Prenatal diagnosis of aberrant right subclavian artery in an unselected population
Article information
Abstract
Purpose
The purpose of this study was to determine the frequency of aberrant right subclavian artery (ARSA) among unselected fetuses and to evaluate its association with chromosomal abnormalities and other congenital anomalies.
Methods
In all, 7,547 fetuses (gestational age, 20 to 34 weeks) were examined using routine antenatal sonography at our institution between April 2014 and September 2015. The right subclavian artery was assessed using grayscale and color Doppler ultrasonography in the transverse 3-vessel and tracheal view, and confirmed in the coronal plane.
Results
ARSA was found in 28 fetuses (0.4%). Further, 27 of these 28 fetuses were euploid (96.4%). Trisomy 18 was the only chromosomal anomaly (3.6%) found in the study sample. ARSA was an isolated finding in 23 of the 28 cases (82.1%). In the remaining three cases (10.7%), ARSA was accompanied with extracardiac anomalies. Other cardiac defects were present in three cases (10.7%).
Conclusion
Isolated ARSA does not seem to be associated with a significantly increased risk of aneuploidy. However, the possibility of fetal karyotyping, which is a more invasive procedure, should be discussed in the light of the overall risk of the fetus.
Introduction
Aberrant right subclavian artery (ARSA) is the most common congenital abnormality of the aortic arch [1-6]. In ARSA, the right aortic arch regresses between the right common carotid and right subclavian arteries, instead of being distal to them. Therefore, this hinders the fusion of the right common carotid and right subclavian arteries to form the brachiocephalic artery. Ultimately, this results in the left aortic arch giving rise to four arteries: the right common carotid, the left common carotid, the left subclavian, and the aberrant right subclavian arteries. ARSA, arising most distally from the aortic arch, must course from the left side of the spine, behind the esophagus and the trachea, to the right upper arm. It is also known as aberrant retroesophageal right subclavian artery [2]. It should be noted that ARSA is, in general, an asymptomatic benign finding; however, esophageal compression, resulting in dysphagia, has been reported in some cases [7,8].
The identification of ARSA in fetuses with Down syndrome was first reported in 2005 by Chaoui et al. [9]. Since then, a few other studies have confirmed the feasibility of prenatally diagnosing this benign aortic arch branching anomaly [2,4,5,10-12]. Prenatal ultrasonography studies have revealed that the incidence of ARSA in the second trimester ranges between 0.4% and 1.5% in chromosomally normal fetuses [1,4,12-16].
The first aim of our study was to estimate the prevalence of fetal ARSA in our unselected population, the second aim was to evaluate the association of ARSA with chromosomal abnormalities, and the third aim was to investigate the coexistence of ARSA and extracardiac or cardiac anomalies.
Materials and Methods
Approval from the Institutional Review Board (CGH-IRB-2015-38) was obtained for this retrospective study, and the need for informed consent was waived. We investigated the course of the right subclavian artery during fetal echocardiography in 7,547 consecutive patients with a gestational age of between 20 and 34 weeks. Routine transabdominal ultrasonography was performed on unselected patients who visited our clinic between April 2014 and September 2015. Examinations were performed using Voluson 730 Expert (GE Healthcare, Milwaukee, WI, USA) and iU 22 (Philips Medical Systems, Bothell, WA, USA) machines equipped with 1- to 5-MHz curvilinear transducers. Right subclavian artery assessment was carried out by five sonographers who are experts in obstetric ultrasonography. During fetal echocardiography, the course of the right subclavian artery was observed after the assessment of the 4-chamber view, outflow tracts, and the 3-vessel and tracheal view. In addition to the B-mode segmental view approach, color Doppler ultrasonography was used for visualizing the transverse 3-vessel and tracheal view, as previously described by Chaoui et al. [9]. In order to accomplish this, the color Doppler velocity settings were adjusted downward (range, 15 to 30 cm/sec) to facilitate the visualization of the peripheral vessels. The normal right subclavian artery in the axial plane was visualized as an S-shaped vessel passing anterior to the trachea at the clavicle level (Fig. 1A). ARSA arose as the last vessel before the aortic isthmus and took a retrotracheal course behind the trachea to the right arm (Fig. 1B). The course of ARSA was straight, without an S-shape proximal concavity surrounding the trachea anteriorly. In order to assess ARSA, we obtained a coronal view of the fetal thorax, posterior to the trachea and anterior to the spine, until we could see the thoracic descending aorta. Color Doppler ultrasonography showed ARSA as a vessel arising from the descending aorta at the level of the aortic isthmus [11]. ARSA then followed an oblique course towards the right clavicle and shoulder (Fig. 2).
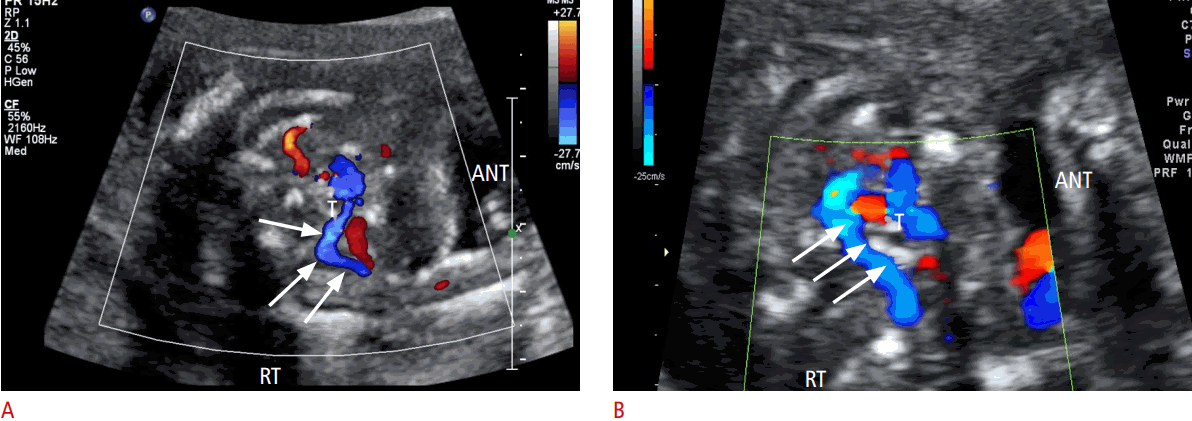
Comparison of color Doppler images of normal and aberrant right subclavian artery (ARSA) detected during the second trimester.
A. Color Doppler axial image shows the course of the normal right subclavian artery (arrows) anterior to the trachea (T). The typical “S” shape can be observed here. B. ARSA (arrows) arises from the junction of the aortic arch and ductus arteriosus and passes behind the trachea (T) towards the right arm. ANT, anterior; RT, right.
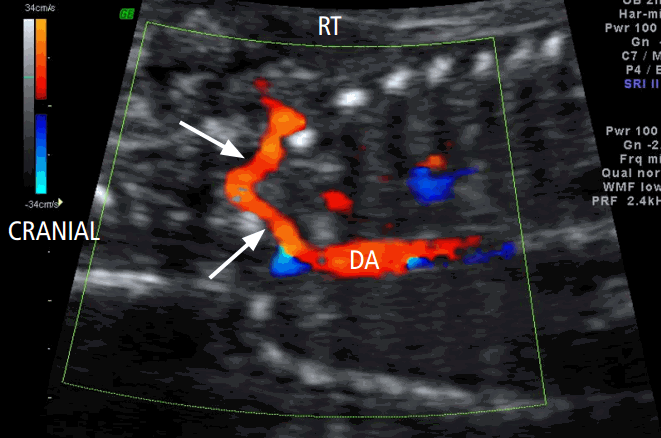
Color Doppler ultrasonographic coronal image showing an aberrant right subclavian artery (arrows) arising from the descending aorta (DA) with an oblique course towards the right shoulder.
RT, right.
Prenatal karyotype analysis was proposed in each case of ARSA. The outcomes of each pregnancy, including the presence of fetal abnormalities, cardiac defects, and the karyotype, were collected. The karyotype was obtained prenatally from an amniotic fluid sample. If the karyotype was not analyzed prenatally, but the newborn appeared clinically normal, then the karyotype was considered normal for major aneuploidies. Additional case information, including the gestational age at the time of diagnosis and postnatal echocardiogram, was also collected.
Results
In the 18-month period between April 2014 and September 2015, we assessed 7,547 routine patients in the second and third trimesters. ARSAs were detected in 28 cases (0.4%), including three cases of twins. The mean gestational age at the time of detection was 24 weeks (range, 20 to 34 weeks). Twenty cases were detected during the second trimester. The other six cases were missed during ultrasonography performed in the second trimester, and were detected in the third trimester. Two cases were diagnosed at 32 weeks at the patients’ first visit to our institution.
Twenty-seven fetuses with ARSA were chromosomally or morphologically normal, but one fetus had trisomy 18 (case 19). The karyotype was obtained from an amniotic fluid sample prenatally in 12 cases. Amniocentesis was performed for the following indications: advanced maternal age (6 cases, 50%), integrated test screening positive for Down syndrome (4 cases, 33.3%), and abnormal second trimester ultrasonography findings (2 cases, 16.7%).
Moreover, 23 of the ARSA cases (82.1%) were isolated findings. Extracardiac anomalies were present in three fetuses (10.7%). These included bilateral choroid plexus cysts and unilateral cerebral ventriculomegaly (case 6); echogenic bowel and unilateral renal pelvis dilatation (case 7); and bilateral choroid plexus cysts, bilateral cerebral ventriculomegaly, small ears, and clenched right hand (case 19). In three cases (10.7%), ARSA was accompanied by other cardiac malformations, including persistent left superior vena cava and aneurysmal dilatation of the proximal portion of the ARSA, called Kommerell diverticulum (case 14) (Fig. 3); ventricular septal defect (case 19); and coarctation of the aorta (case 28).

Aneurysmal dilatation of the descending aorta and proximal portion of the aberrant right subclavian artery (ARSA) at 29 weeks of gestation.
A-C. Axial (A) and sagittal (B) images show the dilated descending aorta and proximal portion of the ARSA (arrows) with a turbulent flow (C). POST, posterior; RT, right.
All 28 children were live-born. Seventeen were girls, and 11 were boys (60.7% vs. 39.3%). Postnatal echocardiography was performed in the 24 affected cases to confirm the prenatal findings. A small atrial septal defect was additionally detected on postnatal echocardiography in one case.
Discussion
Recently, ARSA has become prominent as an ultrasonographic marker of Down syndrome [9]. There have been many relevant studies on the prenatal ultrasonography of ARSA, and descriptions of its prevalence and correlation with Down syndrome [1,4,7,9,12,14-19]. ARSA can be a useful ultrasonographic marker of Down syndrome in first-trimester screenings [5,17]. However, the prevalence of ARSA may decrease with advancing gestation [1]. The ultrasonographic assessment of ARSA is not routinely performed in the first trimester, and it is possible that cases of fetal aneuploidy with ARSA were missed because of spontaneous miscarriage or termination of the pregnancy after prenatal confirmation. The performance of a fetal karyotype test when ARSA is an isolated finding is still a debatable issue.
The most common aortic arch anomaly is ARSA [2,5,6,8]. The incidence of ARSA in the normal population in prenatal and postmortem studies has been reported to be approximately 0.4%- 2% [1,4,15,16,18]. In the prospective study of Rembouskos et al. [5], ARSA was observed in 89 of 6,605 fetuses (1.4%) with normal karyotypes. Gul et al. [15] investigated the course of the right subclavian artery in 4,125 consecutive low-risk pregnancies between 17 and 33 weeks of gestation. The prevalence of ARSA in their study population was 17 of 4,120 (0.4%). The average prenatal prevalence of ARSA in the normal population was 1.0%. Our prenatal prevalence of ARSA was 0.4%.
Prenatal studies reported an ARSA prevalence of 6.8%-37.5% in Down syndrome fetuses [7,12]. There was no case of Down syndrome with ARSA in our study; this is a much lower incidence than that reported in the previous studies [9,12,14]. We examined fetal echocardiograms during the second trimester among unselected patients. We did not perform fetal echocardiography in first-trimester fetuses. Therefore, patients diagnosed with Down syndrome during the first trimester were not included. During the study period, 44 cases of Down syndrome were recorded at our institution. Forty of these 44 cases of Down syndrome were confirmed before 16 weeks of gestation, and they were all lost to follow-up before performing fetal echocardiography in the second trimester. Among the four live-born neonates with Down syndrome, three revealed several soft markers such as short long bone, echogenic bowel, and mild cerebral ventriculomegaly, and one showed persistent left superior vena cava. However, ARSA was not detected in them.
In this study, in 23 of the 28 cases, ARSA (82.1%) was an isolated finding. Previous studies have reported that ARSA was diagnosed as an isolated finding in 52.9%-65% of cases [7,15,16]. The higher rate of isolated ARSA in our study can be explained by the exclusion of early-diagnosed fetal anomalies. We hypothesized that an undetermined fraction of cases with severe abnormalities with ARSA in the first trimester were either stillborn babies or were lost to follow-up.
In this study, three cases (10.7%) of ARSA were accompanied with extracardiac anomalies. The detection rate of extracardiac anomalies with ARSA has thus far been reported in approximately 5%-26.7% of cases [5,7,15,16]. These extracardiac anomalies include arachnoid cysts, cataracts, choroid plexus cysts, cleft lip and palate, cloacal dysgenesis, corpus callosum agenesis, cystic hygroma, Dandy-Walker malformation, echogenic bowel, esophageal duplication cyst, hypoplasic nasal bone, intrauterine growth restriction, low-set ears, micrognatia, polycystic kidney disease, polydactyly, pyelectasis, short femur and humerus, single umbilical artery, strawberry head, thick nuchal fold, and thymic hypoplasia [5,7,15].
An additional cardiac defect was present in three cases (10.7%) in our study. Thus far, the most frequently reported structural abnormality coexisting with ARSA has been cardiac defects (5.4%- 23.5%) [5,7,15,16]. These cardiac defects include aortic stenosis, atrioventricular septal defect, dextrocardia, dilated ductus arteriosus, double-outlet right ventricle, persistent left superior vena cava, rhabdomyoma, tetralogy of Fallot, tricuspid atresia, and ventricular septal defect [5,7,15]. A correlation between ARSA and congenital heart defects (CHDs) was reported earlier. Zapata et al. [3] performed a postnatal study of 11,000 pathologic specimens. They found that the incidence of ARSA was 2.9% in patients with a CHD and 0.1% in patients with normal hearts, suggesting a correlation between ARSA and CHDs. However, definitive conclusions on the prenatal correlation between CHDs and ARSA cannot be drawn because the data were heavily weighted with cardiovascular abnormalities. In patients with complex heart anomalies, efforts might be focused mainly on the evaluation of intracardiac defects than on the arch abnormalities outside the heart. Therefore, it is possible to miss cases of ARSA in such patients. With this in mind, when complex heart anomalies are detected, efforts should be made to evaluate the presence of ARSA.
Aneurysmal dilatation of the proximal segment of ARSA is not infrequent [8,20]. In 3%-15% of cases, its origin in the thoracic aorta is saccular, which is known as Kommerell diverticulum [8,20-22]. In this study, we diagnosed Kommerell diverticulum with aneurysmal dilatation of the proximal segment of ARSA in a dichorionic diamniotic twin (3.6%). Furthermore, the presence of ARSA is believed to increase one’s susceptibility to atherosclerosis and its complications, including aneurysm, dissection, and stenosis [7,20].
ARSA has a female predominance [5,8]. Our results also reflected a higher incidence of ARSA in females than in males. In contrast, another study revealed an equal gender distribution of ARSA [3].
The present study has some limitations. We performed only second and third trimester ultrasonography without transvaginal first trimester ultrasonography. This might have resulted in a lower incidence than that reported by previous studies. The other limitation was that the postnatal confirmation of the diagnosis of ARSA relied solely on the findings of postnatal echocardiography, without computed tomography angiography. Four children did not undergo postnatal echocardiography.
We now have data reflecting the prevalence of ARSA in an unselected population. In the presence of an isolated case of ARSA, it is possible to discuss the option of karyotyping with the child’s parents.
Notes
No potential conflict of interest relevant to this article was reported.
