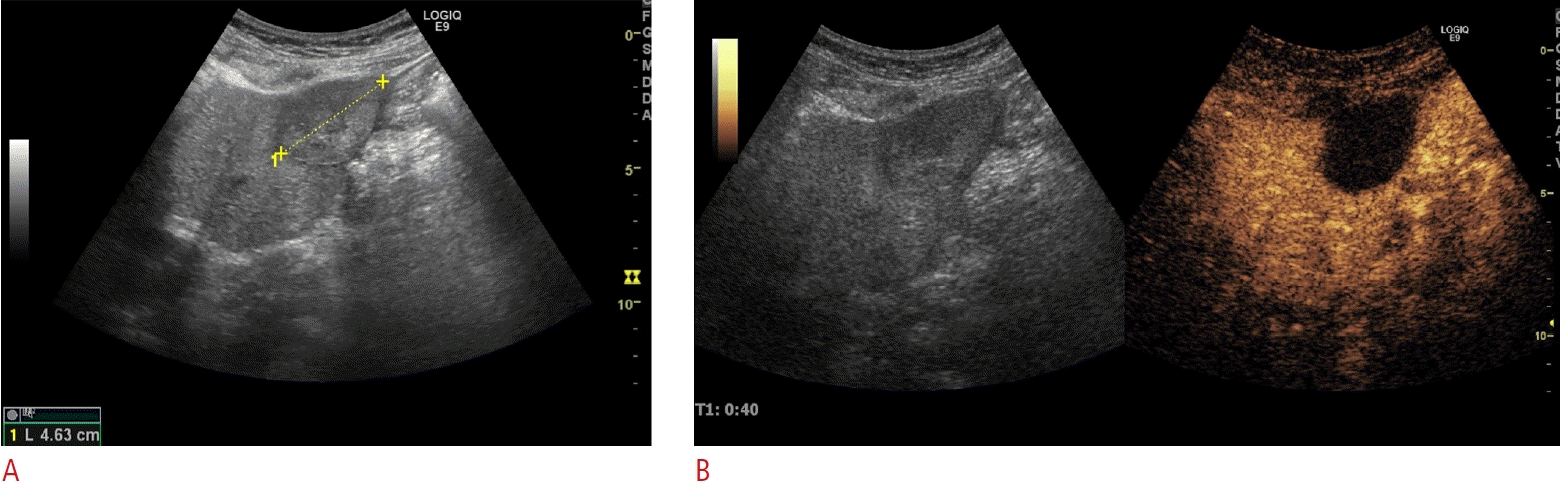
Appearance at a 6-week examination after microwave ablation of a solitary colorectal liver metastasis.
A. With conventual B-mode imaging, only the size of the lesion can be evaluated. No information regarding tissue viability can be extracted. B. In an examination using contrast-enhanced ultrasound, the ablated area shows a sharply demarcated total non-enhancing area, which indicates successful ablation without any signs of viable remaining tumor tissue.
Statistical Analyses
Overall survival was calculated from time of the ablation procedure. Survival curves were estimated by Kaplan-Meier statistics. To determine factors affecting the prognosis, the hazard ratio (HR) and 95% confidence interval (CI) for overall survival were calculated using a Cox proportional hazard regression model. Multivariate analysis for all patients was performed including variables with P<0.1 from the univariate analysis. In patients with and without extrahepatic metastases, all univariate factors were included in the multivariate analysis. To investigate the prognostic importance of extrahepatic metastases, a propensity score–matched analysis was conducted using 1:3 greedy nearest-neighbor matching. The covariates included age, sex, and chemotherapy. Subsequently, univariate Cox regression for extrahepatic metastases with robust covariance matrix estimation was conducted. For the statistical analyses, the median tumor size was used as the cut-off for defining the groups for this variable. Neoadjuvant and adjuvant chemotherapy given with ablation were combined into the same group and referred to as "chemotherapy". This variable was used in the statistical analyses. The level of statistical significance was set to P<0.05. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
This study included 284 patients with 582 liver metastases from colorectal cancer. Of these, 163 were men and 121 were women; the median age was 65 years (range, 36 to 93 years) (Table 1). In the group of patients with extrahepatic metastases, 31 were men and 18 were women; the median age was 64 years. The primary tumor was treated with surgery in 279 cases (98%), either alone or in combination with chemotherapy and/or radiotherapy. Furthermore, 195 patients (68.7%) received chemotherapy either before or after the first ablation treatment, and 36 patients (12.7%) underwent hepatic resection for liver metastases prior to the initial ablation treatment (Table 1). Eleven patients were excluded due to the absence of information in their medical records regarding the treatment of the primary tumor or due to missing medical records.
Survival Analyses for All Patients
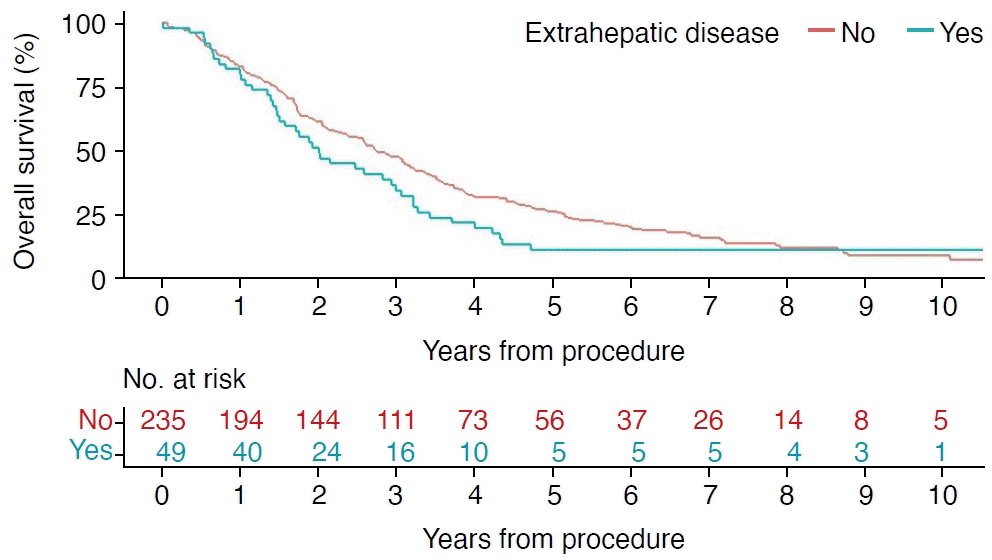
In total, 208 patients were treated with radiofrequency ablation and 76 patients with microwave ablation (Table 1). The median size of the largest metastasis ablated was 2.6 cm (range, 0.5 to 8.0 cm) and the median number of treated metastases was 1 (range, 1 to 11). The median follow-up was 31 months (range, 0.1 to 173 months). The median survival for all included patients was 31 months with 1-, 3-, and 5-year survival rates of 82%, 45%, and 21%, respectively (Fig. 3). Complete ablation was obtained in 258 patients (91%) evaluated within 6 weeks after the procedure. The 1-year local recurrence rate was 29%, and both the 3- and 5-year local recurrence rates were 36%.
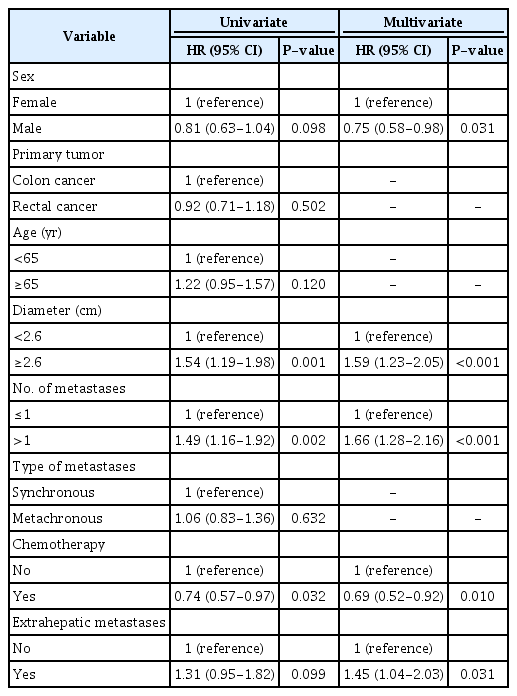
Univariate Cox regression analyses for all patients showed that a diameter ≥2.6 cm (HR, 1.54; 95% CI, 1.19 to 1.98) and >1 metastasis (HR, 1.49; 95% CI, 1.16 to 1.92) were significantly associated with increased mortality (Table 2). Receiving chemotherapy was associated with decreased mortality (HR, 0.74; 95% CI, 0.57 to 0.97). Non-significant relationships with survival were found for male sex (HR, 0.81; 95% CI, 0.63 to 1.04), the location of the primary tumor (HR, 0.92; 95% CI, 0.71 to 1.18), age ≥65 years (HR, 1.22; 95% CI, 0.95 to 1.57), time from primary tumor to metastasis (HR, 1.06; 95% CI, 0.83 to 1.36), and extrahepatic metastases was (HR, 1.31; 95% CI, 0.95 to 1.82).
In multivariate Cox regression analysis for all patients, an increased risk of mortality was associated with a diameter ≥2.6 cm (HR, 1.59; 95% CI, 1.23 to 2.05), >1 metastasis (HR, 1.66; 95% CI, 1.28 to 2.16), and extrahepatic metastases (HR, 1.45; 95% CI, 1.04 to 2.03). Male sex (HR, 0.75; 95% CI, 0.58 to 0.98) and receiving chemotherapy (HR, 0.69; 95% CI, 0.52 to 0.92) were associated with decreased mortality. When comparing the data for women versus men, no significant differences were found for age, the diameter of the largest metastasis, the number of metastases, chemotherapy, or extrahepatic metastases (Pearson chi-square).
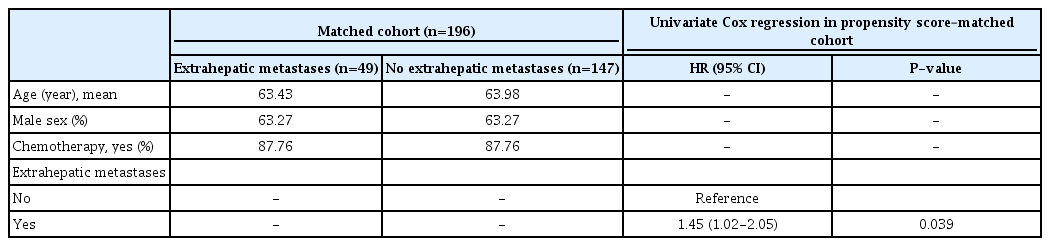
Furthermore, a propensity score–matched analysis was performed at a ratio of 1:3 including the covariates age, sex, and chemotherapy (Table 3). The propensity score-adjusted Cox regression analysis showed that having extrahepatic metastases was associated with an increased risk of mortality (HR, 1.45; 95% CI, 1.02 to 2.05; P=0.039).
Survival Analyses for Patients without Extrahepatic Metastases
Among patients without extrahepatic metastases, the median size of the largest metastasis ablated was 2.6 cm (range, 0.5 to 8 cm) and the median number of treated metastases was 1 (range, 1 to 11). The median survival was 33 months (Fig. 3).
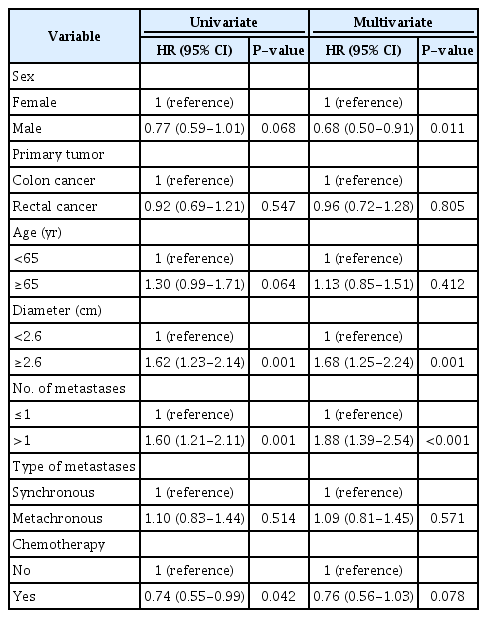
In the Cox regression univariate analyses, a diameter ≥2.6 cm (HR, 1.62; 95% CI, 1.23 to 2.14) and >1 metastasis (HR, 1.60; 95% CI, 1.21 to 2.11) were significantly associated with increased mortality (Table 4). Furthermore, chemotherapy (HR, 0.74; 95% CI, 0.55 to 0.99) was associated with decreased mortality. The multivariate analyses showed that male sex was associated with a decreased risk (HR, 0.68; 95% CI, 0.50 to 0.91), while an increased risk of mortality was associated with a diameter ≥2.6 cm (HR, 1.68; 95% CI, 1.25 to 2.24) and >1 metastasis (HR, 1.88; 95% CI, 1.39 to 2.54).
Survival Analyses for Patients with Extrahepatic Metastases
At the time of the first ablation treatment, 49 of 284 patients (17%) had extrahepatic metastases, with the lungs (24 [8.5%]) and lymph nodes (7 [2.5%]) being the most frequent sites (Supplementary Table 1) and 25 patients had a simultaneous diagnosis of another cancer (Supplementary Table 2). Among patients with extrahepatic metastases, the median size of the largest metastasis ablated was 2.5 cm (range, 1.0 to 5.5 cm) and the median number of treated metastases was 1 (range, 1 to 7). The median survival for patients with extrahepatic metastases was 24 months, compared with 33 months for patients without extrahepatic metastases (log-rank P=0.142) (Fig. 3).
In Cox regression analyses in patients with extrahepatic metastases, chemotherapy was associated with decreased mortality in the multivariate analysis (HR, 0.19; 95% CI, 0.06 to 0.62) (Table 5). However, very few patients did not receive chemotherapy (n=6). None of the other factors were significantly associated with mortality in univariate or multivariate Cox regression analyses.
Complications
Details on complications related to the procedure are reported in Supplementary Table 3. Most of the procedures (71%) did not lead to complications. The most common complication was post-ablation syndrome, and 22 patients were hospitalized with this complication. Of the 284 patients in this cohort, three patients (1%) died shortly after ablation due to complications, one because of thermal damage to the colon and small intestine and two because of multiorgan failure.
Discussion
In this cohort of patients with liver metastases from colorectal cancer, including patients with multiple metastases and extrahepatic metastases, the median survival was 31 months and the 3-year survival rate was 45% for all patients. No significant difference was found in median survival between patients with (24 months) and without extrahepatic metastases (33 months) (P=0.142). However, a propensity score-adjusted Cox regression analysis did find significantly higher risk of mortality for patients with extrahepatic metastases than for those without. In addition, a multivariate Cox regression for all included patients showed that female sex, a diameter of the largest metastasis ≥2.6 cm, the presence of multiple metastases, not receiving chemotherapy, and having extrahepatic metastases were all associated with increased mortality. No significant results were found that could explain the differences in survival between men and women regarding age, the diameter of the largest metastasis, the number of metastases, chemotherapy, or extrahepatic metastases (Pearson chi-square test). Thus, the data suggested a better prognosis for men that cannot be explained by the included confounding variables.
This article presents the results of a single-center study with a long follow-up. The 3- and 5-year survival rates of 45% and 21%, respectively, are comparable to the reported 3- and 5-year survival rates after hepatic resection of 43%-67% and 30%-58%, respectively [16,17]. The 3- and 5-year survival rates reported in other studies concerning ablation were 26%-100% [3,18,19]. However, this study included patients with extrahepatic metastases and multiple and/or large metastases, which might explain the difference. The optimal patient for ablation has a single or few small metastases and disease limited to the liver. However, this study contributes evidence that patients with limited and/or controllable extrahepatic metastases may benefit from ablation, although less than patients without extrahepatic metastases, as found in other studies [20-22]. The propensity score–matched did find a higher risk of mortality for patients with extrahepatic metastases than for those without. Other studies have also found lower overall survival in patients with extrahepatic disease than in patients without [20,22,23]. Nonetheless, a study found that patients with extrahepatic disease limited to the lungs had a longer median overall survival than patients with more than one site of extrahepatic disease (35 months vs. 14 months, P=0.003) [22]. These results may reflect that patients with extrahepatic disease undergoing ablation in the liver represent a selected population with favorable tumor biology. Furthermore, a meta-analysis found a 30-day mortality rate of 0.4% in the RFA group and 1% in the resection group (P=0.32) [14]. The 30-day mortality rate in this cohort of 1% may reflect the inclusion of patients with advanced disease.
The strengths of the present study include a long follow-up and a non-selected study population. Moreover, limited data have previously been published on ablation for patients with extrahepatic metastases. Some potential limitations should be mentioned. Firstly, this study included all patients referred for ablation with both curative as well as palliative intent. Additionally, including patients with and without extrahepatic metastases made the study population rather heterogeneous. However, the results for the two groups were reported separately. Secondly, considering the long period of inclusion, there have inevitably been improvements in technique and a gradual change in indications for ablation that could have impacted the comparison. Thirdly, it was beyond the scope of this study to describe the chemotherapy regimens, although changes have occurred over the years in systemic treatments as well. Therefore, not all patients were treated with the same chemotherapy. Chemotherapy was a prognostic factor for all patients and for patients with extrahepatic metastases. However, the results may have been affected by the small number of patients in the latter group who did not receive chemotherapy (Table 1).
This study differs from comparable studies through its long follow-up and limited exclusion criteria. Patients with extrahepatic metastases have limited treatment options. If the liver metastases are left untreated, the prognosis is poor, with a median survival of less than 12 months, and a 5-year survival of less than 6% [3,24-27]. This study underscores that ablation is a valuable treatment option for patients with extrahepatic metastases. Furthermore, the 30-day mortality rate found herein for the entire group of patients is comparable with those after hepatic resection. In addition, another study reported comparable survival between ablation and resection for solitary metastases <3 cm [7].
In conclusion, ablation for colorectal liver metastases is a valuable treatment with acceptable survival rates, including for patients with extrahepatic metastases who are left with few or no other surgical treatment options. In addition, a combination of ablation therapy and chemotherapy given in combination with ablation (neoadjuvant or adjuvant) was associated with improved survival for patients with CRLM.
Notes
AUTHOR CONTRIBUTION
Conceptualization: Klubien J, Rosenberg J, Skjoldbye BO, Nolsøe CP, Pommergaard HCL. Data acquisition: Klubien J, Rosenberg J, Skjoldbye BO, Lorentzen T, Nolsøe CP, Pommergaard HCL. Data analysis or interpretation: Klubien J, Rosenberg J, Skjoldbye BO, Lorentzen T, Nolsøe CP, Pommergaard HCL. Drafting of the manuscript: Klubien J, Rosenberg J, Skjoldbye BO, Lorentzen T, Nolsøe CP, Pommergaard HCL. Critical revision of the manuscript: Klubien J, Rosenberg J, Skjoldbye BO, Lorentzen T, Pommergaard HCL. Approval of the final version of the manuscript: all authors.
Christian Pállson Nolsøe serves as Editor for the Ultrasonography, but has no role in the decision to publish this article. All remaining authors have declared no conflicts of interest.
Supplementary Material
Supplementary Table 1.
Extrahepatic disease at time of ablation (https://doi.org/10.14366/usg.22208).
Supplementary Table 2.
Other cancers at time of ablation (https://doi.org/10.14366/usg.22208).
Supplementary Table 3.
Complications following ablation (https://doi.org/10.14366/usg.22208).
References
Article information Continued
Notes
Key point
Ultrasound-guided ablation is a valuable treatment option for patients with colorectal cancer with extrahepatic metastases who have limited other treatment options.