Value of contrast-enhanced ultrasonography in microwave ablation treatment of symptomatic focal uterine adenomyosis
Article information
Abstract
Purpose
This study evaluated the value of contrast-enhanced ultrasonography (CEUS) in the ultrasound-guided microwave ablation (MWA) treatment of symptomatic focal uterine adenomyosis.
Methods
This retrospective study was conducted between March 2020 and January 2023, enrolling 52 patients with symptomatic focal uterine adenomyosis who had undergone MWA. All patients were examined with CEUS before and after MWA. The non-perfused volume (NPV) was compared between CEUS and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) following ablation. Therapeutic efficacy and safety were evaluated at 3-, 6-, and 12-month follow-ups. Additionally, this study explored the correlations between pre-treatment CEUS features and a volume reduction ratio indicating sufficient ablation, defined as 50% or more at the 3-month follow-up.
Results
No significant differences in NPV were noted between CEUS and DCE-MRI immediately after MWA and during follow-up (all P>0.05). At the 3-month follow-up, the median VRRs for the uterus and adenomyosis were 33.2% and 63.9%, respectively. Sufficient ablation was achieved in 69.2% (36/52) of adenomyosis cases, while partial ablation was observed in the remaining 30.8% (16/52). The identification of non-enhancing areas on pre-treatment CEUS was associated with sufficient ablation (P=0.016). At the 12-month follow-up, significant decreases were observed in both the uterine and adenomyosis volumes (all P<0.001). Dysmenorrhea and menorrhagia were significantly alleviated at 12 months, and no major complications were encountered.
Conclusion
CEUS can be used to evaluate the ablation zone of focal adenomyosis that has been treated with MWA, similarly to DCE-MRI. The identification of non-enhancing areas on pretreatment CEUS indicates satisfactory treatment outcomes.
Introduction
Adenomyosis is a common condition in women of reproductive age, with incidence rates of 8% to 27% [1,2]. This disease can cause clinical symptoms such as dysmenorrhea, menorrhagia, chronic pelvic pain, and infertility [3]. Patients with adenomyosis experience these symptoms on a chronic basis, substantially impacting their quality of life [2,3]. Currently, the treatment options for adenomyosis encompass medical treatment, surgical procedures, and thermal ablation therapy [4]. Medical treatment for adenomyosis typically involves nonsteroidal anti-inflammatory drugs, combined oral contraceptive pills, and gonadotropin-releasing hormone agonists. However, oral pills may not always effectively alleviate symptoms and can sometimes have intolerable side effects [5]. Hysterectomy is a viable option for patients without fertility concerns, but postoperative recovery may be slow [6-8]. Consequently, increasingly many adenomyosis patients are opting for minimally invasive techniques as their preferred method of treatment.
Ultrasound (US)-guided microwave ablation (MWA) is a novel minimally invasive technique that offers several benefits, including ease of operation, minimal trauma, rapid recovery, and few complications. In recent years, US-guided MWA has become increasingly popular for treating a variety of solid tumors, particularly those in the thyroid and liver [9,10]. The thermal effect produced by MWA induces coagulative necrosis in the viable tissue and micro-vessels within the targeted areas, leading to the reduction or elimination of the targeted lesions [11]. Numerous studies have demonstrated that MWA is a safe and effective alternative for the clinical treatment of symptomatic adenomyosis [12-15].
While most patients experience substantial symptom improvement, the inadequate reduction of lesions often produces dissatisfaction with the efficacy of ablation. Zhang et al. [16] previously suggested that the 3-month mark following MWA could serve as an evaluation point for lesion absorption rates. Typically, the efficacy of thermal ablation is assessed using contrast-enhanced T1-weighted images (T1WI) as part of magnetic resonance imaging (MRI) to determine the non-perfused volume (NPV). However, MRI is time-consuming and can be hindered by metallic implants. Furthermore, the European Society of Urogenital Radiology’s contrast media guidelines highlight the potential for serious adverse reactions, such as severe cardiac dysrhythmias, arrest, and cardiovascular and pulmonary collapse, due to molecules of the MR contrast medium [17]. Contrast-enhanced US (CEUS) is a non-invasive imaging tool that provides information on the blood perfusion of the lesion. It has been extensively used to diagnose and evaluate the efficacy of thermal ablation through scans of the tumor before and after the procedure [18,19]. The microbubble US contrast agent consists of inert gas and a phospholipid layer. It is known to be hypoallergenic and well-tolerated, with few serious adverse reactions. Moreover, CEUS offers several advantages over MRI, including speed, real-time imaging, easy repeatability, and an absence of renal toxicity. Clinically, CEUS has been used to evaluate NPV immediately after MWA for adenomyosis [20], but no investigation has thoroughly assessed the value of CEUS when employed before and after this procedure. Therefore, the primary objective of this study was to determine whether CEUS could serve as a viable alternative to MRI in assessing NPV following MWA. The secondary objective was to investigate whether pre-treatment CEUS could be used to predict the adequacy of MWA treatment outcomes for adenomyosis.
Materials and Methods
Compliance with Ethical Standards
The ethics committee of the institution (Zhongshan Hospital, Fudan University) granted approval for this study (No. B2022-222R). Given the retrospective design and the fact that only clinical and imaging data were collected, the requirement for written informed consent was waived.
Patients
This retrospective study was conducted at three tertiary hospitals in China between March 2020 and January 2023.
To qualify for the study, participants had to meet the following inclusion criteria: (1) a diagnosis of focal adenomyosis, confirmed through medical history, clinical presentation, laboratory tests, US imaging, and MRI; (2) the presence of clinical symptoms related to adenomyosis; (3) no desire for future fertility; (4) refusal to undergo hysterectomy; and (5) no history of treatment. The exclusion criteria were as follows: (1) patients who did not participate in follow-up; (2) patients with incomplete imaging data or clinical information; and (3) patients who had other malignant neoplasms within the past 5 years. Ultimately, the cohort consisted of 52 patients who underwent MWA. A flowchart detailing the patient selection process is provided in Fig. 1.
Pre-treatment Assessment
During the initial consultation, patients’ demographic data and clinical symptoms were meticulously recorded. The severity of dysmenorrhea was quantified using a visual analog scale (VAS), which had a score range of 0-10 (with 0 representing no pain and 10 indicating the most severe pain) [21]. Menstrual blood volume was evaluated based on the pictorial blood loss assessment chart (PBAC). A PBAC score within the range of 45-100 is considered normal, while a score exceeding 100 indicates menometrorrhagia [22,23]. The pre-treatment imaging examination included both US and MRI scans. The US examinations were performed with a LOGIQ E9 US machine (GE Healthcare, Milwaukee, WI, USA) and a LOGIQ E10 US machine (GE Healthcare), equipped with a 1-5 MHz convex array transducer. Using conventional US, the location and the three orthogonal diameters (a, b, and c) of the uterus and adenomyosis were recorded. The volume was calculated according to the following formula: volume (V)=π/6×a×b×c. CEUS was employed to evaluate the blood perfusion of adenomyosis prior to MWA. Two radiologists (L.X.L., with 8 years of CEUS experience, and J.X.L., with 3 years of CEUS experience) independently evaluated the characteristics. SonoVue (Bracco, Milan, Italy), which consists of phospholipid-stabilized shell microbubbles filled with sulfur hexafluoride gas, was used as the contrast agent. After mixing with 5 mL of 0.9% normal saline and vibration blending, 2.0 mL of SonoVue was injected into the antecubital vein as a bolus. Then, 5 mL of 0.9% normal saline was injected to flush the vein. Contrast enhancement was observed for at least 6 minutes after injection of the contrast agent, and images were stored in the longitudinal and transverse planes. During the scan, the following lesion features were observed and recorded (Fig. 2): fast-in (absent/present), enhanced homogeneity (homogeneous/heterogeneous), early hyperenhancement (absent/present), enhancement margin (circumscribed/non-circumscribed), non-enhancing areas in the lesion (absent/present), penetrating vessel (absent/present), and fast-out (absent/present). The fast-in pattern was defined as the appearance of microbubbles in the adenomyosis earlier than in the surrounding uterine tissue, 10-20 seconds post-injection. The fast-out pattern was characterized as the enhancement intensity of the adenomyosis appearing lower than that of the surrounding tissues, 40–60 seconds post-injection. Non-enhancing areas were identified based on the absence of enhancement in all phases during the CEUS. The enhancement margin was considered non-circumscribed when the distinct lesion boundary was less than 50%. For MRI examinations, 3.0-T MRI scanners (Magnetom Verio, Siemens AG, Munich, Germany and GE750, GE Healthcare) were applied. Gadopentetate dimeglumine (Magnevist, Bayer AG, Leverkusen, Germany) was used as the contrast medium. Dynamic contrast-enhanced MRI (DCE-MRI) was used as a reference standard to evaluate NPV.

Examples of contrast-enhanced ultrasonography features.
A. Adenomyosis (arrows) exhibits "fast-in" enhancement: microbubbles appear in the lesion 12 seconds after injection of the contrast agent (thick arrow). B. Adenomyosis (arrows) displays homogeneous enhancement and a circumscribed enhancement margin; the degree of enhancement across the entire lesion is uniform, with clear separation from the surrounding myometrium. C. The adenomyosis (arrows) shows heterogeneous enhancement and a non-circumscribed enhancement margin. The degree of enhancement across the entire fibroid (arrows) is not uniform, with an unclear boundary between the lesion and the surrounding myometrium. D. The adenomyosis (arrows) contains non-enhancing areas: some regions (thick arrows) within the lesion exhibit no perfusion during the early phase (30 seconds after the contrast agent is injected). E. The adenomyosis (arrows) features a penetrating vessel. Specifically, a coarse or twisted penetrating vessel (thick arrows) is visible within the lesion during the early phase. F. The adenomyosis (arrows) demonstrates rapid washout: the enhancement intensity of the lesion appears lower (thick arrows) than that of the surrounding tissues 49 seconds after injection.
Percutaneous MWA
Prior to MWA, a series of laboratory tests were conducted, including tests of blood routine, coagulation function, liver function, and kidney function and levels of electrolytes, serum tumor markers, and sex hormones. To rule out contraindications, chest X-rays and electrocardiograms were also performed before treatment. All MWA procedures were conducted under real-time US guidance by radiologists (Y.S.Y., E.J.X., and Z.B.J.) with over 10 years of experience in interventional treatment. MWA was performed using a monopolar water-cooling MWA system (MTI-5A, 2,450 MHz, GreatWall Medical Equipment Co. Ltd., Nanjing, China) equipped with a 2-mm-thick, 18-cm-long monopolar MWA antenna (XR-A2018W) or an MWA system (KY-2000, 2,450 MHz, Canyon Medical Inc., Nanjing, China) equipped with a 1.9-mm-thick, 18-cm-long MWA antenna (KY-2450B). For better visualization of adenomyosis on US, the patient was placed in the supine position with full abdominal exposure. The MWA treatments were administered under either general anesthesia or local anesthesia with sedation. Local infiltration anesthesia was achieved by injecting 2% lidocaine, while midazolam was administered for sedation 10 minutes prior to MWA. Once the optimal path was confirmed, the microwave antenna was inserted into the adenomyosis. For lesions located near the intestinal tract, blood vessels, and other critical structures, the hydrodissection technique was employed. A normal saline solution was injected to create a liquid isolation area of more than 5 mm between the adenomyosis and other structures. The entire ablation procedure was performed under real-time US guidance using the "moving shot" technique. When the tip was properly positioned, the microwave device was activated and set to 50-60 W. A hyperechogenic signal could be observed within the lesions. Following the procedure, an immediate treatment response evaluation of the ablation zone was conducted using CEUS. The ablation procedure was considered complete if the non-enhanced regions covered the lesion. If not, additional MWA was necessary to treat the enhanced part of the lesion.
Evaluation of Clinical Results
All study participants underwent CEUS and MRI examinations 1-3 days following MWA. Patients were clinically evaluated at 3-, 6-, and 12-month post-treatment, with tests including imaging examinations (conventional US, CEUS, and DCE-MRI), laboratory tests, and an assessment of symptom relief. Using conventional US, the three orthogonal diameters (a, b, c) of the uterus and adenomyosis post-MWA were documented. Postoperative lesions appeared as inhomogeneous echoes, clearly distinguishable from the myometrium. In contrast, with CEUS and DCE-MRI, the three orthogonal diameters of the non-perfused area were also documented. The volume was computed using the formula previously mentioned. The volume reduction ratio (VRR) of adenomyosis was determined using the equation: VRR=[(initial volume-final volume)×100%]/initial volume. Additionally, any side effects from MWA were recorded and classified according to the Clavien-Dindo complication grading system [24]. At the 3-month follow-up, patients with adenomyosis VRRs of ≥50% were deemed to have achieved sufficient ablation [18].
Statistical Analysis
SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was employed for statistical analysis. Categorical variables were expressed as percentages, while continuous variables were presented as medians and interquartile ranges (IQRs; representing the 25th to 75th percentile). The Wilcoxon signed-rank test was utilized to examine differences in continuous data at the time of enrollment and at the 3-, 6-, and 12-month follow-up points. P-values of less than 0.05 were considered to indicate statistical significance. Cohen kappa tests were used to evaluate inter-observer variation in characteristics of adenomyosis on CEUS. The agreement was categorized as excellent (kappa, 0.81-1.0), good (kappa, 0.61-0.80), fair (kappa, 0.41-0.60), or poor (kappa, 0.00-0.40).
Results
Patient Characteristics
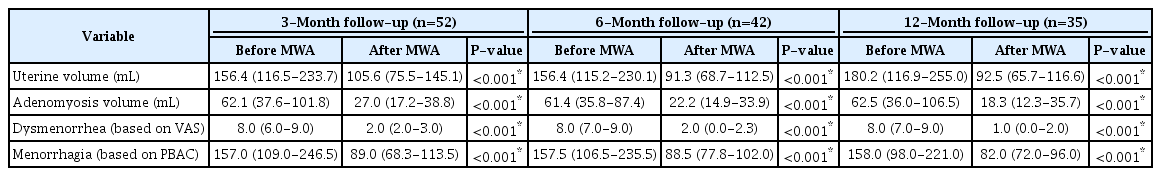
Prior to MWA, the median uterine volume was recorded as 156.4 mL (IQR, 116.5 to 233.7), while the median volume of adenomyosis was 62.1 mL (IQR, 37.6 to 101.8). The median longest diameter of adenomyosis was measured at 58.0 mm (IQR, 50.0 to 69.8). A total of 47 patients reported dysmenorrhea and/or menorrhagia. The severity of dysmenorrhea, as measured on the VAS, was 8.0 (IQR, 6.3 to 9.0). The menstrual volume score, as determined by the PBAC, was 157.0 (IQR, 109.0 to 246.5). Among the patients, 28.8% (15 of 52) reported a diagnosis of anemia or a hemoglobin level below 110 g/L. Regarding the location of adenomyosis, the majority (43 of 52, or 82.7%) were found in the posterior wall, while the remainder (9 of 52, or 17.3%) were in the anterior wall. The characteristics of all patients prior to MWA are outlined in Table 1.
Post-ablation Assessment of NPV
Following MWA, the ablated zone did not exhibit enhancement on CEUS. The immediate median NPV ratio of the 52 adenomyosis cases was 94.0% (IQR, 92.3% to 95.0%) on contrast-enhanced T1WI and 95.0% (IQR, 93.0% to 96.0%) on CEUS. When comparing the NPV ratios of the two techniques, the difference was not statistically significant (P=0.055). No significant differences in NPV were found between DCE-MRI and CEUS immediately after MWA or during follow-up (all P>0.05) (Table 2).
Post-ablation Follow-up Outcomes
In this study, all 52 patients successfully underwent MWA. Changes in uterine volume, adenomyosis volume, dysmenorrhea, and menorrhagia at the 3-, 6-, and 12-month follow-up points are detailed in Table 3. The median percentage reduction in adenomyosis was recorded as 58.7%, 61.9%, and 70.4% at the 3-, 6-, and 12-month follow-ups, respectively. In line with the statistically significant decrease in adenomyosis volume over the follow-up period, the median percentage reduction in uterine volume was 33.2%, 42.8%, and 45.8% at 3, 6, and 12 months, respectively. However, none of the lesions had completely disappeared at the 12-month follow-up. The patients’ pain scores for dysmenorrhea showed a statistically significant decrease at each follow-up stage (all P<0.001). All patients reported a reduction in menstrual blood volume at the 3-, 6-, and 12-month follow-up assessments.
Pre-treatment CEUS Features for Evaluation of Efficacy
At the 3-month follow-up, 36 lesions (69.2%) exhibited VRRs exceeding 50%, indicating sufficient ablation. Of these, 2 lesions (5.6%) demonstrated VRRs of above 70%. The median VRRs for the 36 cases involving sufficiently ablated adenomyosis and the 16 cases of partial ablation were 60.4% (IQR, 58.5% to 64.2%) and 33.0% (IQR, 24.3% to 39.5%), respectively. The inter-observer agreements for the CEUS features of fast-in, homogeneous enhancement, non-circumscribed enhancement margin, non-enhancing area, penetrating vessel, and fast-out were respectively categorized as good-to-fair (kappa value, 0.75), excellent (0.84), good-to-fair (0.61), excellent (0.86), good-to-fair (0.74), and good-to-fair (0.73) (Table 4). The rate of non-enhancing area identification on pre-treatment CEUS differed significantly between the group with sufficient ablation and the group with partial ablation (P=0.016). This was identified as a key factor influencing mid-term ablative efficiency (Fig. 3). Apart from non-enhancing areas, the other six pre-treatment CEUS features—fast-in, hyperenhancement, homogeneous enhancement, enhancement margin, penetrating vessel, and fast-out—exhibited no statistical differences (all P>0.05) (Table 5).

Images of a 34-year-old woman with adenomyosis who underwent microwave ablation (MWA) treatment, demonstrating a volume reduction rate of 50% or more at the 3-month follow-up.
A. Conventional B-mode ultrasonography (US) indicates adenomyosis located in the posterior wall of the uterus, which measured 280.6 mL in volume prior to MWA (arrows). B. Color Doppler US reveals blood flow evident within the lesion (arrows). C. A US image displays the appearance of hyperechoic gas around the antenna during the ablation procedure, which occurs when MWA is initiated (arrows). D. Following MWA, contrast-enhanced ultrasonography was employed to evaluate the necrotic area, with the non-perfused area appearing as a non-enhancing region (arrows). E. Axial T1-weighted contrast-enhanced magnetic resonance imaging after ablation reveals the adenomyosis without enhancement (arrows). F. At 3-month follow-up, the volume of the adenomyosis (arrows) had decreased to 88.4 mL, representing a 68.5% reduction from the initial lesion size.
Periprocedural Complications
Only 23.1% of patients (12 of 52) underwent MWA treatment under general anesthesia. No major complications were detected, and all patients were discharged within 2-4 days following treatment. Minor complications, including abdominal pain, fever, nausea, and vaginal discharge, were observed. A total of 25 patients (48.1%) experienced abdominal pain, classified as a grade I complication. Among these patients, 13 (52.0%) experienced mild pain that did not necessitate medical intervention, while 12 (48.0%) required an additional 500 mg of oral acetaminophen for 1-2 days after MWA. Slight fever, indicated by body temperature ranging from 37.3oC to 38.5oC, was reported in 16 patients (30.8%). This mild fever, also a grade I complication, lasted for 1-2 days and did not require medical treatment. Mild nausea, another grade I complication, was experienced by 12 patients (23.1%) and resolved spontaneously. Vaginal discharge, observed in 22 of the 52 patients (42.3%), completely disappeared within 40 days post-MWA. This was also classified as a grade I complication.
Discussion
In the context of adenomyosis treatment, patients do not broadly accept traditional surgical procedures and long-term medication regimens. In recent years, in situ ablation treatment has been extensively researched and implemented, offering an effective alternative for preserving the uterus in cases of adenomyosis. In 2011, Zhang et al. [25] published the first report on the use of US-guided percutaneous MWA to treat adenomyosis. Percutaneous MWA, a minimally invasive local treatment technique, has been applied in clinical settings for the treatment of adenomyosis. As demonstrated in a meta-analysis by Liu et al. [26], which included 38 studies with 15,908 patients, MWA has emerged as an effective method for the clinical treatment of symptomatic adenomyosis. Unlike previously published studies, the present research investigated the value of CEUS features in assessing and predicting treatment response.
In the present study, US-guided MWA for symptomatic adenomyosis resulted in significant decreases in median uterine volume and lesion volume (45.8% and 70.4%, respectively) at 12 months. These results are superior to those achieved with high-intensity focused US treatment for adenomyosis, as reported by Long et al. [27], which showed decreases of 22% and 30%. Another method for inducing necrosis in adenomyosis tissue is radiofrequency ablation (RFA). Hai et al. [28] reported that the VRRs of mean uterine and adenomyosis volume were 41.2% and 54.7%, respectively, at 12 months after RFA. When comparing the outcomes of MWA and RFA, the present results appear more promising. Therefore, it is clear that MWA, as a minimally invasive technique, is anticipated to become an increasingly practical approach in the treatment of adenomyosis.
The present findings suggest that CEUS can be used to evaluate the ablation zone immediately following MWA and during subsequent follow-up, yielding results comparable to those obtained through MRI. In a clinical setting, it is crucial to promptly determine if an adequate ablation zone has been established. If post-ablation MRI or computed tomography scans reveal that the target lesion has not been sufficiently ablated, a secondary operation may be required. This can prolong the patient’s hospital stay and inflict additional trauma. CEUS addresses these issues by facilitating immediate assessment of treatment responses for thermal ablation of adenomyosis and guiding any necessary supplementary ablation. Furthermore, while MRI has traditionally been employed as a follow-up assessment tool after tumor ablation, it does have certain limitations. This research indicates that CEUS could serve as an alternative method for follow-up after adenomyosis ablation.
No previous studies have been published on the correlation between the enhancement mode of adenomyosis and the efficacy of MWA. However, the findings of this study suggest that cases involving adenomyosis with non-enhancing areas on pre-treatment CEUS tend to exhibit satisfactory treatment outcomes 3 months after MWA. The presence of non-enhancing areas in the lesion is considered indicative of sufficient ablation. One possible explanation for this result could be the existence of cystic spaces within the lesions. Bergeron et al. [29] reported that partial adenomyosis can contain cystic spaces filled with hemolyzed red blood cells and siderophages. When the microwave antenna is inserted into these cystic spaces of adenomyosis under real-time US guidance, and heat energy is subsequently introduced, the liquefactive area is instantaneously vaporized. Consequently, the volume of the lesion is reduced.
Symptomatic relief is consistently a primary concern during MWA treatment for symptomatic adenomyosis. The current study demonstrates that the median preoperative VAS and PBAC scores had significantly decreased by the 3-month follow-up evaluation (all P<0.001). This indicates that patients’ symptoms improved in the early stages following treatment. Generally, a larger extent of coagulative necrosis correlates with more effective clinical symptomatic relief from treatment [30].
In the current study, no major complications were observed. The most frequently encountered minor complication was lower abdominal pain, experienced by 48.1% of patients following MWA. A recent review article by Zhang et al. [15] reported an incidence rate of lower abdominal pain of between 43.9% and 100%. The present findings were promising, with only 30.8% of patients experiencing low-grade fevers. Fortunately, these patients recovered spontaneously, without the need for medical intervention. In general, these patients did not exhibit signs of postoperative infections. This could be explained by the absorption of heat following tissue trauma. Mild nausea is a common clinical symptom following anesthesia. Furthermore, 42.3% of patients reported experiencing vaginal discharge; this finding aligns with previous studies, in which the incidence rate ranged from 7.1% to 88.2%. It is important to highlight strategies for avoiding complications in current clinical practice. Factors such as real-time US guidance and monitoring, an emphasis on the surgical skills involved in MWA, and the use of artificial ascites may play an important role.
The present study did have certain limitations. First, the sample size was relatively small, consisting of only 52 patients (36 patients with VRR ≥50% and 16 with VRR <50%). This limited sample size may have introduced bias. Second, not all patients underwent consistent follow-up, and results were available for only 67.3% (35/52) of participants at the 12-month follow-up mark. Therefore, a need exists for observation of long-term follow-up results. Third, the retrospective nature of this study may limit the generalizability of the findings. Consequently, to confirm the efficacy of CEUS in the US-guided MWA treatment of symptomatic focal uterine adenomyosis, a prospective study with a larger sample size and extended follow-up period is necessary.
In conclusion, CEUS can be used to accurately evaluate the ablation zone of focal adenomyosis treated with MWA, positioning it as a potential alternative to MRI. The identification of non-enhancing areas on pre-treatment CEUS is correlated with satisfactory treatment outcomes.
Notes
Author Contributions
Conceptualization: Li XL, Li JX, Guan SN. Data acquisition: Yu SY, Jin YJ, Xu EJ, Deng EY, Qi JL. Data analysis or interpretation: Fan PL, Li QY, Ji ZB, Xu HX. Drafting of the manuscript: Li XL, Li JX, Yu SY, Fan PL, Jin YJ, Xu EJ, Guan SN, Deng EY, Li QY, Qi JL. Critical revision of the manuscript: Ji ZB, Xu HX. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (Grant 81901753), the Shanghai Municipal Health Commission (Grant SHSLCZDZK 03502), the Science and Technology Commission of Shanghai Municipality (Grants 21Y21901200 and 19DZ2251100), and the Scientific Research and Development Fund of Zhongshan Hospital of Fudan University (Grant 2022ZSQD07).
References
Article information Continued
Notes
Key point
Contrast-enhanced ultrasonography (CEUS) can be used to evaluate the ablation zone of focal adenomyosis treated with microwave ablation, similar to magnetic resonance imaging. The non-enhancing area on pre-treatment CEUS is correlated with sufficient treatment outcome.