Modified CEUS LI-RADS using Sonazoid for the diagnosis of hepatocellular carcinoma
Article information
Abstract
This review outlines several modified versions of the contrast-enhanced ultrasonography Liver Imaging Reporting and Data System (CEUS LI-RADS) that utilize Sonazoid. Furthermore, it discusses the advantages and challenges of diagnosing hepatocellular carcinoma using these guidelines, as well as the authors’ expectations and opinions regarding the next CEUS LI-RADS version. It is possible that Sonazoid could be incorporated into the next version of CEUS LI-RADS.
Introduction
Hepatocellular carcinoma (HCC) is the only type of cancer that can be non-invasively diagnosed in high-risk patients based on typical imaging features on computed tomography (CT), magnetic resonance imaging (MRI) [1], or contrast-enhanced ultrasonography (CEUS) [2-4]. Various guidelines for diagnostic evaluation are presented in Table 1. Among the imaging modalities, CEUS holds unique advantages over CT and MRI, as it provides pure vascular and real-time dynamic images along with excellent safety for patients with impaired renal function or allergies to iodine or gadolinium [5].
The American College of Radiology (ACR) recently developed the CEUS Liver Imaging Reporting and Data System (CEUS LI-RADS) to enhance the diagnostic accuracy of HCC and to streamline communication among radiologists and between radiologists and other physicians. This standardized reporting system is designed for liver nodules in patients at risk for HCC [6].
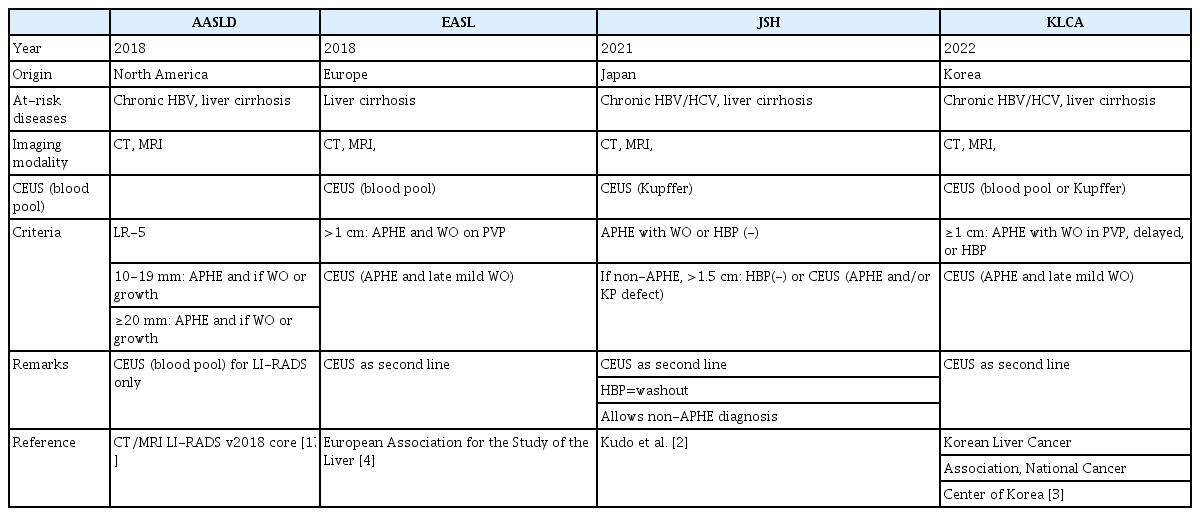
Unfortunately, although the diagnostic performance of contrast agents for HCC is nearly identical [7], the current version of CEUS LI-RADS (version 2017) can be applied only to examinations using so-called pure blood pool contrast agents, not to those using Kupffer cell contrast agents. To overcome this obstacle, several modified versions of the CEUS LI-RADS for diagnosing HCC using Kupffer cell agents or Sonazoid have been proposed and evaluated [8-11].
This review provides an overview of these versions, their advantages and pitfalls in diagnosing HCC, and the authors’ opinions and expectations for the next version of the CEUS LI-RADS.
Ultrasound Contrast Agents for Liver
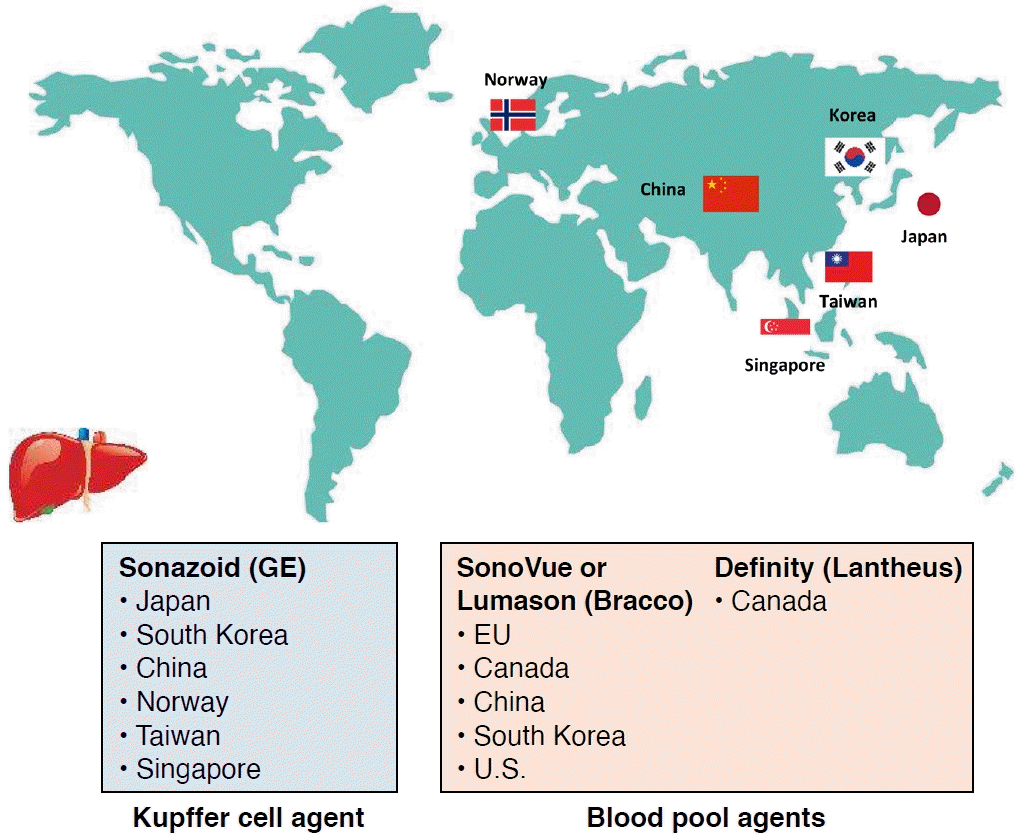
SonoVue has been widely used as an ultrasound (US) contrast agent for the liver in European and Asian countries. It is also used in the United States (under the brand name Lumason) and Canada (under the name Definity). Sonazoid is utilized in Norway and various Asian countries, such as Japan, South Korea, Taiwan, China, and Singapore. The current approval status of US contrast agents for the liver is shown in Fig. 1. Although classified as a second-generation contrast agent, Sonazoid differs from other contrast agents in that it is phagocytosed and incorporated into Kupffer and/or reticuloendothelial cells [12]. This property allows Sonazoid to be retained in the normal liver parenchyma for over 120 minutes [13], enabling both vascular and Kupffer phase (KP) imaging [14]. As a result, Sonazoid is referred to as a Kupffer cell agent. In contrast, SonoVue and Definity are less likely to be phagocytosed and incorporated by Kupffer and/or reticuloendothelial cells [12]. These agents tend to remain in the blood pool and are therefore considered pure blood pool agents.
SonoVue versus Sonazoid
Few studies have directly compared SonoVue and Sonazoid, as both contrast agents are available in a limited number of countries. Only two studies were identified that reported a direct comparison between the two contrast agents in the same individuals on the same days, both of which were carried out by the same group of authors [15,16].
The first study [15] was a preliminary report involving 59 observations in 59 patients with HCC (n=43), non-HCC malignancies (n=10), and benignancies (n=6). The other study was an extended report of 105 observations in 105 patients with HCC (n=61), non-HCC malignancies (n=19), and benignancies (n=25). The first study indicated that when utilizing a 5-minute washout criterion, the diagnostic performance of Sonazoid-enhanced US in diagnosing HCC was superior to that of SonoVue-enhanced US without compromising specificity. This was primarily because late and mild washouts were more frequent with Sonazoid-enhanced US than with SonoVue-enhanced US. In the other study [16], the authors concluded that when the 5-minute washout criterion was employed, the accuracy of Sonazoid-enhanced US (72.4%; 95% confidence interval [CI], 64.1% to 79.3%) was marginally better than that of SonoVue-enhanced US (71.4%; 95% CI, 63.1% to 78.6%). However, this difference was not statistically significant. Interestingly, the Sonazoid-enhanced US 5-minute criterion was found to be slightly more accurate than the 10-minute criterion (KP).
CEUS LI-RADS
The proposal of different imaging diagnostic criteria has the potential to cause confusion among radiologists, hepatologists, and surgeons who manage patients at risk of HCC. To address this issue, the ACR developed the Liver Imaging Reporting and Data System (LI-RADS) for CT or MRI in patients at risk of HCC in 2011, then updated it to a modified version in 2018 [17]. In addition, the ACR established the CEUS LI-RADS in 2016 and further revised it in 2017 [18]. Both the CT/MRI and the CEUS LI-RADS classify hepatic observations in high-risk patients based on the probability of HCC, ranging from LR-1 (definitely benign) to LR-5 (definitely HCC). Observations that are likely or definitely malignant but not specific to HCC are classified as LR-M. The purpose of the LR-5 category is to provide high specificity for HCC, so that observations classified as LR-5 can be considered representative of HCC for making treatment decisions without requiring pathologic confirmation. The CT/MRI LI-RADS has already been incorporated into the American Association for the Study of Liver Disease (AASLD) guidelines [1]. However, the CEUS LI-RADS is not currently available within those guidelines.
Modified CEUS LI-RADS Using Sonazoid
Although the CEUS LI-RADS may be useful, its current edition (version 2017) is applicable only to pure blood pool contrast agents. To address this limitation, several modified versions of the CEUS LI-RADS utilizing Sonazoid have been published for clinicians using this contrast agent in daily practice (Table 2) [8-11].
Sugimoto et al. [8] were the first to propose a simple modified CEUS LI-RADS, which primarily differed from the CEUS LI-RADS (version 2017) in that the former incorporated portal- and late-phase washouts as major imaging features, while the latter emphasized KP findings as the main imaging feature. The other criteria in the modified CEUS LI-RADS were largely similar to those in the CEUS LI-RADS (version 2017). Sugimoto et al. assessed the diagnostic performance of the modified CEUS LI-RADS in identifying HCC in patients at risk for the disease. However, that study lacked a control arm with which to compare the performance of the modified CEUS LI-RADS. The authors reported that under the modified CEUS LI-RADS with Sonazoid, LR-5 was a good predictor of HCC. The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the modified CEUS LI-RADS in diagnosing LR-5 for HCC were 70.3% (95% CI, 57.6% to 81.1%), 92.5% (95% CI, 79.6% to 98.4%), 83.8% (95% CI, 82.8% to 98.7%), 66.1% (95% CI, 52.2% to 78.2%), and 78.7% (95% CI, 69.7% to 86.2%), respectively.
Takahashi et al. [9] compared the diagnostic performance of Sonazoid-enhanced US for noninvasive diagnosis of HCC between the CEUS LI-RADS (version 2017) with a 5-minute washout criterion and the modified CEUS LI-RADS with a 10-minute criterion (i.e., KP) in the same patients at risk for HCC. Early washout (<60 seconds) was observed in 17.3% of HCCs (9 of 52). When the 5-minute criterion was used, 73.1% of HCCs (38 of 52) showed mild washout. In contrast, when using the 10-minute criterion or during the KP, 90.4% (47 of 52) of the HCCs exhibited hypoenhancement. Representative images are shown in Fig. 2. Accordingly, the washout ratio increased with time. However, five HCCs did not show washout for 10 minutes. Based on these findings, the sensitivity of the modified CEUS criteria (63.7%; 35 of 52; 95% CI, 52.9% to 79.7%) was determined to be higher than that of the CEUS LI-RADS criteria (51.9%; 27 of 52; 95% CI, 37.6% to 66.0%) (P=0.0047). The specificity was similar for both criteria (92.0%; 46 of 50; 95% CI, 80.8% to 97.8%). Additionally, the accuracy of the modified CEUS LI-RADS criteria (79.4%; 81 of 102; 95% CI, 70.3% to 86.8%) was found to be higher than that of the CEUS LI-RADS criteria (71.6%; 73 of 102; 95% CI, 61.8% to 80.1%) (P=0.0047). The results are summarized in Table 3. Therefore, the authors concluded that the modified CEUS LI-RADS was more useful than the original CEUS LI-RADS (version 2017) for diagnosing HCC.
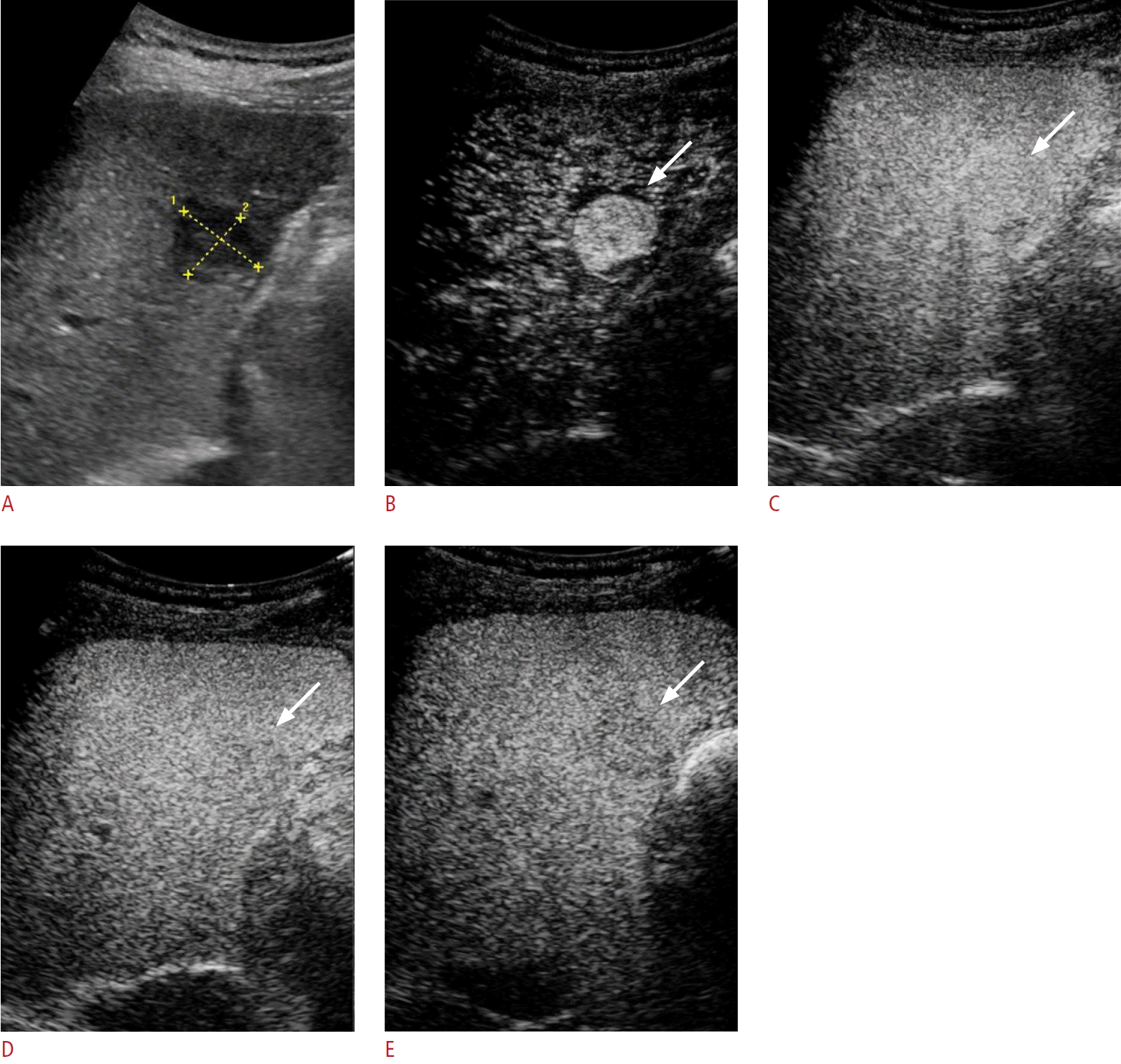
A 64- y ear-old man wi th pathologically confirmed moderately differentiated hepatocellular carcinoma in segment 6 of the liver.
A. A 2.0-cm hypoechoic nodule is visible (yellow calipers) on B-mode ultrasonography (US). B-D. On Sonazoid-enhanced US, the nodule exhibits arterial phase hyperenhancement (B, arrow) and does not demonstrate washout until 1 (C, arrow) and 5 minutes (D, arrow) after contrastmedium injection. E. The nodule exhibits mild hypoenhancement in the Kupffer phase (arrow).
Hwang et al. [10] also proposed a modified CEUS LI-RADS, termed the arterial phase hyperenhancement (APHE) and Kupffer (AK) criteria, which was similar to the one proposed by Sugimoto et al. [8]. They compared the diagnostic performance of the CEUS LI-RADS (version 2017) criteria, which use only vascular phase information such as the arterial phase (10-40 seconds after contrast injection), the portal venous phase (60-90 seconds after contrast injection), and the late phase (3-4 minutes after contrast injection), with the AK criteria for diagnosing HCC in the same at-risk patients. The AK criteria (89.6%; 95% CI, 80.6% to 95.4%) demonstrated higher sensitivity than the LI-RADS criteria (81.8%; 95% CI, 71.4% to 89.7%; P=0.041) (Table 4). However, the specificity was 84.8% (95% CI, 71.1% to 93.7%) for both methods. Six additional lesions were diagnosed as HCC when the KP defect was applied instead of late and mild washout. They included a grayscale image feature, which was unlike HCC (i.e., an ill-defined margin without a hypoechoic halo), in both criteria to enhance their diagnostic performance. Although the performance of both criteria was improved, no significant difference was observed in their diagnostic performance before and after adjustment.
Li et al. [11] proposed a modified CEUS LI-RADS that differed slightly from the two previous proposed versions. Their modified algorithm incorporated two changes compared to the CEUS LI-RADS (version 2017). First, observations measuring at least 10 mm with no rim APHE and no washout were classified as LR-5 if they exhibited a KP defect, which was consistent with the prior two versions. Second, measurements of at least 10 mm with no rim APHE, early washout, and a mild KP defect were classified as LR-5 instead of LR-M, representing a unique new classification. The diagnostic performance of the two different LR-5 criteria for predicting HCC is displayed in Table 5. When using CEUS LI-RADS (version 2017), LR-5 demonstrated a sensitivity of 69%, a specificity of 87%, and an accuracy of 71% in predicting HCC. Incorporating the first modification to upgrade observations with no rim APHE, no washout, and a KP defect from LR-4 to LR-5 led to a significant increase in sensitivity (81%, P<0.001), a non-significant decrease in specificity (83%, P>0.99), and a significant increase in accuracy (82%, P<0.001). Including both the first and second modifications to reclassify observations with no rim APHE, early washout, and a mild KP defect from LR-M to LR-5 resulted in a further increase in sensitivity (89%, P<0.001), no change in specificity (remaining at 83%), and an increase in accuracy (89%, P<0.001).
Although those studies suggested that KP hypoenhancement is useful for accurately diagnosing HCC, Kang et al. [19] explored the optimal washout criteria of CEUS using Sonazoid for HCC diagnosis and found that extending the washout time window beyond 6 minutes did not offer additional benefits to the diagnostic performance for HCC. This is attributed to the pseudo-washout effect, particularly for liver hemangiomas. This finding contrasts with aforementioned studies that demonstrated increased sensitivity for HCC diagnosis when KP hypoenhancement was considered a washout [9-11]. Therefore, although the KP may be valuable for diagnosing HCC, further research on the appropriate time window for the KP and its role in HCC diagnosis appears to be necessary.
Pitfalls and Challenges of Modified CEUS LI-RADS
HCCs Exhibiting Isoenhancement during the KP
Takahashi et al. [9] reported that among all HCCs, five (9.6%) displayed isoenhancement in the KP, resulting in their classification as LR-4. The researchers examined the relationship between KP enhancement and grayscale features, finding that all HCC nodules with isoenhancement in the KP appeared hyperechoic on grayscale US images. Consequently, hyperechoic lesions may not be visible as defects during the KP in moderate mechanical-index (MI) (i.e., MI values ranging from 0.1-0.2) contrast imaging mode due to the persistence of tissue harmonic signals. To address this issue and improve the diagnostic accuracy of HCC, high-MI contrast imaging using the so-called loss of correlation method [20] may be more beneficial, as it demonstrates greater sensitivity compared to low-and medium-MI contrast imaging in detecting the presence or absence of microbubbles in HCC nodules (Fig. 3).
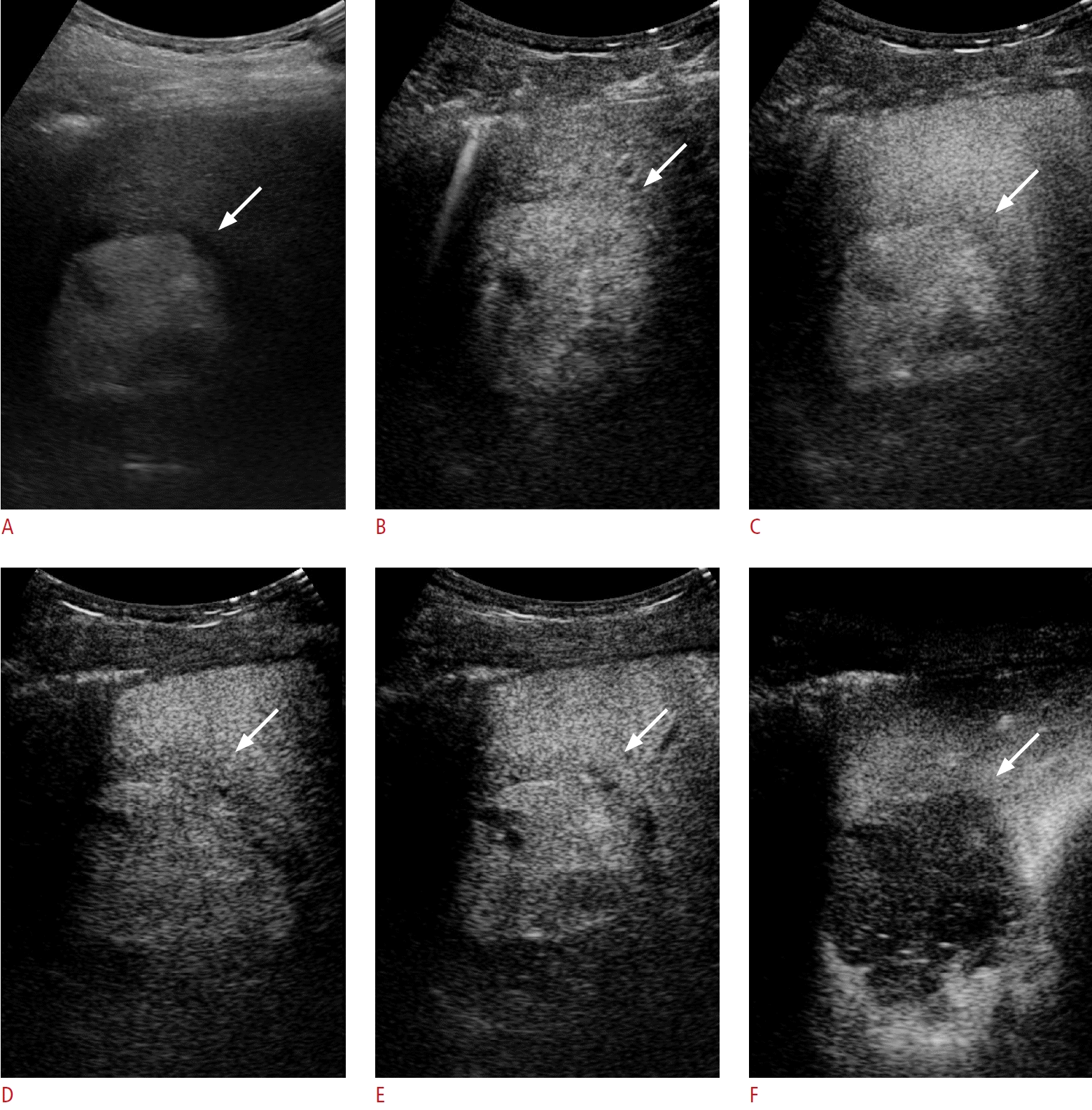
A 67-year-old man with pathologically confirmed well-differentiated hepatocellular carcinoma in segment 8 of the liver.
A. A 5.8-cm hyperechoic nodule can be observed (arrow) on B-mode ultrasonography (US). B-E. On Sonazoid-enhanced US, the nodule demonstrates arterial phase hyperenhancement (B, arrow) and does not exhibit washout until 1 (C, arrow), 5 (D, arrow), and 10 minutes or during the Kupffer phase (E, arrow) after contrast-medium injection. F. The nodule exhibits marked hypoenhancement in the Kupffer phase on high-mechanical-index contrast imaging using the loss of correlation method (arrow).
Takahashi et al. [9] also stated that, of 37 histopathologically confirmed HCCs, four (10.8%) exhibited isoenhancement in the KP and were classified as LR-4. Among these four HCCs, three were well-differentiated. In contrast, nearly all moderately differentiated (22 of 23 nodules: 95.7%) and all poorly differentiated HCCs (5 of 5 nodules: 100%) displayed hypoenhancement in the KP. This can be attributed to the potential retention of Kupffer cells in early HCCs and the fact that some well-differentiated HCCs contain blood spaces resembling normal sinusoids. Consequently, it is important to recognize that well-differentiated HCCs are likely to exhibit isoenhancement in the KP (Fig. 4).

An 81-year-old woman with pathologically confirmed well - differentiated hepatocellular carcinoma in segment 6 of the liver.
A. A 1.4-cm hyperechoic nodule can be observed (yellow caliper) on B-mode ultrasonography (US). B-E. On Sonazoidenhanced US, the nodule exhibits arterial phase hyperenhancement (B, arrow) and does not demonstrate washout until 1 (C, arrow), 5 (D, arrow), and 10 minutes or during the Kupffer phase (E, arrow) after contrast-medium injection.
HCCs Exhibiting Early Washout
Takahashi et al. [9] reported that of 37 histopathologically confirmed HCCs, eight (21.6%) exhibited early washout, resulting in their classification as LR-M. Furthermore, among these eight HCCs with early washout, five were poorly differentiated. In contrast, nearly all moderately differentiated HCCs (20 of 23 nodules: 87.0%) and all well-differentiated HCCs (9 of 9 nodules: 100%) did not display early washout. Consequently, it is important to recognize that poorly differentiated HCCs are relatively likely to exhibit early washout (Fig. 5).
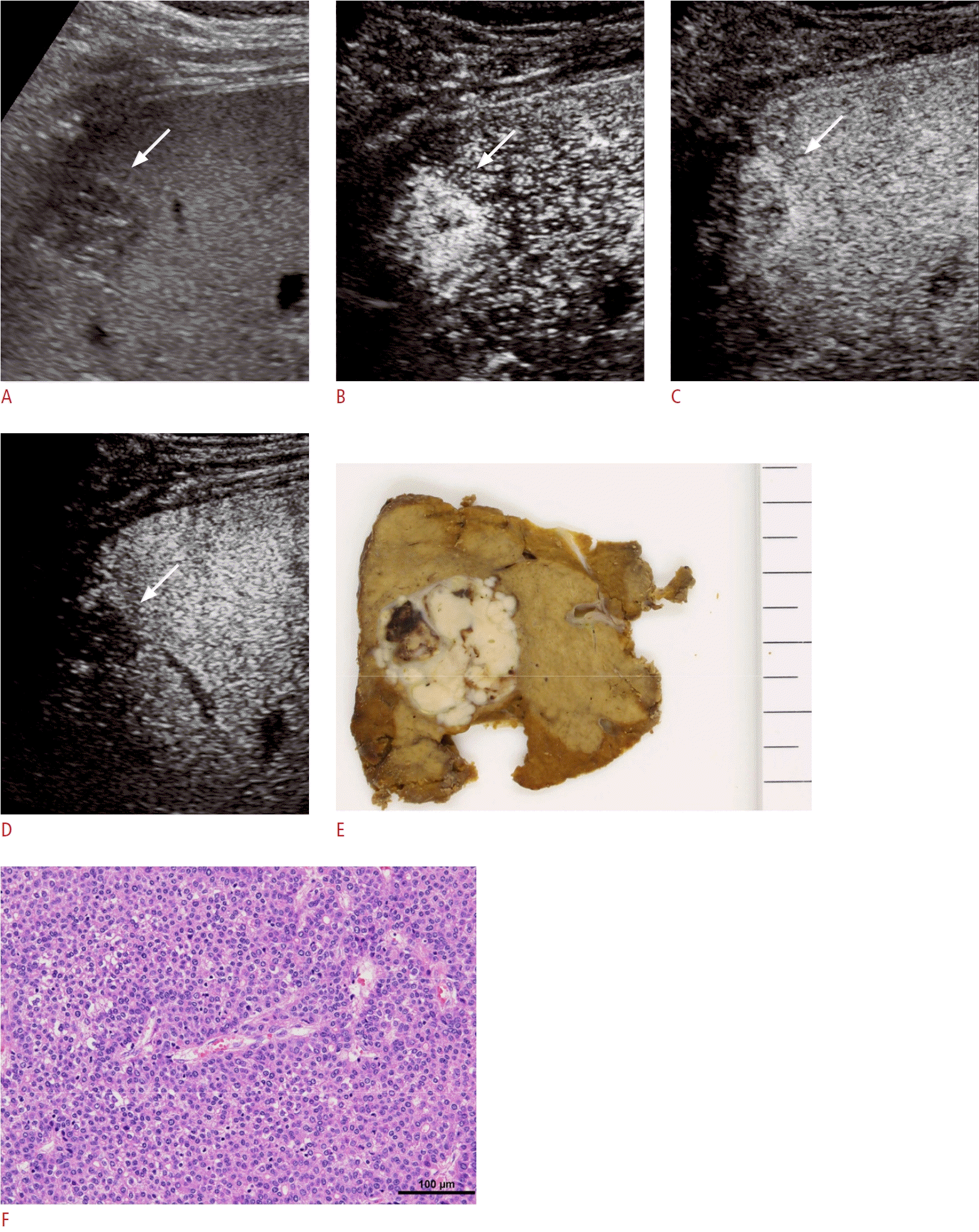
A 71-year-old man with pathologically confirmed poorly differentiated hepatocellular carcinoma (HCC) in segment 7 of the liver.
A. A 2.7-cm hypoechoic nodule is visible (arrow) on B-mode ultrasonography (US). B, C. On Sonazoid-enhanced US, the nodule demonstrates arterial phase hyperenhancement (B, arrow) and exhibits early washout within 60 seconds (C, arrow). D. The nodule displays marked hypoenhancement in the Kupffer phase (arrow). E. Grossly, the HCC presents as a simple nodular type with extranodular growth. F. The HCC exhibits a solid growth pattern (hematoxylin and eosin, ×200).
High-Flow Hemangioma
Sugimoto et al. [8] reported that their study involved 10 hemangiomas, of which two were miscategorized as LR-5. These two hemangiomas were high-flow and exhibited non-peripheral, non-globular APHE as well as hypoenhancement in the KP. If portal- and late-phase information had been considered, these lesions would not have been categorized as LR-5. Therefore, it is important to recognize that high-flow hemangiomas are likely to display APHE and hypoenhancement in the KP (Fig. 6). To differentiate high-flow hemangiomas from HCC using CEUS, micro–B-Flow imaging (GE Healthcare, Chicago, IL, USA) is useful, as it can reveal small intratumoral vasculatures, particularly when using contrast agents. When this imaging technique is applied to HCC lesions, intra-tumoral vasculatures are visible, whereas in high-flow hemangiomas, intra-tumoral vasculature is not observed (Fig. 6F).

A 43-year-old woman with a high-flow hemangioma in segment 7 of the liver.
A. A 1.9-cm hyperechoic nodule can be observed (yellow caliper) on B-mode ultrasonography (US). B-E. On Sonazoid-enhanced US, the nodule exhibits non-peripheral, non-globular arterial phase hyperenhancement (B, arrow) and does not display washout until 1 minute (C, arrow). The nodule demonstrates mild hypoenhancement up to 5 minutes (D, arrow) and marked hypoenhancement in the Kupffer phase (E, arrow). F. On micro–B-Flow imaging with Sonazoid 50 seconds after injection, the nodule does not reveal any intra-tumoral vasculature (yellow caliper).
CEUS LI-RADS: the Next Version
These authors are not currently involved in the development of CEUS LI-RADS and thus cannot speculate on its future updates. Nevertheless, the authors would like to share their perspectives and expectations regarding this matter.
First, CEUS is identified as a secondary diagnostic tool in several guidelines, including those of the Japan Society of Hepatology [2], the Korean Liver Cancer Association-National Cancer Center [3], and the European Association for the Study of the Liver [4]. However, CEUS has not been included in the AASLD guidelines [1]. Although the AASLD is an American institution, it exerts a strong global influence, and its guidance documents would assist clinicians worldwide in understanding and applying the available evidence in practice. As a result, the authors strongly recommend incorporating CEUS LI-RADS into the AASLD guidelines.
Second, geographical variations in treatment practices influence the balance between sensitivity and specificity required for HCC diagnosis. In the United States and certain European nations, where liver transplantation is the primary treatment for HCC, high specificity for diagnosis is preferred. However, in some Asian nations, where radical resection or local therapy is more common, high sensitivity is preferred [21]. As a result, it is expected that the next version of the guidelines will take both sensitivity and specificity into account. The modified CEUS LI-RADS could be a promising option, as it retains high specificity while enhancing sensitivity.
Finally, in several Asian countries, Sonazoid is utilized for the diagnosis and monitoring of liver lesions in patients at risk for HCC. If Sonazoid is incorporated into the next updated version of CEUS LI-RADS and adopted globally, it will significantly impact the daily treatment and management of HCC. Consequently, the authors believe that not only blood pool agents but also Kupffer cell agents like Sonazoid will soon be integrated into the CEUS LI-RADS. Although Sonazoid is a second-generation US contrast agent, its properties are entirely distinct from those of other blood pool agents. Therefore, while combining the criteria for both US contrast agents would be ideal, as is the case with CT/MRI LI-RADS, the authors encourage all working group members to adopt a more flexible approach.
Conclusion
This review examined several proposed modifications to the CEUS LI-RADS for diagnosing HCC using Sonazoid. All of these modified versions utilized KP hypoenhancement findings as the primary imaging feature, rather than late-phase mild washout. By employing this finding as the main imaging feature, all referenced studies demonstrated an improvement in the sensitivity of HCC diagnosis while maintaining specificity, compared to the CEUS LI-RADS with a 5-minute criterion. Consequently, the authors expect that Sonazoid will soon be integrated into the next updated CEUS LI-RADS, and KP or post-vascular-phase findings will serve as the primary imaging feature for HCC diagnosis.
Notes
Author Contributions
Conceptualization: Sugimoto K, Itoi T. Data acquisition: Sugimoto K, Kakegawa T, Takahashi H (Hiroshi Takahashi), Wada T, Abe M, Yoshimasu Y, Takeuchi H (Hirohito Takeuchi). Data analysis or interpretation: Sugimoto K, Kamiyama N. Drafting of the manuscript: Sugimoto K. Critical revision of the manuscript: Sugimoto K, Kamiyama N, Kakegawa T, Takahashi H (Hiroshi Takahashi), Wada T, Abe M, Yoshimasu Y, Takeuchi H (Hirohito Takeuchi), Itoi T. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Key point
This review covers some of the proposed modifications to contrast-enhanced ultrasonography Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of hepatocellular carcinoma (HCC) using Sonazoid. These modified versions all utilize Kupfferphase hypoenhancement as the primary imaging feature, rather than focusing on late-phase mild washout. Using this finding as the main imaging feature, all studies demonstrated improved sensitivity for HCC diagnosis while maintaining specificity, compared to the CEUS LI-RADS with a 5-minute criterion.