Clinical applications of shear wave dispersion imaging for breast lesions: a pictorial essay
Article information
Abstract
Shear wave dispersion (SWD) imaging is a newly developed ultrasound technology designed to evaluate the dispersion slope of shear waves, which is related to tissue viscosity. This advanced imaging technique holds potential for distinguishing malignant lesions from benign lesions and normal breast tissue. The SWD slope, as determined by shear wave elastography (SWE), could offer crucial insights into the characterization of breast lesions. This article presents SWE and SWD images of both malignant and benign breast lesions, in addition to normal breast tissue.
Introduction
Shear wave elastography (SWE) is widely used to evaluate breast lesions [1,2]. The addition of SWE to B-mode ultrasound (US) can improve the diagnostic performance of US in differentiating benign and malignant breast lesions [3-5]. SWE is a US technique that quantifies tissue elasticity by using acoustic radiation forces to generate laterally propagating shear waves. It can measure the propagating shear wave velocity, which differs depending on tissue stiffness [1-5]. Most current SWE approaches use a linear elastic model that only quantifies elasticity, assuming that the tissue is purely elastic. However, human soft tissues show viscoelastic properties.
Shear wave dispersion (SWD) imaging is a newly developed US technique that evaluates the dispersion slope of shear waves, which is related to the viscosity of biological tissues [6-8]. Dispersion refers to the frequency dependence of both the speed and attenuation of shear waves in the viscous component. As the shear wave speed depends on the frequency of shear waves, the dispersion curve can be obtained from an analysis of shear wave speed according to frequency [9]. The dispersion slope is correlated with viscosity. Therefore, an analysis of SWD characteristics can measure tissue viscosity indirectly.
Several recent studies have evaluated the usefulness of SWD imaging in human and rat liver tissues [6-8,10,11]. SWD can be used for evaluating necroinflammation in the liver [6,7]. Lee et al. [7] investigated the role of SWD imaging in detecting allograft damage after liver transplantation. The SWD slope determined using SWE was related to both liver fibrosis and the degree of hepatic necroinflammatory changes, and the SWD slope showed better diagnostic performance than the SWE value in detecting allograft damage after liver transplantation. However, few studies have used SWD imaging to evaluate breast lesions [11,12]. Ormachea et al. [11] obtained SWD images to evaluate breast phantoms and two women (one with a fibroadenoma and one with dense breast tissue). Kumar et al. [12] found a significant difference in shear viscosity between malignant and benign breast masses, with a higher median value for the malignant masses.
This pictorial review aims to assess the feasibility of breast viscosity evaluation using SWD imaging in patients with benign and malignant breast lesions. All images were obtained from women who underwent breast imaging at Korea University Ansan Hospital.
SWD Imaging
In 1951, long before the advent of clinical elastography, Hans Oestreicher proposed that tissues behave as linear and isotropic viscoelastic media, with corresponding stiffness and viscosity [13]. Currently, SWE can measure the group velocity of a collection of shear waves with reasonable diagnostic performance. Tissue stiffness, measured with two-dimensional (2D) SWE, is a widely recommended parameter. However, stiffness and viscosity may provide distinct sets of clinical data because viscosity is a qualitatively different parameter.
SWD maps can be created using an imaging technique incorporated into a US system (Aplio i-series, Canon Medical Systems, Tokyo, Japan) [6-8]. This technique can estimate the dispersion slope of shear wave speed versus frequency to evaluate changes in tissue viscosity. SWD processing involves four main steps (Fig. 1). In the first step, the displacement induced by shear waves is obtained using a technique based on color Doppler scanning. Second, displacement at each location is transformed from the time to the frequency domain through the Fourier transform algorithm to estimate the phase changes in shear waves at different frequencies. Third, the shear wave speed is calculated using the phase-difference method between displacements at each frequency from the following equation:
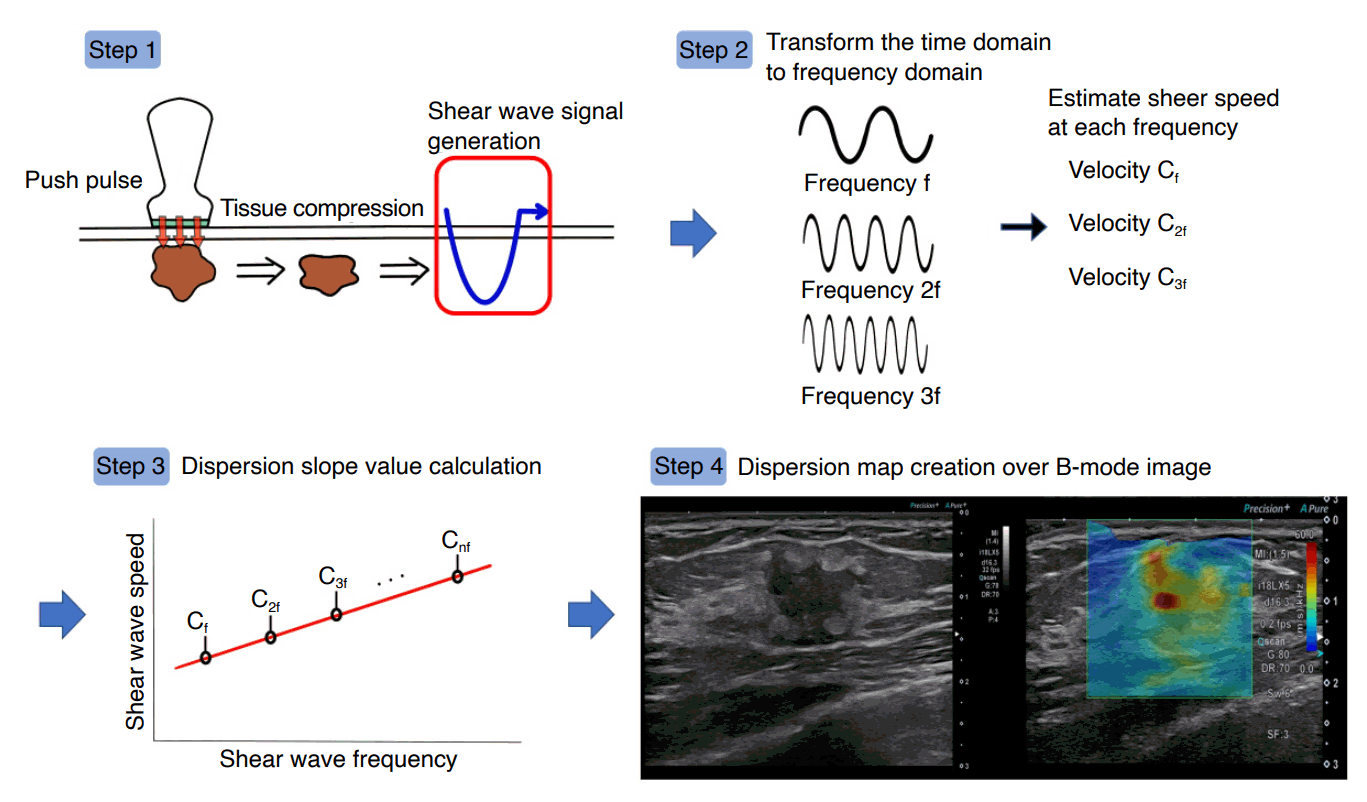
Four main steps of shear wave dispersion map processing.
First, the displacement induced by the shear waves is obtained. Second, displacement at each location is transformed from the time to frequency domains by the fast Fourier transform to estimate phase changes in the shear waves at several frequencies. Third, the shear wave speed is calculated using a phase-difference method. Fourth, the gradient of the shear wave speed is calculated based on the distribution of the shear wave speed versus frequency. The calculated gradient values are then superimposed on the measurement locations to create a dispersion map.
where π is the circular constant, f is the frequency, ∆L is the displacement between the frequencies at each point, ∆ø is the difference between the phases at each point, Cs(nf) is the shear speed at each frequency, and ° defines each element in the equation [7]. Finally, the slope of the shear wave speed is calculated based on the distribution of shear wave speed versus frequency. The shear wave viscosity value can be estimated from the slope over the shear wave frequency bandwidth (Fig. 2). The slope of the dispersion slope is then superimposed on each measurement location.
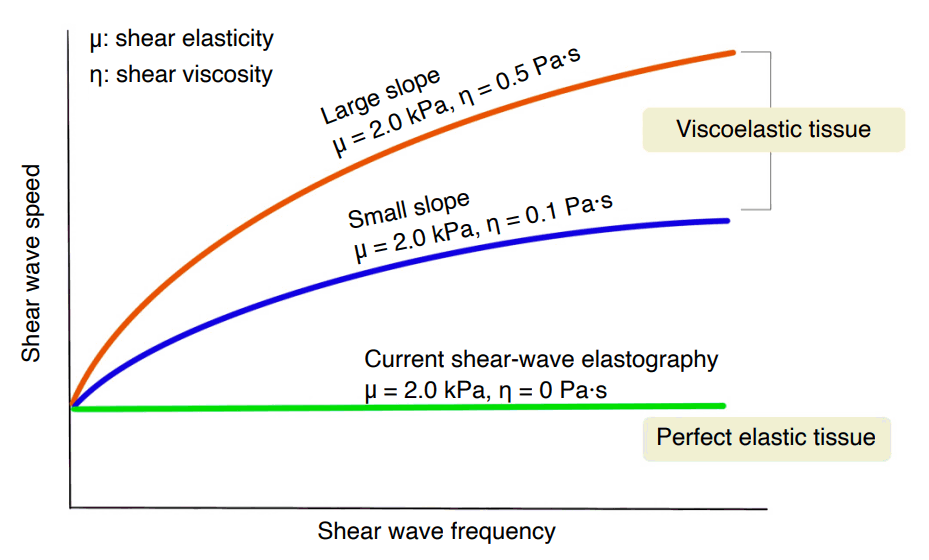
Relationship between shear wave speed and frequency.
In perfectly elastic tissue, the shear wave speed is constant regardless of shear wave frequency. However, in viscoelastic tissue, shear wave speed varies depending on shear wave frequency. If the shear elasticity is fixed at 2.0 kPa and the shear viscosity varies from 0.1 to 0.5 Pa·s, the slope increases according to the shear viscosity level. The slope correlates with the viscosity coefficient.
SWD imaging can be activated automatically in SWE mode using the Aplio i-series (Canon Medical Systems). In SWE quad-view mode, it is possible to simultaneously view the shear wave elasticity (elasticity map), SWE arrival time contours (propagation map), grayscale US, and SWD slope (dispersion map) (Fig. 3). The dispersion slope value ([m/s]/kHz), elasticity value (kPa), and their standard deviations are displayed for quantitative assessment.
SWD Evaluation of Breast Lesions
A radiologist experienced in breast imaging (24 years of experience in breast US) performed 2D SWE and SWD imaging examinations using an Aplio i800 system with a linear probe (US frequency range, 5-18 MHz; PLI-1205BX, Canon Medical Systems). The SWD imaging technology for breast examination was a prototype. For quantitative measurements, the radiologist placed a 2-mm circular region of interest on the fibroglandular breast tissue in ultrasonographic negative patients and the areas with the highest SWE and SWD responses in patients with breast lesions [1]. Subcutaneous fat was selected as the reference area for both the elasticity and dispersion ratios. All SWE and SWD measurements were repeated at least three times per patient [14].
In normal breast tissue, tissue elasticity and viscosity tended to be similar to each other in the fibroglandular tissue, and were slightly higher than the corresponding values in the subcutaneous fat tissue (Fig. 4). In cases of benign breast lesions, the elasticity value was in the range of 10-20 kPa, while the dispersion slope value was approximately 10 (m/s)/kHz, which is similar to that of fibroglandular tissue (Figs. 5, 6). However, the elasticity values of malignant breast lesions notably exceeded 50 kPa, and the dispersion slope values were above 20 (m/s)/kHz in most malignant lesions (Figs. 7-14), which may help distinguish malignant lesions from both benign lesions and normal fibroglandular tissue. Several prior studies have evaluated the SWE characteristics among different histologic types or molecular subtypes of invasive breast cancers [15-18]. However, no studies have assessed these features in SWD imaging.

Shear wave dispersion imaging in normal breast tissue.
A circular 2-mm region of interest was placed on the fibroglandular tissue and subcutaneous fat. Homogeneous background echotexture (A) and heterogeneous background echotexture (B) are shown on breast ultrasonography.

A 50-year-old woman with a benign breast mass.
Grayscale ultrasound (US) shows an oval, microlobulated, hypoechoic mass at 1 o’clock in the left breast. The stiffness value on the elasticity map is 6.8 kPa, and the dispersion slope value on its map is 7.81 (m/s)/kHz. US-guided core-needle biopsy diagnosed this mass pathologically as sclerosing adenosis with fibrocystic change.
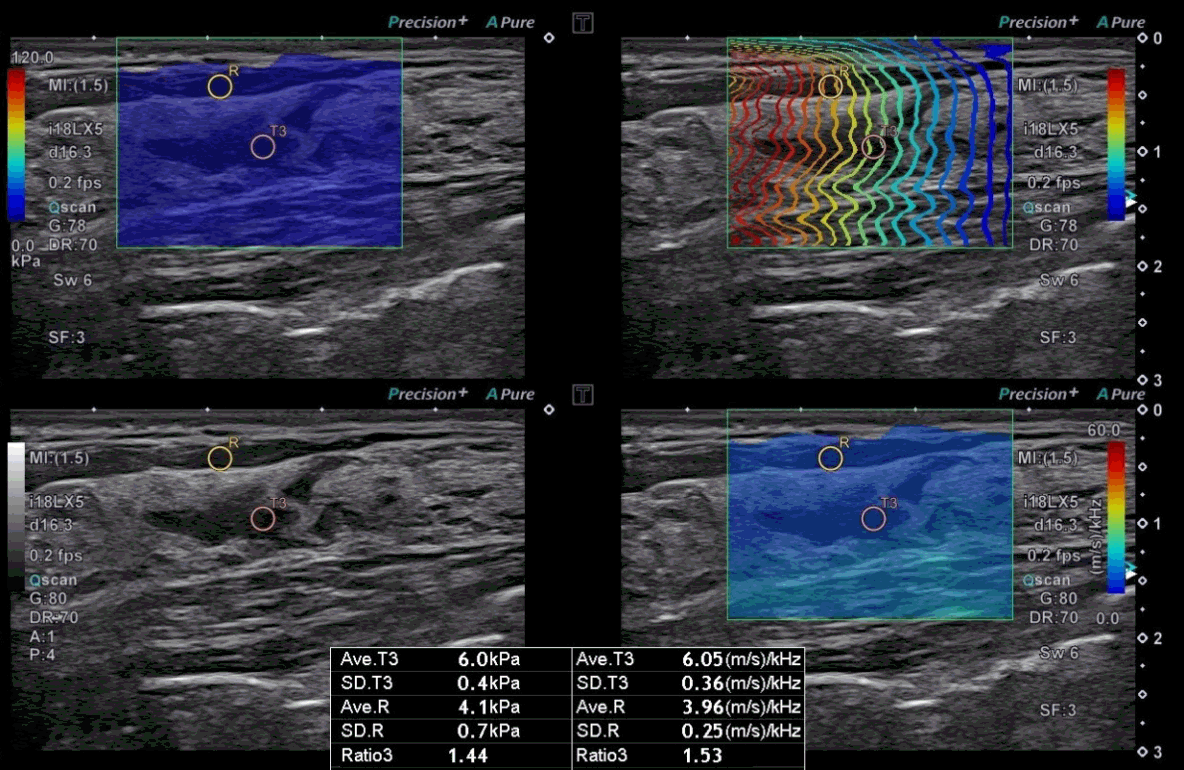
A 50-year-old woman with a benign non-mass lesion.
On grayscale ultrasound (US), a non-mass lesion is seen at 2 o’clock in the left breast. The stiffness value on the elasticity map is 6.0 kPa, and the dispersion slope value on its map is 6.05 (m/s)/kHz. US-guided coreneedle biopsy pathologically confirmed that the lesion was adenosis with microcalcifications and fibrocystic change.
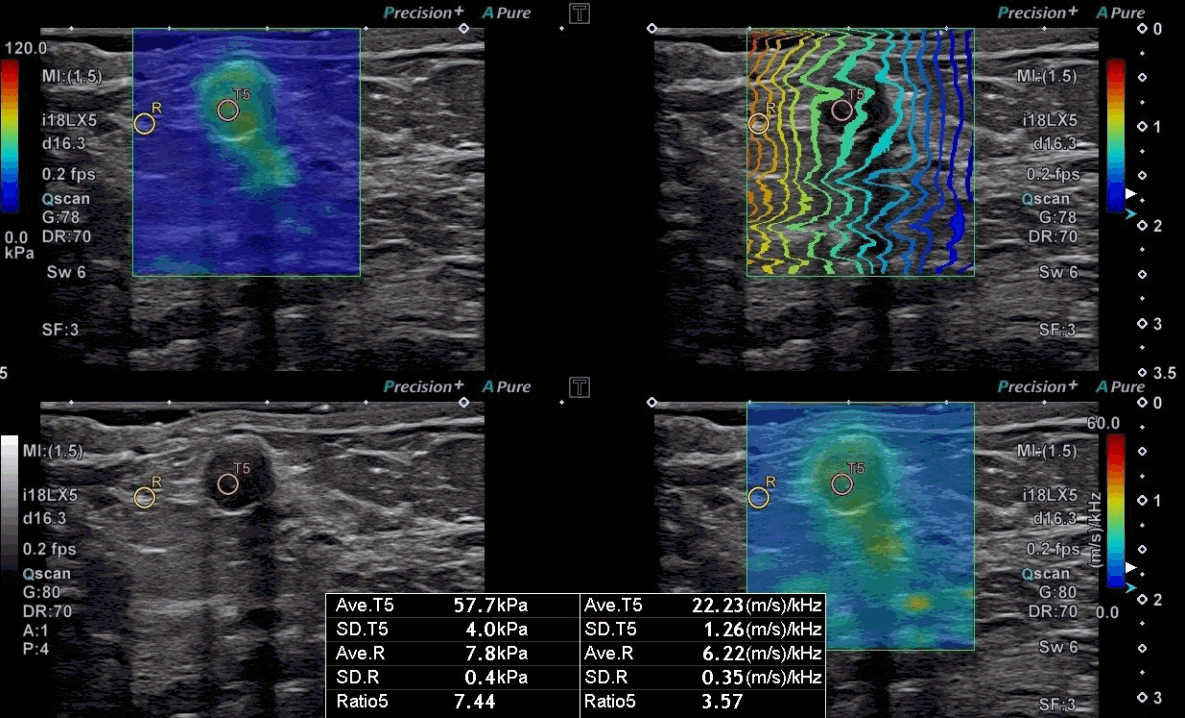
A 57-year-old woman with ductal carcinoma in situ.
A grayscale ultrasound image shows an oval hypoechoic mass with partially indistinct margins at 3 o’clock in the right breast. The stiffness value on the elasticity map is 57.7 kPa, and the dispersion slope value on its map is 22.23 (m/s)/kHz. The mass was diagnosed as ductal carcinoma in situ by surgical excision.
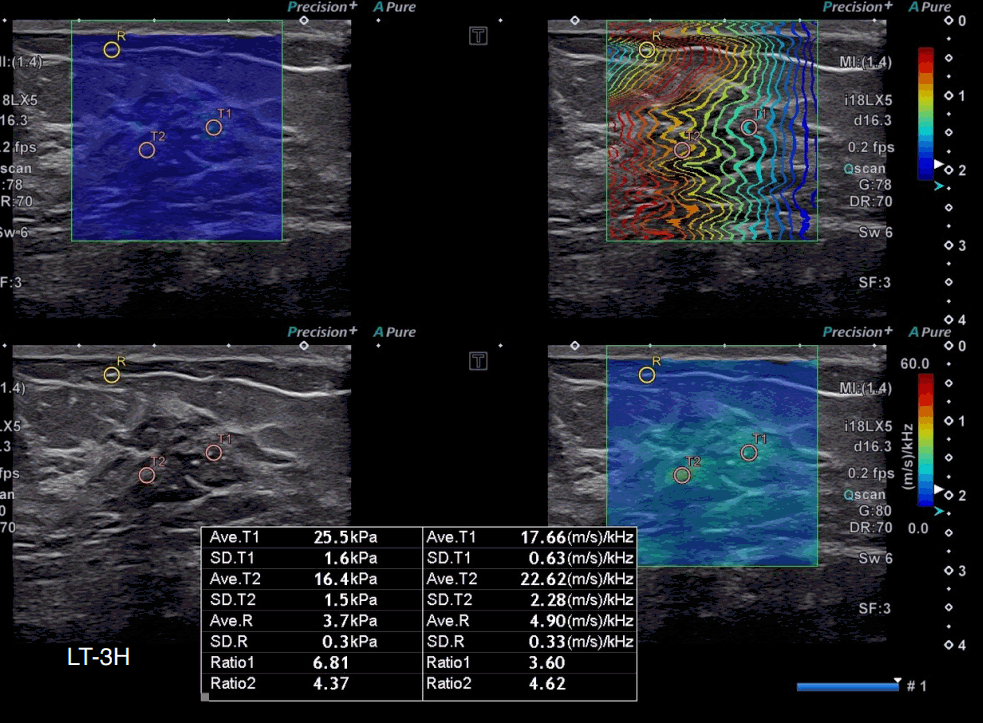
A 48-year-old woman with microinvasive carcinoma.
On grayscale ultrasound (US), an indistinct hypoechoic non-mass lesion with internal calcifications is seen at 3 o’clock in the left breast. The highest stiffness value on the elasticity map (T1) is 25.5 kPa, and the highest dispersion slope value on its map (T2) is 22.62 (m/s)/kHz. US-guided core-needle biopsy diagnosed the lesion as ductal carcinoma in situ, and surgical excision confirmed microinvasive carcinoma.
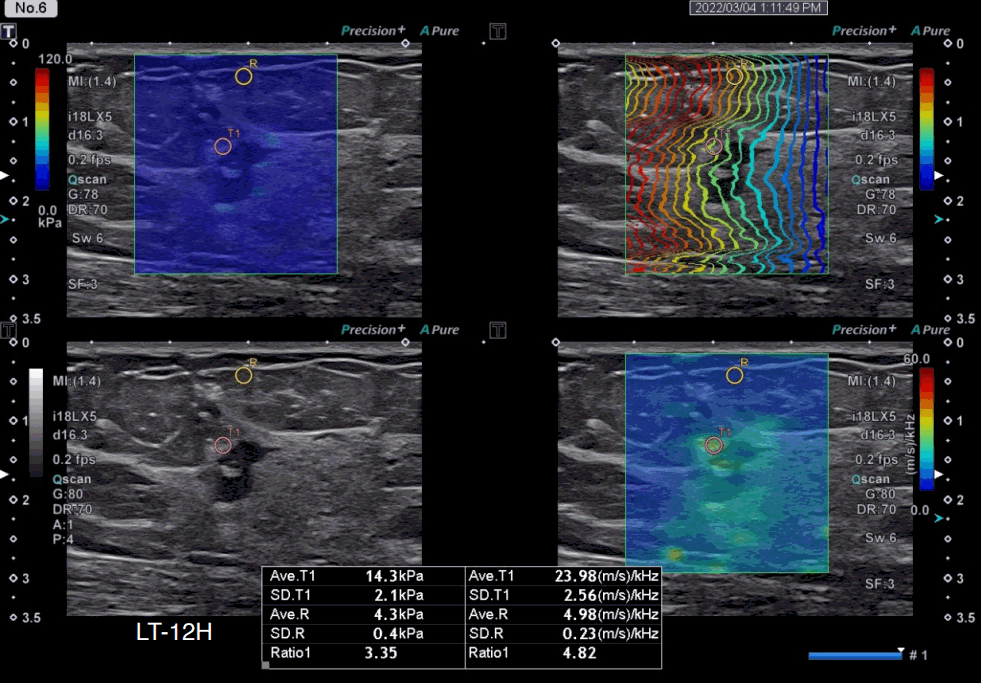
A 50-year-old woman with invasive lobular carcinoma, luminal A subtype.
A grayscale ultrasound image shows an irregular hypoechoic mass with angular margins at 12 o’clock in the left breast. The stiffness value on the elasticity map is 14.3 kPa, and the dispersion slope value on its map is 23.98 (m/s)/kHz. The breast cancer molecular subtype was luminal A (estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, and Ki-67 index <5%).
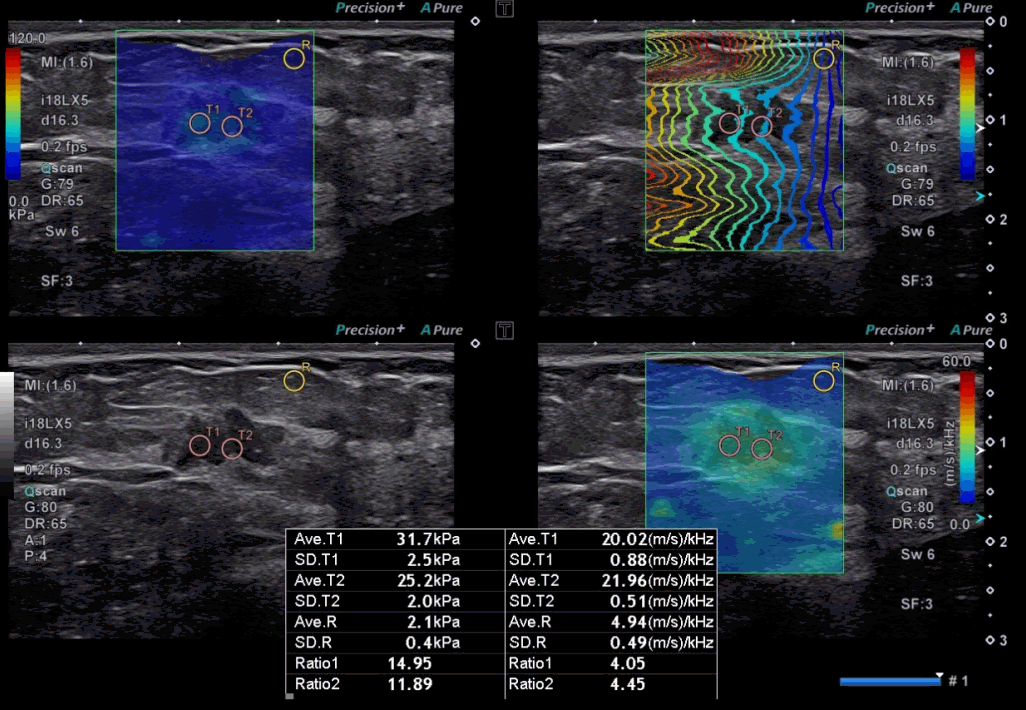
A 49-year-old woman with invasive ductal carcinoma, luminal A subtype.
A grayscale ultrasound image demonstrates an irregular indistinct hypoechoic mass at 1 o’clock in the left breast. The highest stiffness value on the elasticity map (T1) is 31.7 kPa, and the highest dispersion slope value on its map (T2) is 21.96 (m/s)/kHz. The breast cancer molecular subtype was luminal A (estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, and Ki-67 index <10%).

A 67-year-old woman with invasive ductal carcinoma, luminal B subtype.
A grayscale ultrasound image shows a spiculated irregular hypoechoic mass at 10 o’clock in the right breast. The stiffness value on the elasticity map is 57.8 kPa, and the dispersion slope value on its map is 24.47 (m/s)/kHz. The breast cancer molecular subtype was luminal B (estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, and Ki-67 index >14%).

A 62-year-old woman with mucinous carcinoma, luminal B subtype.
A grayscale ultrasound image shows an indistinct oval isoechoic mass at 7 o’clock direction of the right breast. The highest stiffness value on the elasticity map (T1) was 136.7 kPa, and the highest value of dispersion slope on its map was 48.10 (m/s)/kHz. The area showing low value on the elasticity map is correlated with the area of high value on dispersion map (yellow arrows), indicating high viscosity of the mass. Surgical pathology revealed a 1.8-cm mucinous carcinoma, luminal B subtype (estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, and Ki-67 index >14%).
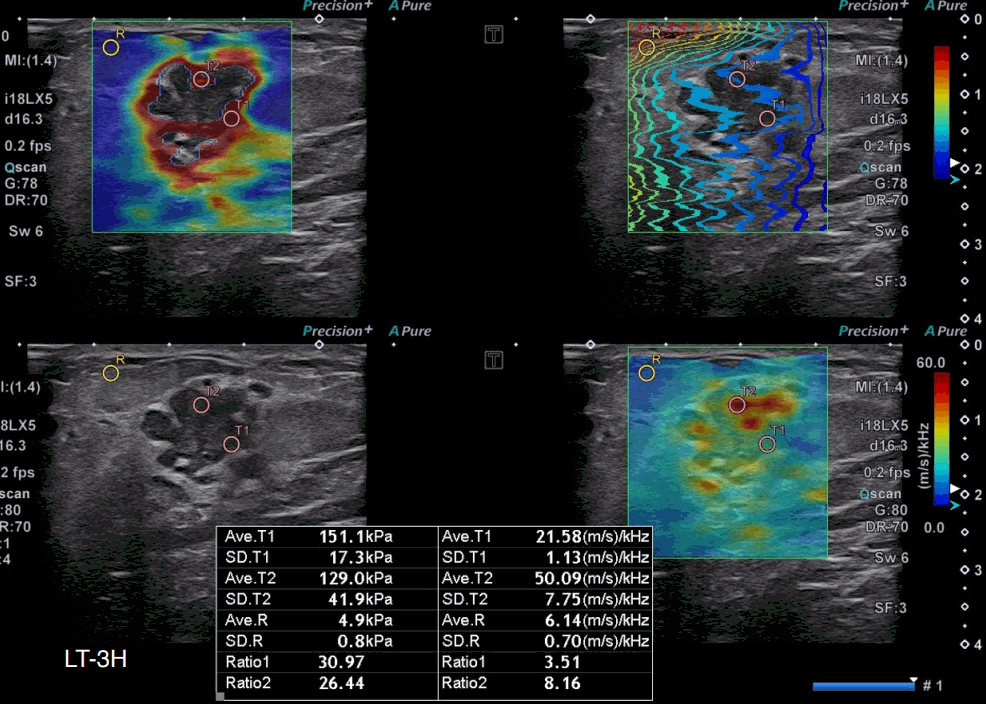
A 40-year-old woman with invasive ductal carcinoma, human epidermal growth factor receptor 2 (HER2)–enriched subtype.
A grayscale ultrasound image shows an irregular, microlobulated, hypoechoic mass at 3 o’clock in the left breast. The highest stiffness value on the elasticity map (T1) is 151.1 kPa, and the highest dispersion slope value on its map (T2) is 50.09 (m/s)/kHz. The molecular subtype was the HER-enriched subtype (estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-positive).
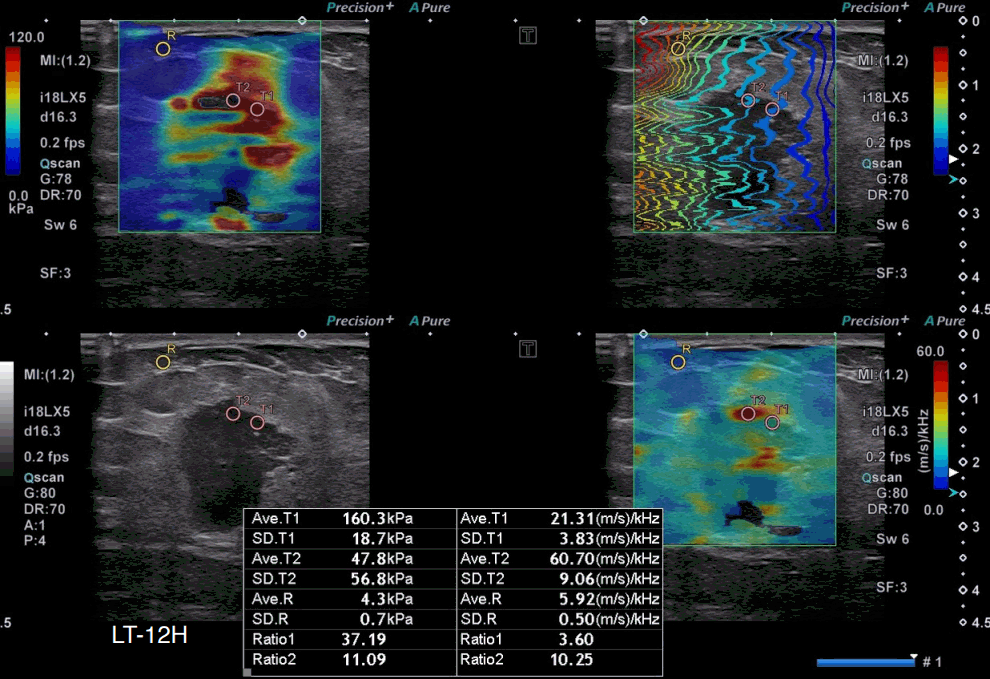
A 51-year-old woman with invasive ductal carcinoma, triple-negative subtype.
A grayscale ultrasound image shows an indistinct oval hypoechoic mass with a nonparallel orientation at 12 o’clock in the left breast. The highest stiffness value on the elasticity map (T1) is 160.3 kPa, and the highest dispersion slope value on its map (T2) is 60.70 (m/s)/kHz. Surgical pathology revealed a 2.5-cm invasive ductal carcinoma of no specific type, which was confirmed as triple-negative (estrogen receptor–negative, progesterone receptor–negative, and human epidermal growth factor receptor 2–negative).
Conclusion
This pictorial essay shows the SWE and SWD images of benign and malignant lesions on breast US. The SWD slope determined by 2D SWE may provide more detailed and useful information regarding the characterization of breast lesions. We suggest that SWD imaging can help differentiate malignant from benign breast lesions. This possibility needs to be verified in further studies with larger numbers of participants.
Notes
Author Contributions
Conceptualization: Seo BK. Data acquisition: Seo BK. Data analysis or interpretation: Bae MS, Kim HY, Oh H, Seo BK. Drafting of the manuscript: Bae MS, Kim HY, Oh H, Seo BK. Critical revision of the manuscript: Bae MS, Seo BK. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) grant (No. NRF-2021R1A2C1010565) funded by the Korean government (Ministry of Science and ICT) and Canon Medical Systems.
References
Article information Continued
Notes
Key point
Shear wave dispersion (SWD) imaging is a new ultrasound technique for assessing the dispersion slope of shear waves. This article reports the authors’ preliminary clinical experience with SWD imaging for breast lesions.

