Two-dimensional shear wave elastography (ElastQ) accurately rules out liver fibrosis and rules in advanced chronic liver disease across liver disease etiologies: a prospective multicenter study
Article information
Abstract
Purpose
This study evaluated ElastQ, a two-dimensional shear wave elastography (2D-SWE) technique, for the non-invasive assessment of liver fibrosis risk using liver stiffness measurement (LSM). The aim was to determine its diagnostic accuracy and establish LSM cutoffs for clinical risk stratification.
Methods
A prospective multicenter study was conducted, employing vibration-controlled transient elastography (VCTE) as a reference standard. The statistical analysis utilized Pearson correlations and Lin concordance correlation coefficients, diagnostic areas under the curve (AUCs), and 90%-specific rule-in and 90%-sensitive rule-out ElastQ cutoffs.
Results
The study included 875 patients at risk for liver disease, of whom 816 (376 women, 46.1%; median age, 57.0 years [interquartile range, 19.0]) had successful and reliable VCTE- and ElastQ-LSMs. The median LSM was 13.0 kPa (range, 2.0 to 75.0 kPa) for VCTE and 6.6 kPa (range, 2.9 to 26.5 kPa) for ElastQ. The correlation between VCTE-LSM and ElastQ-LSM was adequate for VCTE-LSM <15 kPa (Pearson r=0.63) but lower for VCTE-LSM ≥15.0 kPa (Pearson r=0.27). VCTE-LSM indicated no fibrosis risk (<5.0 kPa) in 178 cases (21.8%), gray zone (5.0-9.9 kPa) in 347 cases (42.5%), and advanced chronic liver disease (ACLD; ≥10.0 kPa) in 291 cases (35.7%). The diagnostic AUC for ElastQ-LSM was 0.82 for fibrosis risk and 0.90 for ACLD. The clinically relevant ElastQ cutoffs for ruling out fibrosis risk and ruling in compensated ACLD (cACLD) were <5.0 kPa and ≥9.0 kPa, respectively.
Conclusion
ElastQ 2D-SWE enables accurate, non-invasive assessments of liver fibrosis and cACLD risk. In clinical practice, ElastQ-LSM <5.0 kPa rules out fibrosis, while ElastQ-LSM ≥9.0 kPa rules in cACLD.
Introduction
Liver disease causes 2 million deaths per year worldwide, with the highest mortality rates found in patients with advanced liver fibrosis [1]. Liver biopsy can be performed percutaneously or via the transjugular route [2,3], but it has considerable limitations for fibrosis staging [4], including sampling error [5] and a risk for severe complications, including mortality [6]. Therefore, a need exists for accurate, non-invasive alternatives to assess liver fibrosis risk. Liver stiffness measurement (LSM) using shear wave elastography (SWE) techniques provides a quantitative surrogate biomarker for fibrosis staging, and this has already been implemented in clinical practice for diagnosing patients with diffuse liver disease and assessing the risk of compensated advanced chronic liver disease (cACLD) [7]. Vibration-controlled transient elastography (VCTE)–based LSM has been widely validated for various etiologies of liver disease [8], but it requires a dedicated device. While VCTE may be ineffective in difficult-to-scan individuals, such as those with obesity, alternative methods such as point shear wave elastography (pSWE) [9] and two-dimensional shear wave elastography (2D-SWE) [10] can overcome these limitations. These tools are integrated into modern ultrasound platforms, which also allow for evaluation with B-mode, Doppler, and contrast if necessary. However, due to inherent differences in elastography software and technical implementation by different manufacturers, the LSM cutoffs for determining distinct categories of liver fibrosis risk and severity may vary depending on the SWE technique employed [11]. Importantly, according to the 2020 Society of Radiologists in Ultrasound (SRU) guidelines [12], within the range relevant for distinguishing different fibrosis risk strata (specifically, significant fibrosis, advanced fibrosis, and cACLD), which is below <15 kPa, the discordance between LSMs using SWE systems from different manufacturers is small and not clinically relevant. Therefore, the same liver stiffness cutoff values may be used across devices from different manufacturers [12]. Current clinical guidelines recommend using VCTE-LSM to rule out any liver fibrosis and rule in cACLD [12,13]. While studies on various 2D-SWE techniques have previously demonstrated diagnostic accuracy, largescale and prospective real-world data on 2D-SWE using ElastQ are limited. Consequently, a prospective multicenter study was conducted utilizing the ElastQ 2D-SWE technique, with the aim of deriving clinically useful LSM cutoffs for evaluating fibrosis and advanced chronic liver disease (ACLD).
Materials and Methods
Compliance with Ethical Standards
Three European centers contributed data: (1) Fondazione I.R.C.C.S. Policlinico San Matteo, University of Pavia, Pavia, Italy, (2) Center for Advanced Research in Gastroenterology and Hepatology "Victor Babes" University of Medicine and Pharmacy Timisoara, Timisoara, Romania, and (3) Division of Gastroenterology & Hepatology, Department of Medicine III, Medical University of Vienna, Vienna, Austria. The study received approval from the local ethics committees of all participating centers (respective EC-numbers: P-20170031748, 5/2018, and 1650/2017). All participants were informed about the study procedures and provided written informed consent. This study was conducted in adherence with the most recent version of the Declaration of Helsinki.
Study Centers and Study Population
For this study, adult participants with suspected or diagnosed liver disease were prospectively recruited from three European centers. These patients underwent simultaneous VCTE (FibroScan, Echosens, Paris, France) and ElastQ 2D-SWE (EPIQ-7, Philips Medical Systems, Amsterdam, The Netherlands) assessments. Patients with hepatic malignancy, severe liver inflammation (aspartate transferase/alanine transaminase >5× upper limit of normal), mechanical cholestasis, post-hepatic liver congestion, or prior liver transplantation were excluded from the study.
Elastography Protocol
ElastQ was performed utilizing the C5-1 convex probe, while VCTE was carried out using the M or XL probe, as recommended by the probe selection tool of the VCTE device. Measurements were conducted in accordance with published recommendations [14]. The participants were placed in the supine position, with the right arm abducted to facilitate intercostal access to the right lobe of the liver [12]. A total of ≥10 VCTE-LSM and ≥5 2D-SWE-LSM assessments were performed on the same day, in a fasted state (at least 4 hours), following 10 minutes of rest. 2D-SWE LSMs were obtained during a mid-breath hold, while no specific breathing instructions were given for VCTE LSMs; measurements were taken at a depth of 1.5-2.0 cm below the liver capsule, avoiding vessels and other sources of artifacts. All elastography operators were specifically trained on their respective devices and conducted initial measurements under supervision. Most operators had years of ultrasound experience and a record of over 300 successful elastographic examinations. This study included only individuals with successful and reliable VCTELSM (defined by VCTE-LSM interquartile range [IQR]/median ≤0.3 or VCTE-LSM <7.1 kPa [15]) and reliable ElastQ-LSM (defined as ElastQ-LSM IQR/median ≤0.3 [12]. Failed LSM was characterized by the inability of the respective device to produce any LSM value after several attempts.
Statistical Analysis
The demographic information and clinical characteristics of the patients are presented as absolute and relative frequencies for categorical variables, mean±standard deviation for parametric variables, and median (IQR) for non-parametric variables. The normality of the distribution was assessed using the Shapiro-Wilk test.
Significance testing and correlation analysis
The Fisher exact test or chi-square test was employed to assess differences between groups defined by categorical variables, depending on group size. For parametric variables, one-way analysis of variance was utilized, while the Kruskal-Wallis test was applied for non-parametric variables. A P-value of <0.05 was considered to indicate statistical significance. Positive likelihood ratios were calculated using the following formula: sensitivity/(100-specificity).
To evaluate the correlation between ElastQ and the reference method (VCTE), both Pearson and Spearman correlation coefficients were calculated. Due to the observed decrease in the correlation as VCTE-LSM increased, a choice was made to separately calculate the Pearson correlation coefficients for two categories: individuals with VCTE-LSM <15 kPa and those with VCTE-LSM ≥15.0 kPa. This binary classification holds clinical significance, as according to the Baveno guidelines, VCTE-LSM values ≥15.0 kPa indicate ACLD [7]. Consequently, only values below this approximate threshold would be utilized to determine fibrosis risk. A Bland-Altman-Leh analysis was conducted to generate the corresponding graphs [16]. Furthermore, the concordance between the comparator LSM techniques was assessed using Lin concordance correlation coefficients [17].
Statistical analysis software
Microsoft Excel (Microsoft Corp., Redmond, WA, USA) was utilized for data collection and storage, while Microsoft Word (Microsoft Corp.) was employed for writing and GrammarlyGO (Grammarly, San Francisco, CA, USA) for editing purposes. Statistical analysis and graph plotting were conducted using the R programming language for statistical computing and graphics (R 4.2.1+, R Foundation for Statistical Computing, Vienna, Austria), with the dplyr, openxlsx, table1, ggplot2, agRee, and BlandAltmanLeh packages. Additionally, Microsoft PowerPoint (Microsoft Corp.) was used to create Fig. 1. The OptimalCutpoints and pROC packages were employed for receiver operating characteristic (ROC) calculations. In this study, VCTE served as the reference standard for defining fibrosis categories, with <5.0 kPa indicating no fibrosis risk, 5.0-9.9 kPa indicating a gray zone result, and ≥10.0 kPa indicating ACLD [7]. Liver biopsy, which is often considered the gold standard for fibrosis assessment, was not utilized in this study; the technique is frequently no longer indicated and therefore would have been unethical to perform for many of the included participants.

Study population flow chart.
A. Flow chart of the study population is shown. B. After the use of shear wave elastography the FIB-4 score was applied in a two-step approach to further refine fibrosis risk assessment. FIB-4, Fibrosis-4 score; VCTE, vibration-controlled transient elastography; LSM, liver stiffness measurement; EQ, ElastQ; ACLD, advanced chronic liver diseases; cACLD, compensated ACLD.
In this cohort, the diagnostic performance of ElastQ was assessed using ROCs and the area under the curve (AUC). Rule-in and rule-out cutoffs for ACLD were selected based on diagnostic performance, aiming for >90% specificity and >90% sensitivity, respectively. Subsequently, patients were categorized into three fibrosis risk groups according to the newly derived ElastQ cutoffs: no fibrosis (fibrosis rule-out), ACLD rule-in, and the gray zone. For those in the gray zone, the Fibrosis-4 score (FIB-4) [18] was applied with a cutoff of <1.3 to rule out and ≥2.67 to rule in ACLD [19]. Since some patients in this study had ascites, a subgroup analysis was conducted of patients without ascites to ensure that the results remained valid for those screened for cACLD.
Results
Study Cohort
In this prospective, comparative, European multicenter study involving 875 participants, ElastQ failed in 24 cases (2.7%), VCTE in five cases (0.6%), and both methods in two cases (0.2%). ElastQ-LSM results were deemed unreliable in 24 participants (2.7%), while VCTE-LSM results were unreliable in four participants (0.3%). Ultimately, paired VCTE-LSM and ElastQ-LSM measurements from 816 individuals with various liver disease etiologies were analyzed. Details are presented in the study flowchart in Fig. 1A.
The distribution of fibrosis risk strata, as defined by VCTE, included 178 (21.8%) individuals with no fibrosis, 347 (42.5%) in the gray zone, and 291 (35.7%) with ACLD. The overall median age was 57.0 (IQR, 19.0) years. Demographic characteristics, both in general and by fibrosis risk category, are presented in Table 1. The distribution of fibrosis risk categories and etiologies of liver disease varied across centers; information on population characteristics by center and by etiology can be found in Supplementary Table 1 and Supplementary Table 2, respectively. Age, body mass index, transaminase levels, international normalized ratio, bilirubin level, and cholestasis parameters (gamma-glutamyl transferase and alkaline phosphatase levels) were higher in advanced fibrosis risk categories, while albumin and platelet levels were lower.
Pearson correlation analysis demonstrated a moderate agreement between reliable VCTE-LSM and ElastQ-LSM (r=0.64; P<0.001; 95% confidence interval [CI], 0.60 to 0.69) (Fig. 2A). Furthermore, the Spearman rho (ρ) was 0.72 (P<0.001; 95% CI, 0.68 to 0.75), and a Lin concordance correlation coefficient of 0.30 (lower bound, 0.25; upper bound, 0.35). In the Bland-Altman analysis, a monotonic increase was observed in the absolute numerical difference between VCTE- and ElastQ-LSM relative to the median LSM, as depicted in Fig. 3. To clarify the impact of liver stiffness values on the correlation between VCTE- and ElastQ-LSM, Pearson correlation coefficients were separately analyzed below and above a VCTE-LSM threshold of 15 kPa. The Pearson r value was 0.63 (P<0.001; 95% CI, 0.58 to 0.68) for VCTE-LSM ≤15.0 kPa and 0.23 (P<0.001; 95% CI, 0.13 to 0.39) for VCTE-LSM >15.0 kPa (Fig. 2B).
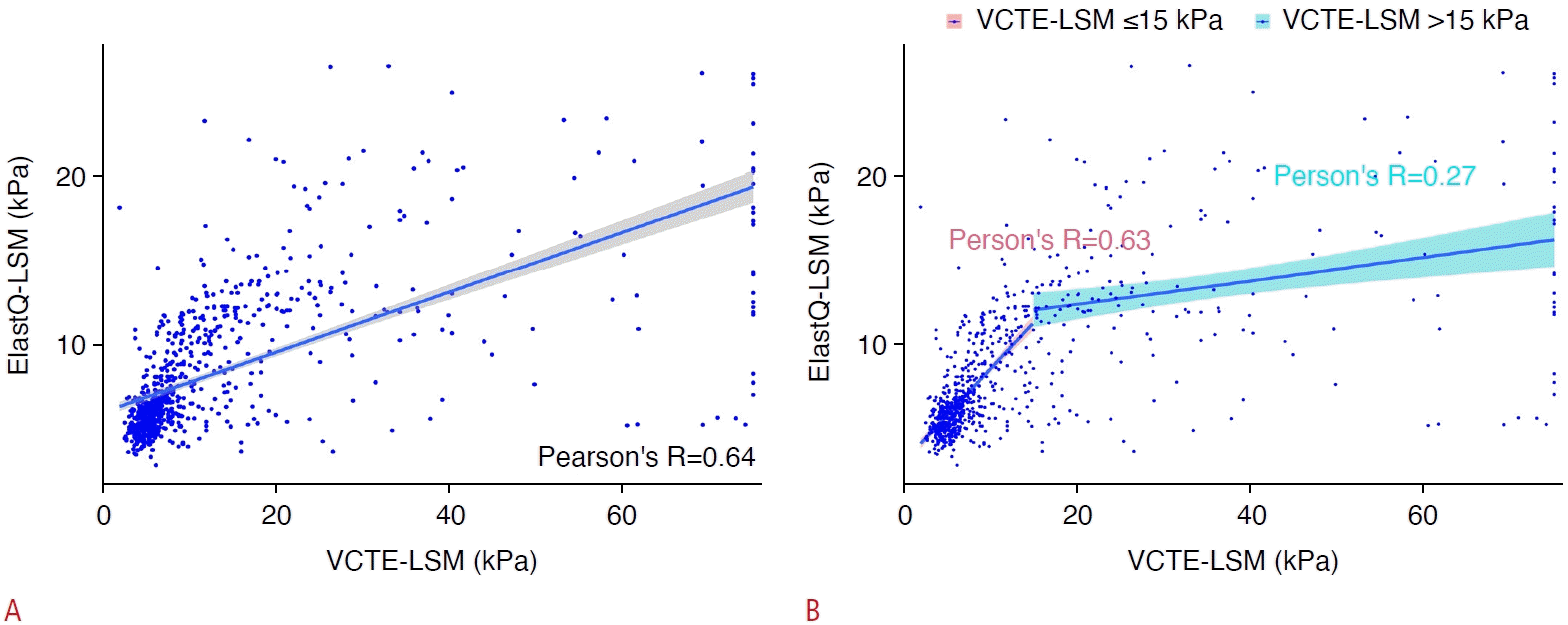
The correlation of ElastQ-LSM with VCTE-LSM overall and below and above 15 kPa.
Scatterplot shows (A) reliable LSM obtained through ElastQ and VCTE, including the correlation line and Pearson correlation results of VCTEand ElastQ-LSM (lower right corner), and (B) reliable LSM obtained through ElastQ and VCTE, featuring Pearson correlation results and correlation lines for values equal to and below 15 kPa (red) as well as above 15 kPa for VCTE-LSM (blue). LSM, liver stiffness measurement; VCTE, vibration-controlled transient elastography.
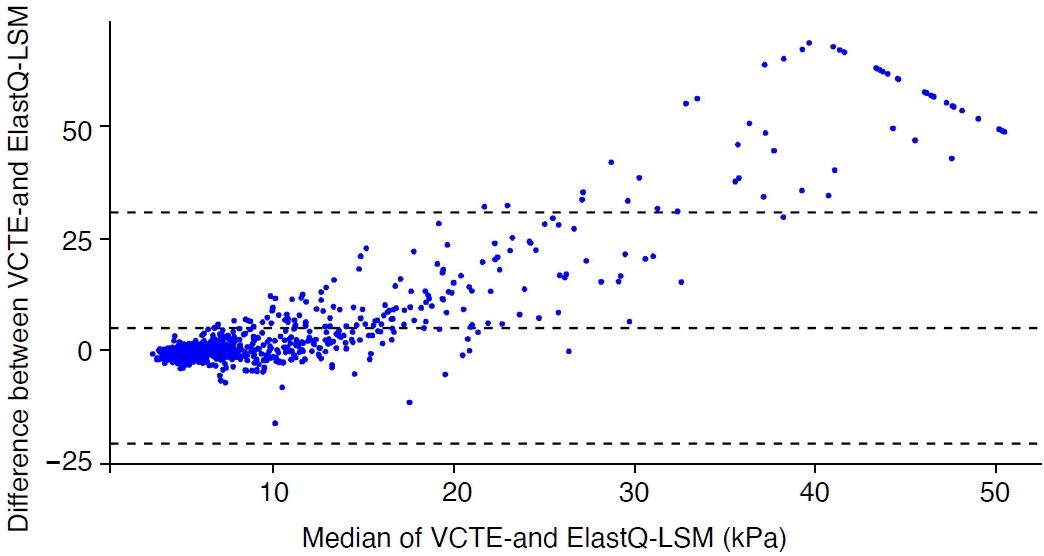
Bland-Altman-Leh plot comparing the differences between reliable VCTE and ElastQ-LSM to the median of VCTE and ElastQ-LSM, with median and 95% confidence intervals displayed (dotted lines).
LSM, liver stiffness measurement; VCTE, vibration-controlled transient elastography.
The decision to separately analyze the Pearson correlation coefficients below and above a VCTE-LSM threshold of 15 kPa was influenced by two main factors. First, a decrease in the correlation between VCTE and ElastQ was observed with increasing liver stiffness (as measured by either VCTE or ElastQ), as shown in Supplementary Fig. 1. Second, international guidelines recognize that a VCTE-LSM of 15 kPa is a decisive cutoff for diagnosing ACLD [7]. Consequently, VCTE-LSM values below this 15 kPa threshold are used to determine fibrosis risk. However, SWE values above this threshold have also been shown to be predictive of decompensation risk and mortality [20].
Supplementary Fig. 1 displays the Pearson correlation coefficients between ElastQ and VCTE below the VCTE threshold on the horizontal axis. Interestingly, the overall Pearson correlation coefficients increased with the VCTE-LSM up to approximately 22.0 kPa, where it peaked at a Pearson r of approximately 0.7 and then only slightly decreased. To further explore how Pearson correlation coefficients varied at different levels of stiffness (and considering that most LSMs in this study were within the VCTE-LSM range of <20.0 kPa), a plot was generated showing the Pearson correlation at a given VCTE-LSM ±10.0 kPa (using a moving window of 20.0 kPa total width) (Supplementary Fig. 2). This revealed that the Pearson correlation coefficients in this moving window were strongest in the range of less than 20.0 kPa, and then decreased. The Pearson correlation coefficients of ElastQ and VCTE, while varying across etiologies, were acceptable in all of them when sufficient patient numbers across the spectrum of fibrosis risk categories were included (see Supplementary Table 3 for comparison). Of the reliable study population of 816 patients, 48 (5.9%) had ascites (n=38 with 2 points on the Child-Pugh scale and n=10 with 3 points). To ensure the validity of the cutoffs in assessing cACLD, a subgroup analysis was performed of patients without ascites. The overall Pearson correlation coefficient between VCTE and ElastQ among patients without ascites was r=0.66. The rule-in and rule-out cutoffs calculated in the subpopulation without ascites remained numerically the same as in the total population, with slight, clinically insignificant differences in the AUC, sensitivity, and specificity (Supplementary Table 4).
ElastQ successfully predicted the VCTE-based fibrosis risk categories with the following AUCs: gray zone, 0.82 (95% CI, 0.79 to 0.85); and ACLD, 0.90 (95% CI, 0.87 to 0.92). The established cutoffs were <5.0 kPa to rule out fibrosis (no fibrosis group/gray zone rule-out), ≥6.7 kPa to rule in the gray zone, <6.2 kPa to rule out ACLD, and ≥9.0 kPa to rule in ACLD. A summary of these cutoffs, including their respective AUC, sensitivity, and specificity values, is presented in Table 2. By applying these newly derived ElastQ-LSM cutoffs, individuals could be classified as follows: no fibrosis, n=133 (16.3%); gray zone, n=418 (51.2%); and ACLD, n=265 (32.5%). Population characteristics based on fibrosis risk classification according to ElastQ-LSM are displayed in Supplementary Table 5. The characteristics of ElastQ-LSM false negatives included older age, elevated markers of hepatic inflammation and cholestasis (aspartate transferase, alanine transaminase, FIB-4, and gamma-glutamyl transferase), increased severity of liver dysfunction (lower platelet count, lower albumin level, higher international normalized ratio, and higher bilirubin), as well as, by definition, higher markers of VCTE-LSM and measurement uncertainty (VCTE-LSM IQR/median and ElastQ IQR/median). Supplementary Table 6 presents the details of the comparison.
As recommended by the latest European Association for the Study of the Liver guidelines on non-invasive testing for evaluating liver disease and severity [21], a laboratory-based non-invasive test, specifically the FIB-4 score, was carried out for individuals within the gray zone. FIB-4 information was not available for 113 of 418 participants (27.0%), as this study also included patients for whom LSM was ordered by the referring physician but who did not undergo laboratory testing at the respective study center, and the availability of FIB-4 component parameters was not defined as an inclusion criterion. Consequently, of the 305 (n=418-113) patients in the gray zone, fibrosis could be ruled out in an additional 131 (43.0%) and ruled in for 53 (17.4%). Ultimately, after utilizing FIB4 and ElastQ in a two-step approach, fibrosis could be ruled out in 264 of 703 (n=816-113) (37.6%) cases and ACLD ruled in for 318 cases (45.2%), while 121 cases (17.2%) finally remained in the gray zone (Fig. 1B).
Discussion
The need for accessible and accurate tools for the identification and monitoring of patients with liver disease is widely acknowledged. Both the SRU [12] and the European Association for the Study of the Liver [21] guidelines recommend the use of LSM via elastography for assessing liver fibrosis risk and identifying ACLD/cirrhosis [22]. Importantly, these guidelines are based on meta-analyses of various 2D-SWE techniques but do not yet incorporate data from 2D-SWE ElastQ for recommendations, which also pertain to this technique. Furthermore, previous studies evaluating 2D-SWE for liver fibrosis risk assessment have primarily focused on patient populations with chronic viral hepatitis C [23-25] or hepatitis B [26,27], whereas this study also included patients with other etiologies, including a considerable proportion of participants with non-alcoholic fatty liver disease or alcoholic liver disease. Additionally, clinical performance data on ElastQ is limited beyond those meta-analyses. Although 2D-SWE–based LSM is widely validated, most studies have employed supersonic shear imaging or 2D-SWE–LOQIG-E9 techniques. Some research has examined the correlation of ElastQ 2D-SWE with other ultrasound-based elastography techniques, without providing specific and clinically relevant cutoffs. To the best of the authors’ knowledge, the accuracy and fibrosis-risk-cutoffs of the ElastQ 2D-SWE technique have only been assessed in a single-center cohort of 178 patients with hepatitis C virus (HCV) infection (i.e., a considerably smaller cohort than the cohort of study) [28]. This HCV-exclusive study suggested Youden cutoffs for low fibrosis risk and liver cirrhosis identification that were somewhat higher (8.15 for F2 and 12.65 for F4) than those presented in this study, a discrepancy that can be attributed to the use of the Youden method for selecting cutoffs, the exclusion of non-HCV liver disease etiologies, and the use of different VCTE cutoffs for fibrosis risk classification.
This study of 875 patients with various liver disease etiologies from three European centers found moderate Pearson correlation coefficients between ElastQ and VCTE. However, this correlation was strongest at lower LSM strata and became increasingly divergent at VCTE-LSM ≥15 kPa. The slightly higher Spearman correlation coefficients emphasized the nonlinear relationship of LSM, as measured by the two methods. Similar findings have been reported in previous studies, which showed an increase in the difference between pSWE- and 2D-SWE-LSM compared to VCTE-LSM as LSM increased [9,11]. Nevertheless, the numerical difference between VCTE and ElastQ at VCTE-LSM ≥15 kPa is of less clinical importance, since the critical distinction for patient management—the diagnosis of cACLD—can still be readily made using both elastography techniques. Interestingly, despite the deviation from VCTE-LSM values, the 2D-SWE-LSM results above the cACLD threshold appear to be predictive of further decompensation and mortality [20].
Although the cutoffs are nearly identical for the same 2D-SWE technique implemented on different machines from the same manufacturer [29], differences exist across manufacturers [11,12,30]. However, the SRU consensus on the use of pSWE and 2D-SWE for the assessment of liver stiffness has emphasized that these differences are generally smaller than the overlap of stiffness values between consecutive stages of liver fibrosis. The SRU has proposed a "rule of four" for LSM cutoffs across systems for viral hepatitis and non-alcoholic fatty liver disease [12]. This rule suggests ruling out fibrosis at ≤5.0 kPa and ruling out cACLD at <9.0 kPa, while values in the range of 9.0-13.0 kPa indicate potential cACLD and values above 13.0 kPa strongly suggest cACLD [12]. Notably, these cutoffs differ from those proposed for VCTE in the "rule of five" introduced by the Baveno VII guidelines [7,12]. The present multicenter study assessed the diagnostic accuracy of ElastQ and explored whether this technique conforms to the "rule of four" recommended by the SRU.
A higher rate of failed or unreliable measurements was observed with ElastQ than with VCTE, which might have been attributable to several factors. First, the VCTE device (FibroScan) used in this study automatically excludes single measurements that are deemed failed or unreliable. Second, the need to follow breath commands can present a challenge for sicker patients when using a 2D-SWE technique such as ElastQ. In contrast, VCTE does not require a breath hold, potentially making it easier to obtain measurements. However, failed or unreliable measurements do not pose an insurmountable clinical issue, as non-invasive measurements can readily be repeated (for instance, under better conditions), or alternative non-invasive tests (such as blood-based scores) can be selected. Undoubtedly, further research on factors affecting the ability to obtain valid and reliable measurements using SWE techniques is warranted.
ElastQ showed high diagnostic accuracy for excluding fibrosis (AUC, 0.82) and determining or excluding ACLD (AUC, 0.90), which remained the case when only compensated patients were evaluated. The cutoffs presented here are also suitable for assessing the risk of cACLD. The data and calculations provided suggest that ElastQ can exclude significant fibrosis below 5 kPa, which is in exact numerical agreement with the rule of four. ElastQ-LSM values of 6.7 kPa or higher indicate a gray zone in which fibrosis cannot be confidently excluded and cACLD cannot be confirmed. cACLD can be ruled out below 6.2 kPa, while ElastQ-LSM values of 9 kPa or higher confirm or strongly suggest cACLD. These cutoffs align well with the "rule of four" cutoffs for excluding fibrosis, with the ElastQ 9-kPa threshold for cACLD presented as an inclusive cutoff to allow for sensitive cACLD screening. In contrast, the "rule of four" cutoff at 13 kPa would be more specific for confirming cACLD. An analysis using the VCTE-based "rule of five" cutoff for confirming cACLD at 15 kPa would indeed result in an ElastQ cACLD confirmation cutoff numerically close to 13 kPa.
The investigation of false-negative in comparison to true-positive ElastQ-LSM findings suggests that particular attention should be given when interpreting low ElastQ-LSM results in patients with advanced age, elevated markers of liver inflammation, cholestasis, and reduced liver function, as well as a high ElastQ-LSM IQR/ median.
After applying the ElastQ rule-out cutoff for fibrosis (<5 kPa) and the rule-in cutoff for cACLD (≥9 kPa), a FIB-4 cutoff of ≥2.67 was utilized in the remaining ElastQ gray zone. Consequently, it was possible to rule out fibrosis in 264 of 703 (816-113) patients (37.6%) and rule in ACLD in 318 (45.2%), leaving only 121 participants (17.2%) with undetermined fibrosis risk based solely on a non-invasive assessment.
The authors believe that for 2D-SWE with ElastQ to become more widely adopted in clinical practice, a straightforward approach with clear and simple recommendations is necessary. Therefore, it is proposed that two clinically relevant ElastQ cutoffs should be used: (1) ElastQ-LSM <5.0 kPa to rule out the risk of fibrosis, and (2) ElastQ-LSM ≥9 kPa to rule in cACLD and prompt referral to a liver specialist/center for further management. For the remaining patients, with ElastQ-LSM values between 5.0 and 8.9 kPa (the gray zone), a non-invasive, simple blood-based test, such as the FIB-4 score, should be applied to guide their further management. Importantly, as with VCTE-LSM results, ElastQ-LSM should be confirmed through a second independent examination and under fasting conditions. The specific outcomes of patients in the different ElastQ-LSM strata, focusing on the incidence rate of liver-related events, remain to be studied. Notably, other 2D-SWE methods have been shown to predict hepatic decompensation and survival in patients with cACLD [20]; therefore, it is crucial to identify patient characteristics where ElastQ produces falsely low LSM values.
This study had several limitations. First, VCTE-LSM was used as a reference standard. Although VCTE-LSM is well-established, noninvasive, and widely available, further studies on ElastQ using liver biopsy as the gold standard and its predictive value for outcomes are warranted. Second, this study included patients from liver care centers, where the prevalence of significant and advanced fibrosis, as well as cirrhosis, is much higher than in the general population. This should be considered when interpreting the ElastQ cutoffs obtained in this study. Third, a divergence was observed between VCTELSM and ElastQ-LSM, which increased monotonously and became pronounced at VCTE-LSM ≥15.0 kPa. This effect has been previously described for ElastQ [29] and other acoustic radiation force imaging-based techniques, especially for 2D-SWE [10,11,29,30]. Importantly, despite the possibility that the difference between elastography techniques is a technical artifact caused by different implementations and technologies, patient factors may contribute to this divergence. Potential confounders include the size of the region of interest [31], body mass index, and skin-to-liver-capsule distance [32]). Additionally, the specific interactions between multiple potential perturbators should be explored. However, this divergence only becomes pronounced above the clinical decision cutoff for cACLD. Furthermore, at the ElastQ cutoffs suggested herein for clinical decision-making, the correlation between ElastQ- and VCTE-LSM remained strong. Fourth, some patients in this study had ascites. However, the cutoffs derived from the subpopulation without ascites were numerically the same as in the overall population, with only clinically irrelevant differences in the associated sensitivity and specificity. Finally, the cutoffs presented here should be validated in an external cohort.
In summary, this study demonstrated a significant correlation between ElastQ-LSM and the widely established VCTE-LSM. Notably, ElastQ-LSM exhibited excellent diagnostic accuracy for the non-invasive assessment of liver fibrosis and cACLD risk. It is recommended to apply the clinically relevant ElastQ-LSM cutoffs at <5.0 kPa to rule out fibrosis and at ≥9.0 kPa to rule in (c) ACLD. While FIB-4 may be applied sequentially in the ElastQ-LSM gray zone of 5.0-8.9 kPa, the authors strongly advise ongoing monitoring of individuals in the ElastQ-LSM gray zone, particularly if ElastQ-LSM values fall within the 6.5-8.9 kPa range.
In conclusion, ElastQ-LSM is a readily accessible and precise tool for non-invasive assessment of liver fibrosis risk, suitable for clinical practice. Further studies are needed to evaluate the occurrence of liver-related events across the various ElastQ-LSM risk categories.
Notes
Author Contributions
Conceptualization: all authors. Data acquisition: all authors. Data analysis or interpretation: Bauer DJM, Mandorfer M, Ferraioli G, Reiberger T. Drafting of the manuscript: Bauer DJM, Ferraioli G, Reiberger T. Critical revision of the manuscript: all authors. Approval of the final version of the manuscript: all authors.
DB served as a speaker and/or consultant and/or advisory board member for AbbVie and Siemens, received travel support from AbbVie and Gilead, and received grant support form Siemens and Gilead. ADS, RM, LM, AR, GS: nothing declared. MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol-Myers Squibb, Gilead, Collective Acumen, and W. L. Gore & Associates and received travel support from AbbVie, BristolMyers Squibb, and Gilead. IS served as speaker for AbbVie, BMS, Gilead, Janssen, Echosens, and Philips; received advisory board fees from AbbVie, Merck; Siemens, Canon, and Toshiba; and received research support from Philips. GF served as a speaker for Canon Medical Systems, Fujifilm Medical Systems, Mindray Medical Systems, Philips Ultrasound, and Siemens Healthineers and as an advisory board member for Philips Ultrasound and Siemens Healthineers. TR served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/ research support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips, and W. L. Gore & Associates as well as travel support from Boehringer Ingelheim and Gilead.
Acknowledgements
Ultrasound devices, support, and training were kindly provided by Philips Healthcare.
Supplementary Material
Supplementary Table 1.
Population characteristics by center (https://doi.org/10.14366/usg.23069).
Supplementary Table 2.
Population characteristics by etiology (https://doi.org/10.14366/usg.23069).
Supplementary Table 3.
Pearson’s correlation per etiology (https://doi.org/10.14366/usg.23069).
Supplementary Table 4.
ElastQ cutoffs for different fibrosis risk groups amongst patients without any ascites (https://doi.org/10.14366/usg.23069).
Supplementary Table 5.
Population characteristics by fibrosis risk group based on ElastQ (https://doi.org/10.14366/usg.23069).
Supplementary Table 6.
Comparison of baseline characteristics of ElastQ-ACLD FN vs. ElastQ-ACLD TN (https://doi.org/10.14366/usg.23069).
Supplementary Fig. 1.
Correlation of LSM by ElastQ and VCTE below the threshold VCTE-LSM given on the x-axis/horizontal axis. LSM, liver stiffness measurement; VCTE, vibration-controlled transient elastography (https://doi.org/10.14366/usg.23069).
Supplementary Fig. 2.
Correlation of LSM by ElastQ and VCTE at given VCTE-based liver stiffness. LSM, liver stiffness measurement; VCTE, vibration-controlled transient elastography (https://doi.org/10.14366/usg.23069).
References
Article information Continued
Notes
Key point
Liver stiffness measurements using ElastQ and vibration-controlled transient elastography showed a significant correlation; however, in alignment with prior publications, this correlation weakened above 15 kPa. The ElastQ shear wave elastography technique can accurately and non-invasively rule out liver fibrosis below 5.0 kPa and rule in compensated advanced chronic liver disease (cACLD) at or above 9.0 kPa. In cases for which cACLD cannot confidently be ruled in or out, patients should be referred to a specialized hepatologist for further evaluation, including tests such as the Fibrosis-4 score, enhanced liver fibrosis, or liver biopsy.


